Abstract
Individuals exhibiting an anxiety disorder are believed to possess an innate vulnerability that makes them susceptible to the disorder. Anxiety disorders are also associated with abnormalities in the interconnected brain regions of the amygdala and prefrontal cortex (PFC). However, the link between anxiety vulnerability and amygdala-PFC dysfunction is currently unclear. Accordingly, the present study sought to determine if innate dysfunction within the amygdala to PFC projection underlies the susceptibility to develop anxiety-like behavior, using an anxiety vulnerable rodent model. The inbred Wistar Kyoto (WKY) rat was used to model vulnerability, as this strain naturally expresses extinction-resistant avoidance; a behavior that models the symptom of avoidance present in anxiety disorders. Synaptic plasticity was assessed within the projection from the basolateral nucleus of the amygdala (BLA) to the prelimbic cortical subdivision of the PFC in WKY and Sprague Dawley (SD) rats. While WKY rats exhibited normal paired-pulse plasticity, they did not maintain long-term potentiation (LTP) as SD rats. Thus, impaired plasticity within the BLA-PL cortex projection may contribute to extinction resistant avoidance of WKY, as lesions of the PL cortex in SD rats impaired extinction of avoidance similar to WKY rats. Treatment with d-cycloserine to reverse the impaired LTP in WKY rats was unsuccessful. The lack of LTP in WKY rats was associated with a significant reduction of NMDA receptors containing NR2A subunits in the PL cortex. Thus, dysfunction in amygdala-PFC plasticity is innate in anxiety vulnerable rats and may promote extinction-resistant avoidance by disrupting communication between the amygdala and prefrontal cortex.
Keywords: Anxiety Vulnerability, Long-term Potentiation, Avoidance, Wistar Kyoto Rat, NR2A, NMDA
Introduction
Stress is often viewed as a trigger leading to the development of anxiety disorders. While stress is capable of exacerbating certain disorders, not all individuals exposed to stress, even prolonged period of stress, will develop anxiety (Mineka and Zinbarg, 2006). Further complicating this issue is the fact that a variety of psychological disorders can result from the same stressor (Jordan et al., 2004; Hussain et al., 2011). This variability raises the question: what puts individuals at risk for developing a specific mental illness? Individuals who develop an anxiety disorder following stress are believed to possess an innate vulnerability that makes them susceptible to develop the disorder (Mineka and Zinbarg, 2006). Thus, understanding the neural underpinnings of anxiety vulnerability can provide valuable insight into the etiology of this complex group of disorders.
Questions about anxiety vulnerability can be addressed using a rodent model of anxiety vulnerability, the Wistar Kyoto (WKY) rat. In the absence of stress, this strain does not exhibit an overtly anxious phenotype. However, WKY rats show an exacerbated response to stress (Pare and Redei, 1993; Pardon et al., 2002; Rittenhouse et al., 2002; Shepard and Myers, 2008), exhibiting behavioral inhibition in response to novelty (Pare, 1994; Servatius et al., 2008; McAuley et al., 2009), and an increased sensitivity to develop stress-related ulcers (Pare, 1989). Most striking is the innate ability of WKY rats to develop extinction-resistant avoidance that is dependent on stressor intensity and modulated by safety signals (Servatius et al., 2008; Beck et al., 2011; Jiao et al., 2011, Beck et al., 2014). Extinction-resistant avoidance is important, given that avoidance is a core symptom of anxiety disorders (American Psychiatric Association, 2013) and often predicts disease severity (Foa et al., 2006; O’Donnell et al., 2007). Compared to less vulnerable rat strains like the Sprague Dawley (SD) rat, WKY rats acquire lever-press avoidance faster and to a higher asymptotic level (Servatius et al., 2008; Beck et al., 2010). However, WKY rats are not capable of extinguishing learned avoidance to the same degree as SD rats (Servatius et al., 2008; Beck et al., 2011; Jiao et al., 2011). In fact, once avoidant behavior is acquired, WKY rats will begin each extinction session with an avoidance response despite showing within session extinction in the previous session (Beck et al., 2011).
Studies in rodents have implicated several key brain regions in the development of anxiety-like behavior specifically, the basolateral nucleus of the amygdala (BLA) (Hitchcock and Davis 1986; LeDoux et al. 1990; Maren 1999, Davis and Whalen 2001, Moscarello and LeDoux 2013) and the prelimbic (PL) subdivision of the prefrontal cortex (PFC) (Morgan and LeDoux 1995; Gewirtz et al. 1997; Corcoran and Quirk 2007; Bravo-Rivera et al. 2014; Wang et al. 2015). Synaptic plasticity between the BLA and the PL cortex, at least in part, underlies emotional learning and memory (Rosenkranz et al. 2003; Maroun Richter-Levin 2003; Vouimba and Maroun 2011). The BLA to PL cortex projection of SD rats expresses NMDA-dependent long-term potentiation (LTP) that is stress sensitive, as prior stress exposure inhibits the induction of LTP (Maroun Richter-Levin 2003). By blocking LTP within the BLA to PL projection, stress may block executive functioning provided by the PL cortex and allow for the amygdala to react more instinctually to the stressor (Maroun Richter-Levin 2003). Given the importance of the BLA to PL projection in modulating emotional learning and memory the present study was undertaken to determine whether an unstressed anxiety vulnerable rat strain, like the WKY rat strain, would display impaired plasticity within the BLA to PL cortex projection, similar to that observed in a stressed SD rat.
In the present study we tested the hypothesis that dysfunction within the BLA to PL cortex projection is an innate characteristic of anxiety vulnerable rats, and contributes to the development of extinction-resistant avoidance behavior in this strain.
Methods
Animals
Sprague-Dawley (n=46) and Wistar Kyoto (n=51) naïve male rats were obtained from Harlan Laboratories (Indianapolis, IN) at three months of age. Rats were individually housed under a 12:12hr light:dark cycle (lights on at 0700) and allowed free access to food and water. Prior to experimentation, all rats were given one week to acclimate. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the IACUC of the Veterans Affairs New Jersey Health Care System.
Electrophysiological Recording
Electrophysiology protocol was as previously described Cominski et al. (2014) with minor adaptations. Naïve rats were anesthetized with urethane (1.5g/kg, i.p.) and placed in a stereotaxic apparatus. A recording electrode (75μm, Teflon coated stainless steel wire) was inserted into the PL cortex (3.20 mm anterior and 0.5mm lateral from bregma, 3.0–3.5mm ventral from the brain surface) and a stimulating electrode (125μm, Teflon coated stainless steel wire) was inserted into the basolateral amygdala (BLA) (2.30 mm posterior and 4.9mm lateral from bregma, 7.0–8.0mm).
Constant current stimulation (biphasic pulse, 300μs duration) was delivered to the BLA (AM Systems Isolated Pulse Stimulator, 2100, Carlsborg, WA) at a rate of 1/10sec (or 1 pair/10s for paired pulse stimulation). Signals were amplified a total of 10,000X and bandpass filtered between 0.1–5000Hz (AM Systems Differential AC Amplifier, Model 1700, Carlsborg WA). Field potentials were visualized on a digital oscilloscope and recorded for off-line analysis (SciWorks software, version 7.2 SP1, DataWave Technologies). All analysis was performed on average waveforms (average of 6 individual responses). An input-output curve was generated using 200 –1000μA BLA stimulation. Paired-pulse responses were assessed for 50–1000ms interpulse intervals at a stimulus intensity that produced 50% maximal response amplitude, as determined by the input-output curve. The effect of paired pulse stimulation is expressed as amplitude of second response/amplitude of first response x 100. Stimulation parameters for the induction of long-term potentiation (LTP) were based on Maroun and Richter-Levin (2003). Theta burst stimulation (TBS) delivered to the BLA consisted of three sets of 10 trains with an interset interval of 1min and an intertrain interval of 200ms; each train consisted of 10 pulses at 100Hz. The effects of TBS were evaluated for 60 or 120min. Data are expressed as the percent of average baseline response: (amplitude of response /amplitude of averaged baseline) x 100. To confirm electrode placement lesions were made (30s, 500μA) at the conclusion of all recordings.
Pharmacological Manipulation
D-cycloserine (15mg/kg, intraperitoneal, i.p, Sigma, USA) was dissolved in sterile saline and administered to naïve rats following electrode optimization. This dose has previously been shown to reverse impairments in NMDA-dependent LTP (Yamamoto et al., 2008; Richter-Levin and Maroun, 2010). Control rats received an equivalent volume of saline (i.p). Recordings started 40min post injection.
Microdialysis and Neurochemical Analysis of Amino Acid Neurotransmitters from the PL Cortex
Surgeries were identical to that described under the electrophysiology procedure. A CMA 11 Guide Cannula and a 1mm CMA 11 Microdialysis Probe (Holliston, MA) was lowered into PL cortex of naïve rats. Sterile CNS Perfusion Fluid (CMA Microdialysis AB, Holliston, MA) was perfused into the PL cortex at a flow rate of 1.2ml/min using a CMA 402 Syringe Pump (Holliston, MA). Sampling occurred 1 hr after insertion of probes. Dialysate samples were collected in 20min intervals and immediately stored at −80°c for later analyses. A total of 3 samples were collected in each subject.
Analysis was performed as previously described (Rowley et al., 1995). Dialysate (24μl) was reacted with o-phthalaldehyde (1.0μl) for 10min at room temperature. Derivatized samples were analyzed using high performance liquid chromatography with electrochemical detection (HPLC-EC). The system consisted of a Waters Breeze pump (Milford, MA) that pumped mobile phase through an autosampler (model #717, Milford, MA), C18 column, and electrochemical detector set to 900nA (model #464, Milford, MA). The mobile phase consisted of monosodium phosphate (1.0M) and EDTA (0.1M) with 25% methanol; pH was adjusted to 4.5 with glacial acidic acid. A PC computer with Waters Empower software was used for data acquisition and analysis. Peak heights were used to determine quantities of d-serine, glycine, glutamate, and GABA. All values were corrected for differences in probe recovery rates. Data are expressed as pmol/μl.
Protein Extraction and Western Blot Analysis of NMDA Receptor Subunits from the PL Cortex
Brains were dissected from naïve rats and immediately placed on ice. Tissue was blocked to expose the prefrontal cortex and PL was extracted by tissue punch. All tissue was immediately placed on dry ice and stored at −80°c for later analysis.
Protein was isolated by homogenizing tissue in 200μl of ice-cold lysis buffer containing 150mM NaCl, 10mM Hepes buffer, 0.02% sodium azide, 0.1% sodium doedecyl sulfate,deoxycholic acid, 50mM NaF, 1% NP40, and a protease inhibitor cocktail. The homogenate was frozen in a slurry of 2-methylbutane and dry ice and thawed at 37°C three times. Lysates were then vortexed and incubated on ice for 15s five times. Then lysates were sonicated and placed on ice for 30min after which it was centrifuged. The supernatant was removed, aliquoted and stored at −80°C until use. Total protein concentrations were determined utilizing the BCA Protein Assay (Thermo Fisher Scientific Inc., Rockford, IL, USA). Samples (20μg) were loaded on each lane of a 3–8% tris-acetate gel (Life Technologies, Grand Island, NY). Electrophoresis was performed for 60min at 150V. The protein was then electrotransferred onto a polyvinylidene difluoride (PVDF) membrane, and stained with Swift membrane stain (G-Bioscience, St. Louis, MO). After blocking for 1hr in 5% milk powder, membranes were probed with primary antibodies against NR2A, NR2B, and NR1, (Millipore, Temecula, CA: 1:1000) overnight at 4°C. The membranes were then incubated with donkey anti-rabbit labeled secondary antibodies (Jackson ImmunoResearch, West Grove, PA: 1/500 dilution) and the signal was visualized using ECL Western Blotting Substrate (Thermo Fisher Scientific Inc., Rockford, IL USA) and by exposure to HyBlot CL®Autoradiography Film (Denville, South Plainfield, NJ). The membranes were then stripped with Re-Blot Plus Western Blot Mild Antibody Stripping Solution (EMD Millipore Corporation, Billerica, MA) and re-probed with an antibody against Beta Tubulin III (Abcam, Cambridge, MA, USA, 1:20000) to account for minor loading variability. The intensity of the bands was quantified using the UNSCAN-IT software (Silk Scientific, Orem, UT, USA).
Prelimbic (PL) Cortex Lesions
Bilateral PL cortex lesions occurred as previously described by Beck et al. (2014). Briefly, rats were anesthetized using sodium pentobarbital (50 mg/kg, i.p.) and placed into a stereotaxic apparatus. A Hamiliton microsyringe was lowered at a rate of 0.2mm/min on a 10° angle (toward midline) at the following coordinates: A/P: +2.9mm, L: ±1.0mm, DV −4.0mm. A total of 0.2 μl of ibotenic acid (5 mg/ml) was injected at each site.
Active Lever-Press Avoidance
The avoidance protocol was identical to previously described reports in Beck et al. (2014) and Pang et al. (2011). Briefly, training sessions were separated by 2–3 days, with 20 trials per session. At the start of each session, rats were placed individually in operant chambers (Coulbourn, Whitehall, PA). Each trial started with the onset of an auditory warning signal. Sixty seconds after the start of the warning signal, intermittent shock (every 3s, 1.0 mA intensity and 0.5s duration) was delivered through a grid floor. A lever press made at this time immediately terminated shock and warning signal and initiated the intertrial interval; this type of response represented an escape response. Lever presses made less than 60s after the start of the trial (i.e., prior to the shock period) prevented shocks from occurring, terminated the warning signal and initiated the intertrial interval; this type of response constituted an avoidance response. The intertrial interval was 3min in duration and cued by a flashing light. Acquisition training consisted of 12 sessions.
During extinction training, all procedures were the same as during the acquisition phase, except foot shock was omitted. Responses during the first 60s of the warning signal were classified as “avoidance” responses, and those with latencies greater than 60s were designated as “escape” responses. Extinction training consisted of 10 sessions.
Histology
Brains were checked for accuracy of lesions, electrodes and microdialysis probes. Rats were sacrificed and brains were quickly extracted, then stored in 10% phosphate buffered formalin. Brains were sectioned using a sliding microtome and mounted onto slides coated with gelatin. Sections were stained with cresyl violet, dehydrated, cover slipped, and viewed under a light microscope. Rats with electrode or probe placement found outside the target area were removed. Similarly, rats with lesions found to significantly disrupt the neighboring infralimbic (IL) cortex or anterior cingulate cortex were removed from the study. Lesion volume was also assessed in SD rats using the Cavalieri method (StereoInvestigator, MBF Bioscience, Williston, VT).
Statistical Analysis
Data are expressed as mean ± 1 standard error of the mean. Statistics were performed using SPSS for Mac (Version 20, IBM) with an alpha level of 0.05 For electrophysiology, statistics were performed on averaged amplitude values of BLA-evoked responses. Violations of sphericity determined by Mauchly’s Test were adjusted using the Greenhouse-Geisser correction. When post-hoc analysis was required, the Fisher’s LSD Multiple Comparison Test was used. The input-output data were assessed using a 2 (Strain) x 5 (Stimulus Intensity) or 2 (Strain) x 2 (Treatment) x 5 (Stimulus Intensity) mixed-design ANOVA. Paired pulse data was evaluated using a 2 (Strain) x 2 (Order) x 6 (ISI) mixed-design ANOVA. The presence of LTP was assessed in each strain using a repeated measures ANOVA with time as a factor. To determine whether long-lasting potentiation differed between the two strains a 2 (Strain) x 2 (Time) mixed-design ANOVA was performed on response amplitudes comparing baseline (5min prior to TBS) to 60 min post-TBS. To determine if BLA-evoked potentials at 60 min were significantly potentiated above baseline (5 min prior to TBS) a paired samples t-test was performed for each strain. In the second experiment, the effects of DCS on LTP were assessed for each strain using a 2 (Treatment) x 27 (Time) mixed-design ANOVA. To determine whether long-lasting potentiation differed between DCS and vehicle treated rats of each strain a 2 (Treatment) x 2 (Time) mixed-design ANOVA was performed comparing baseline (5min prior to TBS) to 120 min post-TBS. To determine if BLA-evoked potentials at 60 min were significantly potentiated above baseline (5 min prior to TBS) a paired samples t-test was performed for all groups. Microdialysis results were assessed by independent samples t-test. NMDA receptor subunit data was assessed by a 2 (Strain) x 2 (Blot) ANOVA. For the analysis of avoidance performance, sessions were blocked into groups of 2 sessions resulting in 6 session blocks for acquisition and 5 session blocks for extinction. Avoidance acquisition was assessed by a 2 (Strain) x 2 (Lesion) x 6 (Session Block) mixed-design ANOVA. Avoidance extinction was assessed by a 2 (Strain) x 2 (Lesion) x 5 (Session Block) mixed-design ANOVA. In addition, a Pearson’s r was calculated to assess the relationship between lesion volume and extinction performance in SD rats. Extinction performance was calculated by computing the percent change in avoidance between the last acquisition session and the last extinction session
Results
WKY and SD Rats Display Similar Presynaptic Plasticity, but WKY Rats Fail to Maintain Long-Term Potentiation (LTP) of BLA-Evoked Responses in the PL Cortex
We first sought to determine if innate dysfunction within the BLA to PL cortex projection exists in the form of abnormal synaptic plasticity in WKY rats. Changes in synaptic plasticity within the BLA to PL cortex projection are believed to be important in learning about aversive events. As previously stated, previous studies have shown that plasticity within the BLA to PL cortex projection is blocked by stress exposure (Maroun and Richter-Levin, 2003) and changes in response to aversive learning paradigms (Vouimba and Maroun, 2011).
Response Latency and Input-Output Response Curve
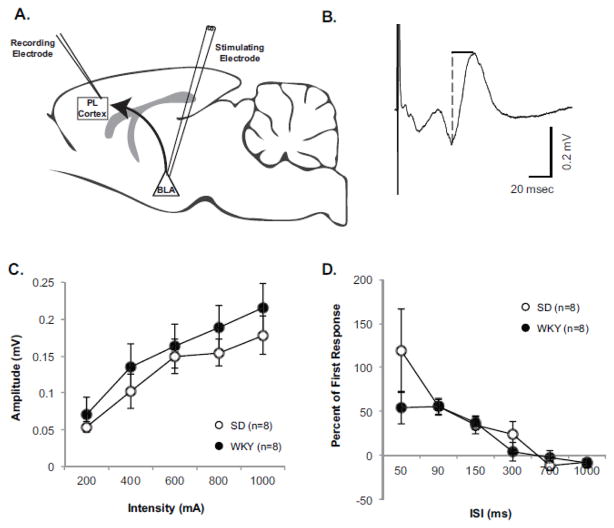
Recording and stimulation sites are illustrated in Figure 1. Stimulation of the BLA elicited a distinct response observed in the PL cortex. BLA-evoked response amplitude were measured from peak negativity to peak positivity (Figure 2B). The average response latency for peak negativity was 21.0 ± 0.8ms (n=16) and for peak positivity was 31.4 ± 1.2ms (n=16), and latencies did not differ significantly between strains. An input-output (I/O) response curve was performed on BLA-evoked responses to identify potential strain differences (n=8 per strain). Increase in stimulus intensity resulted in larger evoked responses [main effect of Stimulus Intensity: F (1.65, 23.08) = 26.43, p<0.001], but evoked responses did not differ between strains (Figure 2C).
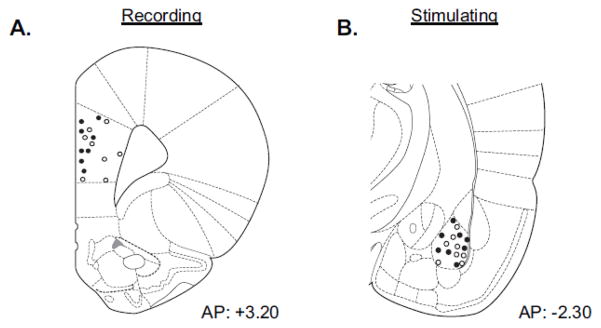
Figure 1. Confirmation of Electrode Placement.
Upon the completion of all recordings, electrode placement was marked with an electrolytic lesion. A–B) Shown are diagrams of recording electrode placement in the prelimbic (PL) cortex and stimulating electrode placement in basolateral amygdala (BLA).
Figure 2. WKY and SD Rats Display Similar Input-Output Response Curves and Presynaptic Plasticity of BLA-Evoked responses in the PL Cortex.
A) Shown is a diagram illustrating the experimental design. B) Representative field recording with dashed line showing how amplitude was measured for each response. (C) An input-output response curve was assessed for BLA-evoked responses in naïve Sprague Dawley (SD, open circles, n=8) and Wistar Kyoto (WKY, closed circles, n=8) rats between the current intensities of 200–1000μA. Data are expressed as averaged raw values. Evoked response amplitudes significantly increased in response to increasing current, but amplitudes did not differ between strains. (D) Paired pulse profiles were also assessed in naïve SD and WKY rats between the interstimulus intervals (ISI) of 50–1000ms. Data are expressed as percent of first response. BLA-evoked responses expressed significant paired pulse facilitation at the IPIs of 50, 90, and 150, but profiles did not differ between strains.
Paired-Pulse Profile
A paired pulse profile of BLA-evoked response amplitudes were assessed in WKY and SD rats (Figure 2D) as a measure of presynaptic plasticity (Fioravante and Regehr, 2011). The paired pulse profile of BLA-evoked responses exhibited robust facilitation at ISI of 50, 90, and 150ms with relatively no change at longer ISIs [main effect of ISI F (1.87, 26.21) = 17.19, p<0.001, main effect of Order F(1,14) = 40.22, p<0.001, and an ISI x Order interaction F (1.49, 20.88) = 19.01 p<0.001, Fisher’s LSD p<0.05]; however, profiles did not differ as a factor of strain. These results indicate that basic functioning and presynaptic plasticity of the BLA to PL cortex projection is similar between SD and WKY rats.
Long-Term Potentiation
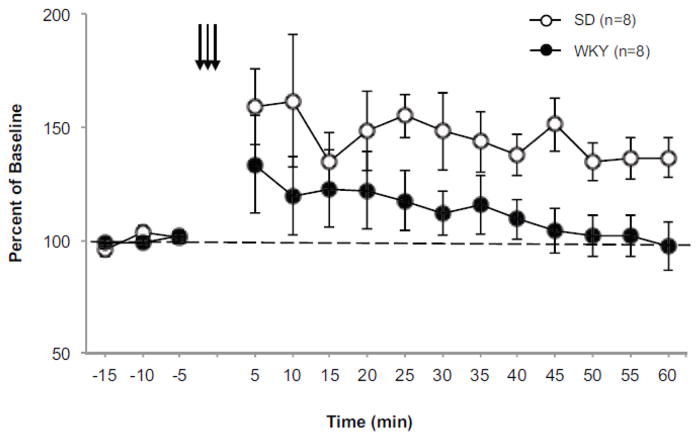
As previously described (Maroun and Richter-Levin, 2003), BLA-evoked response amplitudes were significantly potentiated in SD rats following theta-burst stimulation (TBS) (Figure 3) as evident by a main effect of Time [F (12,84)=2.50, p<0.001]. However, a significant effect of time was not observed in WKY rats following TBS. Further analysis revealed that SD and WKY rats differed in the level of long-lasting potentiation observed at 60 min post TBS [Strain x Time interaction F(1,14) = 4.74, p<0.019]. Paired samples t-test revealed that BLA-evoked response amplitudes were only significantly potentiated from baseline in SD rats [t (7) = −3.78, p<0.002], stabilizing at approximately 36% above baseline. This was in stark contrast to BLA-evoked response amplitudes observed in WKY rats that stabilized at approximately 1% above baseline. Taken together, the data indicate that WKY rats did not show significant long-lasting potentiation of BLA-evoked field potentials in response to TBS. Given that paired pulse profiles were not different between strains, the lack of LTP in WKY rats may result from postsynaptic dysfunction within the PL cortex.
Figure 3. WKY rats lack LTP of BLA-evoked responses in the PL cortex.
LTP was assessed in BLA-evoked responses of naïve Sprague Dawley (SD, open circles, n=8) and Wistar Kyoto (WKY, closed circles, n=8) rats following theta-burst stimulation (TBS). All data is expressed as percent of baseline. Arrows represent three sets of high frequency stimulation used to induce LTP. The dashed line represents baseline. Each data point is the average of 30 consecutive sweeps (5 min). BLA-evoked responses were followed for 60 min after the induction of LTP. BLA-evoked responses expressed robust potentiation following TBS, however, this result was not observed in WKY rats. At 60 min post TBS the evoked response of SD, but not WKY rats was significantly potentiated above baseline.
D-Cycloserine (DCS) Does Not Reverse the Lack of LTP in WKY Rats
Previous studies have shown that LTP in the BLA to PL cortex projection is NMDA-dependent (Maroun and Richter-Levin, 2003); thus we sought to determine if an NMDA receptor agonist would reverse the impaired LTP in WKY rats. The partial NMDA receptor agonist DCS (15mg/kg) was selected because this drug can facilitate extinction learning (Walker et al., 2002; Ledgerwood et al., 2003; Weber et al., 2007) and reverse stress-induced deficits in synaptic plasticity (Yamamoto et al., 2008; Richter-Levin and Maroun, 2010).
Input-Output Response Curve
An I/O response curve was assessed 40min after vehicle (SD: n=4, WKY: n=6) or DCS (SD: n=5, WKY: n=5) injection. BLA-evoked responses increased in response to greater stimulus intensities [main effect of Stimulus Intensity F (1.41, 22.48) = 28.25, p< 0.001]. However, BLA-evoked responses were not affected by treatment and did not differ between strains.
Long-Term Potentiation
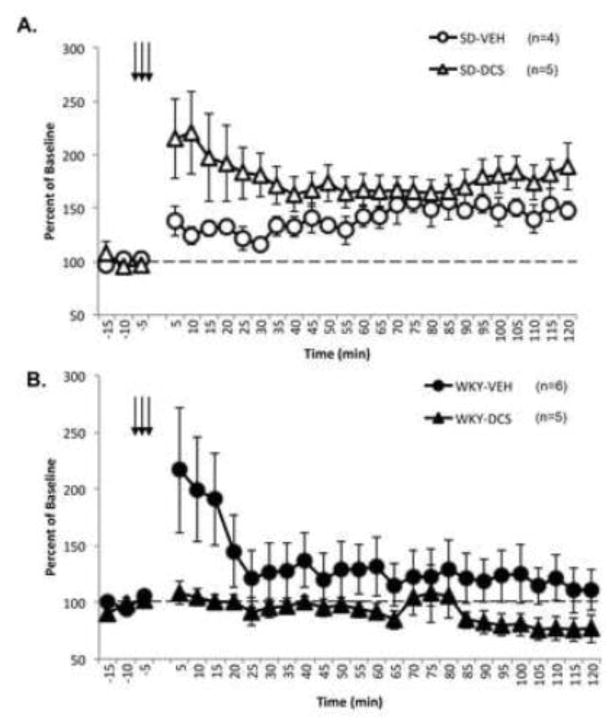
Consistent with our initial results, BLA-evoked responses of SD rats exhibited significant potentiation following TBS (Figure 4A) as evident by a main effect of Time [F (26, 182) = 5.00, p<0.001]. However, the level of potentiation significantly differed between vehicle (n=4) and DCS (n=5) treated SD rats [Time x Treatment interaction F (26, 182) = 1.90, p<0.010]. Further analysis revealed that vehicle and DCS treated rats showed long-lasting potentiation present at 120 min post TBS [main effect of Time F (1,7) = 11.85 p<0.01]. However, a treatment effect was not observed at this time point.
Figure 4. D-Cycloserine (DCS) Does Not Reverse Lack of LTP in WKY Rats.
Naïve Sprague Dawley (SD, open) and Wistar Kyoto (WKY, closed) were given 15mg/kg DCS (triangles) or vehicle (circles) and assessed for LTP following theta-burst stimulation (TBS). All data is expressed as percent of baseline. Arrows represent three sets of high frequency stimulation used to induce LTP. The dashed line represents baseline. Each data point is the average of 30 consecutive sweeps (5 min). TBS was identical to initial experiment and represented by arrows. A) BLA-evoked responses in SD rats were significantly potentiated from baseline, and treatment with DCS further facilitated the observed potentiation above saline controls immediately following TBS. B) However the BLA-evoked response in WKY rats failed to show LTP and DCS had no effect on the BLA-evoked response in this strain. At 120 min post TBS BLA-evoked responses in SD, but not WKY rats were significantly potentiated above baseline.
Interestingly, DCS treatment appeared to produce opposite effects on BLA-evoked responses in WKY rats (Figure 4B). A main effect of Time [F (26, 234) = 2.40, p<0.001] was observed in BLA-evoked responses from vehicle (n=6) and DCS (n=5) treated WKY rats. A main effect of Treatment [ F (1,9) = 5.42, p<0.047] was also observed. Consistent with previous findings, long-lasting potentiation was not observed in vehicle or DCS treated WKY rats at 120 min post TBS. However, BLA-evoked potentials from vehicle and DCS treated WKY rats significantly differed from each other [main effect of Treatment F (1,9) = 7.53, p<0.045] at 120 min post TBS.
Taken together, the data confirms our initial findings that WKY rats fail to express significant long-lasting potentiation of BLA-evoked field potentials following TBS. While DCS treatment appeared to potentiate BLA-evoked field potentials in SD rats at early time points, DCS treatment in WKY rats appears to further blunt any brief potentiation observed in this strain.
WKY and SD Rats Display Similar Basal Levels of Extracellular Amino Acid Neurotransmitters in the PL cortex
In an attempt to determine if abnormalities at the neurotransmitter level contribute to lack of NMDA-dependent LTP observed in the PL cortex of anxiety vulnerable rats, we focused our efforts on determining if strain differences exist in neurotransmitters known to modulate NMDA receptor function (Table 1). Compared to SD rats (n=6), WKY (n=8) rats tended to have lower levels of extracellular glutamate, but this result did not reach significance (p=0.07). The levels of endogenous NMDA receptor co-agonists, glycine and d-serine were not different between strains. Moreover, GABA was assessed to determine the inhibitory tone of the PL cortex, and was not different between strains.
Table 1. Amino acid neurotransmitters levels in the prelimbic (PL) cortex of WKY and SD rats.
Amino acid neurotransmitters levels in the PL cortex do not differ between strains. Basal levels of serine, glycine, glutamate, and GABA were assessed in naïve Sprague Dawley (SD, n=6) and Wistar Kyoto (WKY, n=8) rats by microdialysis and high-performance liquid chromatography with electrochemical detection (HPLC-EC). All values are based on a standard and have been corrected for differences probe efficiency. No significant differences were observed in neurotransmitter levels between strains; however, glutamate did trend lower in WKY rats. P-values were generated by independent samples t-test.
| SD (n=6) | WKY (n=8) | p-value | |
|---|---|---|---|
| Serine | 4.29a±0.51 | 4.54±0.88 | 0.631 |
| Glycine | 146.11±18.13 | 163.23±28.95 | 0.544 |
| Glutamate | 13.89±3.68 | 7.27±0.99 | 0.072 |
Units are pmol/ml
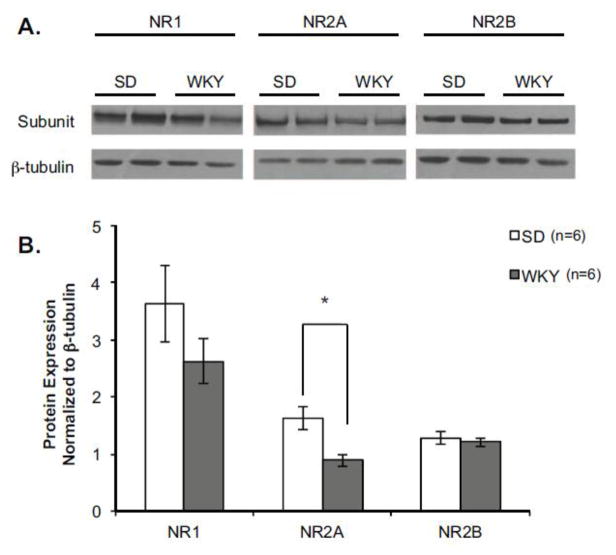
WKY Rats Show Reduced NR2A Protein Expression in the PL Cortex
Next we sought to determine if abnormalities exist at the receptor level that might contribute to a lack of NMDA-dependent LTP observed in the PL cortex of anxiety vulnerable rats (Figure 5). NMDA receptor subunit composition can modulate potential for plasticity (Cull-Candy and Leszkiewicz, 2004). Therefore, the levels of NR1, NR2A and NR2B subunits from the PL cortex were quantified in naïve SD (n=6) and WKY (n=6) rats. Interestingly, WKY rats expressed significantly less NR2A protein in the PL cortex compared to SD rats [main effect of Strain F (1, 8) = 13.22, p<0.01]. NR1 protein expression also trended lower in WKY rats, although this difference failed to reach statistical significance (p=0.07). No strain differences were observed in the expression of NR2B protein.
Figure 5. WKY Rats Show Reduced NR2A Protein Expression in the PL Cortex.
Anxiety vulnerable rats express lower levels of NR2A protein in the prelimbic (PL) cortex. Protein expression of NMDA receptor subunits was assessed in the PL cortex of naïve Sprague Dawley (SD, n=6) and Wistar Kyoto (WKY, n=6) rats. A) Representative blots of NMDA receptor subunits from the PL cortex of 2 SD and 2 WKY rats. B) Compared to SD rats (open bars), WKY rats (closed bars) express significantly less NR2A protein. This result was specific to the NR2A subunit, as strain differences were not observed in NR1 or NR2B protein levels. *p<0.05.
Lesions to the PL Cortex Promote Extinction-Resistant Avoidance Behavior
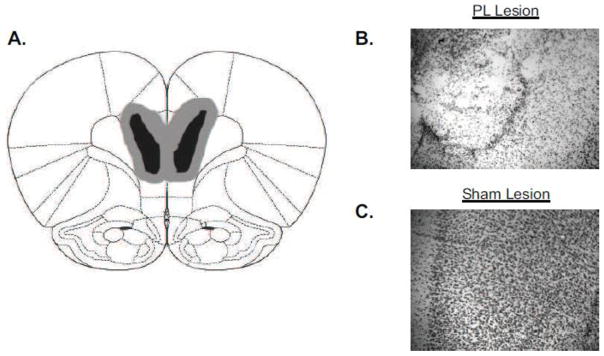
Ibotenic acid lesions were made to the PL cortex of SD (sham n=9, lesion n=8) and WKY (sham n=8, lesion n=10) rats prior to avoidance training to determine the functional consequence of innate PL cortex dysfunction in WKY rats. The extent of the lesions are depicted in Figure 6.
Figure 6. Confirmation of PL Cortex Lesions.
Ibotenic acid lesions were made to the prelimbic (PL) cortex of Sprague Dawley (SD) and Wistar Kyoto (WKY) rats prior to avoidance training. Accuracy of lesions was confirmed for each subject. A) Diagram illustrating the smallest (black) and the largest (gray) lesion observed. Representative photomicrographs of lesion (B) and sham (C) rats reveal that ibotenic acid lesions resulted in significant cell loss and disruption of cortical layers with the PL cortex.
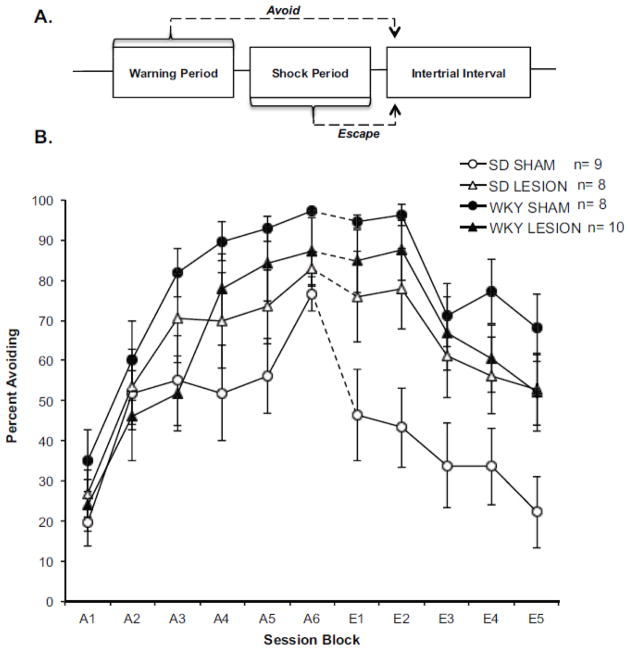
Avoidance Acquisition
All groups successfully acquired avoidant responding (Figure 7B: see A1–A6) as evident by a main effect of Session Block [F(3.00, 91.94) = 50.87 p<0.001]. WKY rats acquired avoidant responding faster than SD rats [Session Block x Strain Interaction: F(3.00, 91.94) =2.742, p<0.048], however, PL cortex lesions had no effect on the rate of acquisition in either strain.
Figure 7. Lesions to the PL Cortex Promote Extinction-Resistant Avoidance Behavior.
A) Shown is a diagram illustrating an avoidance trial. Each trial begins with the presentation of a 60s warning tone. A leverpress response made during this period constitutes an avoidance response. If a rat fails to respond within 60s footshocks are initiated. A lever-press response made during the shock period constitutes an escape response. A lever-press response during either period sends the rat into a 3-min intertrial interval (ITI) that is free of shock. B) Ibotenic acid lesions were made to the PL cortex of Sprague Dawley (SD) and Wistar Kyoto (WKY) rats prior to avoidance training. Graph represents mean percentage of avoidance responses per session block (session block = 2 training sessions) for SD (open) and WKY rats (closed) with ibotenic acid (triangle) or sham (circle) lesions. Acquisition session blocks are denoted with an “A” and extinction session blocks are denoted with an “E”. While WKY rats acquired lever-press avoidance faster than SD rats, PL cortex lesions had no effect on the rate of acquisition in either strain. Conversely, PL cortex lesions had a significant effect on extinction training in a strain dependent manner. WKY sham rats showed a characteristic resistance to extinguish avoidant responding compared to SD sham rats. In WKY rats, PL cortex lesions had no effect on this behavior. However, PL cortex lesions in SD rats impaired extinction similar to levels observed in WKY sham and lesion rats. The asterisk (*) represents a significant difference between the SD lesion and SD sham groups during extinction training.
Avoidance Extinction
During extinction, a decreased in avoidant responding was observed across extinction sessions [main effect of Session Block: F(2.17, 67.13) = 27.20 p<0.001]. Consistent with previous findings, WKY sham rats showed a resistance to extinction compared to SD shams [main effect of Strain F(1, 31) =8.57, p<0.006]. PL cortex lesions significantly impaired avoidance extinction in a strain dependent manner (Figure 7B: see E1–E5). PL cortex lesions in SD rats impaired extinction [Strain x Lesion Interaction: F(1, 31) = 5.28, p<0.028]. Pairwise comparisons revealed a significant difference between SD sham and SD lesion groups, but no difference between SD lesion and either WKY group [Fisher’s LSD p<0.05]. PL cortex lesions had no effect in WKY rats. The strain by lesion interaction was most prominent in the first extinction block (compare A6 and E1). In order to determine whether damage outside the PL cortex could account for extinction performance in SD rats, a correlation analysis was conducted between lesion volume and extinction performance. No correlation was found suggesting that extinction-resistance in SD rats resulted from damage to the PL cortex and not neighboring brain regions.
These results indicate that PL cortex contributes to avoidance extinction although it may not be necessary for avoidance acquisition. PL cortex lesions having no effect on extinction in WKY rats is consistent with the hypothesis that PL cortex dysfunction naturally exists in WKY rats and contributes to the anxiety vulnerable phenotype expressed by this strain.
Discussion
The present study sought to determine if abnormalities exists in synaptic plasticity within the BLA to PL cortex projection of WKY rats and how such dysfunction contributes to the anxiety vulnerable phenotype present in this strain. WKY rats lacked NMDA-dependent LTP in the BLA to PL cortex projection. Impaired LTP may be due to post-synaptic dysfunction, as presynaptic plasticity did not differ between strains. DCS was ineffective in reversing the lack of LTP in WKY rats at a dose that was effective in facilitating early potentiation in SD rats. Amino acid transmitters that modulate NMDA receptors did not differ between strains. The impaired LTP in WKY rats may be due to fewer NR2A subunits in the PL cortex of WKY rats that may also underlie the avoidant extinction-resistance observed in this model of anxiety vulnerability.
Lack of LTP within the BLA to PL projection of WKY rats
WKY rats show a lack of synaptic plasticity within the BLA to PL cortex projection. The BLA and PL cortex are connected by a bi-directional circuit that includes a direct reciprocal projection back to BLA from the PL cortex (McDonald, 1987; Cassell et al., 1989; St Onge et al., 2012) and an indirect reciprocal projection to BLA (via the PL cortex to infralimbic cortex, amygdala intercalated cells to the central amygdala) (Pare and Smith, 1993; McDonald et al., 1996). Through the BLA to PL projection, information from the amygdala reaches cortical levels. The direct and indirect projection from the PL cortex back to amygdala provides cortical modulation of the amygdala circuitry that is important for aversive learning (Corcoran and Quirk, 2007; Laurent and Westbrook, 2008; Knapska and Maren, 2009). Plasticity in these circuits allows an organism to learn about aversive events, their valence and what predicts them. Further, hippocampal input to the PL cortex may provide additional contextual information (Sierra-Mercado et al., 2011). Stress modulates plasticity within the BLA-PL circuits, providing saliency (i.e., presence or absence of danger) to the event (Maroun Richter-Levin 2003). Thus, the lack of LTP within the BLA to PL cortex projection in the present study suggests that anxiety vulnerable rats may have difficulty learning about and predicting when aversive events will occur, giving rise to a lack of temporal and contextual specificity common in anxiety disorders.
The Role of the NR2A Subunit in Plasticity and Behavior
Our data suggests that the lack of synaptic plasticity within the BLA to PL cortex projection is due to limited NR2A subunits causing impaired prefrontal NMDA-dependent LTP. Interestingly, the NR2A subunit plays an essential role in NMDA-dependent LTP in the forebrain. A functional NMDA receptor is a tetramer composed of two subunits: NR1 and NR2 (A–D) (Cull-Candy and Leszkiewicz, 2004). The NR2 subunits express unique conductance properties that contribute to specific forms of synaptic plasticity (Monyer et al. 1994; Loftis and Janowsky, 2003; Cull-Candy and Leszkiewicz, 2004). In the forebrain, most forms of NMDA-dependent plasticity have been attributed to NMDA receptors containing NR2A and NR2B subunits (Monyer et al., 1994; Loftis and Janowsky, 2003; Liu et al., 2004; Massey et al., 2004; Zhao et al., 2005). For example, NR2A subunits in the hippocampus and perirhinal cortex are responsible for mediating LTP while NR2B subunits are responsible for mediating LTD (Liu et al., 2004; Massey et al., 2004; Jin and Feig, 2010). This may be a result of NR2A containing receptors predominantly expressed in the synapse (Stocca and Vicini, 1998), whereas receptors containing NR2B are expressed mostly at extrasynaptic locations (Tovar and Westbrook, 1999; Petralia et al., 2010). However, other studies have shown that the NR2B subunit is also important for mediating plasticity and learning. For example, both NR2A and NR2B receptor subunits in the anterior cingulate contribute to LTP (Zhao et al., 2005) and the NR2B subunit may be important in neuronal reorganization processes associated with various forms of learning (Barria and Malinow, 2005, Tang et al., 1999). Interestingly, SD and WKY rats did not differ in NR2B protein expression. Thus, given the importance of NR2A and NR2B subunits in mediating plasticity, it is possible that the lower quantities of NR2A receptors disrupt the balance of plasticity within the PL cortex and directly contributes to the lack of LTP observed in anxiety vulnerable rats.
Behavioral evidence also supports the notion that NR2A receptors are involved in anxiety-like behaviors. Antagonism of NR2A and NR2B receptors produce different results when administered during the extinction of conditioned fear, a procedure that depends on NMDA receptors in the PFC (Santini et al., 2001; Burgos-Robles et al., 2007). Rats treated with CPP (an NMDA competitive antagonist with greatest affinity for NR2A subunits (Lozovaya et al., 2004) show within session extinction that is not maintained in subsequent sessions (Santini et al., 2001), a pattern that is similar to WKY rats in avoidance extinction (Beck et al., 2011). In contrast, rats treated with ifenprodil, a selective NR2B antagonist (Williams, 2001), do not exhibit within session extinction (Sotres-Bayon et al., 2007).
Treatment of Anxiety Disorders with DCS
The fact that DCS appeared to further impair LTP in WKY rats was surprising. Several studies have shown that DCS can reverse impairments in NMDA-dependent LTP caused by stress exposure (Yamamoto et al., 2008; Richter-Levin and Maroun, 2010). Although our rats were not exposed to stress, one might expect similar results in a stress-sensitive strain. DCS exerts its physiological effects by binding to the glycine-binding site of the NR1 subunit (Hood et al., 1989; Sheinin et al., 2001). However, the NR2 subunits are responsible for mediating LTP. Thus, a reduction in NR2A subunits represents an overall reduction in NMDA subunits capable of inducing LTP. DCS also reduces glutamate transmission (Rouaud and Billard, 2003), which might negate the facilitatory effects of DCS on NMDA-dependent LTP. The reduction of glutamate transmission may be more pronounced in sensitive individuals. The WKY rats trended to have lower levels of glutamate in the PL cortex compared to SD rats. Thus, DCS could potentially make it more difficult to induce LTP in WKY rats rather than facilitate LTP. Moreover, recent clinical studies using DCS, in combination with cognitive behavioral therapy, show that benefits of DCS may be limited to only specific types of anxiety disorders (Storch et al., 2007; Siegmund et al., 2011; Litz et al., 2012; Hofmann et al., 2013).
Involvement of the PL Cortex in Extinction-Resistant Avoidance
The finding that lesions to the PL cortex inhibited avoidance extinction in SD, but not WKY rats is consistent with the hypothesis that dysfunction within the PL cortex is innate in anxiety WKY rats. In SD rats, activation of the PL cortex is required to significantly extinguish avoidant responding. However, in WKY rats innate dysfunction within the PL cortex prevents this brain region from being engaged during extinction training, resulting in extinction-resistant avoidance. Thus, lesions of the PL cortex would not worsen avoidance extinction in WKY rats, as their PL cortex would naturally not be engaged during extinction training. While our results provide support for this hypothesis, it is likely that extinction-resistant avoidance in WKY rats is more complex and results from breakdown in communication between the PL cortex and other brain regions.
To date, several studies have assessed the role of the PFC in the extinction of shock-motivated behaviors; however, not all shock-motivated learning paradigms are equivalent. The overwhelming majority of these studies have utilized Pavlovian fear conditioning. In this task, a tone is paired with shock such that the animal learns the tone-shock association and subsequently displays a reflexive freezing response when the tone is re-experienced (Davis, 1992; Fanselow and Ledoux, 1999). Conditioned fear is an important adaptive response readily acquired in normal rodents. Conditioned fear is often difficult to extinguish and has been shown to involve complex neural reorganization processes (Sah and Westbrook, 2008). However, the active lever-press avoidance paradigm described in the present manuscript differs from conditioned fear. During active lever-press avoidance, a rat must learn several associations: 1) lever-press behavior will terminate foot-shock 2) the (warning) tone is predictive of impending foot-shock; and lever-press behavior during the tone ends the trial, without shock exposure. Freezing in this task impairs a rat’s ability to mount an active avoidance response. Thus, fear conditioning requires a rat to execute a reactive behavior, whereas active avoidance requires rats to perform a proactive behavior.
Fear conditioning and active-lever-press avoidance may also differ neurobiologically. Extinction of conditioned fear is primarily mediated by the infralimbic (IL) cortex, a subregion of the PFC (Laurent and Westbrook, 2009; Milad and Quirk, 2002; Sierra-Mercado et al., 2011). Further, inactivation of the PL cortex facilitates extinction of conditioned fear. This result differs from the findings of the present manuscript as lesions to the PL cortex impair lever-press avoidance extinction. Given the uniqueness of both paradigms it is not surprising that the same brain region may have different effects on extinction training. This point was emphasized in a recent study comparing the role of the IL cortex in the extinction of two different aversive learning paradigms; contextual fear conditioning and conditioned odor aversion. The study found that while the IL cortex is necessary for the consolidation of extinction training of recent and remote contextual fear memories, it is only involved in the consolidation of extinction training for recent conditioned odor aversion (Awad et al., 2015). Thus, this study provides an example of how the same brain region could uniquely contribute to the extinction in two different aversive learning paradigms. Further studies are necessary to determine why certain subregions of the PFC contribute differently to various shock-motivated learning paradigms and what this means in terms of modeling anxiety disorders.
Overlap with Major Depressive Disorder
It is also important to acknowledge that while we have used the WKY rat as a model of anxiety vulnerability, other studies use this strain as a model of major depressive disorder (MDD) (Wieland et al., 1986; Pare, 1994; Tejani-Butt et al., 1994; Lopez-Rubalcava and Lucki, 2000; Will et al., 2003; Belujon and Grace, 2014). Anxiety and depression are often comorbid (American Psychiatric Association, 2013) and both disorders are associated with PFC dysfunction (Baxter et al., 1989; Rajkowska et al., 2007; Koenigs and Grafman, 2009). Postmortem analysis of PFC tissue obtained from individuals with MDD reveals that depressed individuals have significantly less NR2A and NR2B subunits (Feyissa et al., 2009). Therefore, it may be possible that MDD is associated with reduction of both subunits while certain anxiety disorders are associated with a reduction in only NR2A.
Summary
Here, we present evidence that innate dysfunction within the BLA to PL cortex projection exists in a naïve, anxiety vulnerable rat strain. When exposed to stress, our results suggest that vulnerable rats are forced to learn about the stressor without full PL cortex engagement, which in turn leads to the development of anxiety-like symptoms (e.g. extinction-resistant avoidance).
Highlights.
WKY rats fail to express LTP within the BLA to PL projection following TBS
DCS fails to reverse the lack of LTP within the BLA to PL projection of WKY rats
Lack of LTP in WKY rats may be due to a reduction in NR2A subunits in the PL cortex
The WKY rat naturally develops extinction-resistant avoidance
Lesions of the PL cortex in SD rats impair avoidance extinction similar to WKY rats
Acknowledgments
The research presented in the current study was funded by the Biomedical Laboratory Research and Department of Veterans Affairs Office of Research & Development (1I01BX000218 and I01BX000132), the NIH (RO1-NS44373), and funds from the Stress and Motivated Behavior Institute.
Footnotes
Disclosures:
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The opinions and conclusions presented are those of the authors and are not the official position of the U.S. Department of Veterans Affairs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5. American Psychiatric Publishing; Washington, DC: 2013. [Google Scholar]
- Awad W, Ferreira G, Maroun M. Dissociation of the Role of Infralimbic Cortex in Learning and Consolidation of Extinction of Recent and Remote Aversion Memory. Neuropsychopharmacology. 2015;103 doi: 10.1038/npp.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA Receptor Composition Controls Synaptic Plasticity by Regulating Binding of CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Archives of General Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Beck KD, Jiao X, Pang KC, Servatius RJ. Vulnerability factors in anxiety determined through differences in active-avoidance behavior. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34:852–860. doi: 10.1016/j.pnpbp.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Beck KD, Jiao X, Smith IM, Myers CE, Pang KC, Servatius RJ. ITI-Signals and Prelimbic Cortex Facilitate Avoidance Acquisition and Reduce Avoidance Latencies, Respectively, in Male WKY Rats. Frontiers in Behavioral Neuroscience. 2014;8:403. doi: 10.3389/fnbeh.2014.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Jiao X, Ricart TM, Myers CE, Minor TR, Pang KC, Servatius RJ. Vulnerability factors in anxiety: Strain and sex differences in the use of signals associated with non-threat during the acquisition and extinction of active-avoidance behavior. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2011;35:1659–1670. doi: 10.1016/j.pnpbp.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biological Psychiatry. 2014;76:927–936. doi: 10.1016/j.biopsych.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Rivera C, Roman-Ortiz C, Brignoni-Perez E, Sotres-Bayon F, Quirk GJ. Neural structures mediating expression and extinction of platform-mediated avoidance. Journal of Neuroscience. 2014;34:9736–9742. doi: 10.1523/JNEUROSCI.0191-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Chittick CA, Siegel MA, Wright DJ. Collateralization of the amygdaloid projections of the rat prelimbic and infralimbic cortices. Journal of Comparative Neurology. 1989;279:235–248. doi: 10.1002/cne.902790207. [DOI] [PubMed] [Google Scholar]
- Cominski TP, Jiao X, Catuzzi JE, Stewart AL, Pang KC. The role of the hippocampus in avoidance learning and anxiety vulnerability. Frontiers in Behavioral Neuroscience. 2014;8:273. doi: 10.3389/fnbeh.2014.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. Journal of Neuroscience. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Science’s STKE : Signal Transduction Knowledge Environment. 2004;255:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–32. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Progress in Neuropsychopharmacology & Biological Psychiatry. 2009;33:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Current Opinion in Neurobiology. 2011;21:269–274. doi: 10.1016/j.conb.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Stein DJ, McFarlane AC. Symptomatology and psychopathology of mental health problems after disaster. The Journal of Clinical Psychiatry. 2006;67:15–25. [PubMed] [Google Scholar]
- Gewirtz JC, Falls WA, Davis M. Normal conditioned inhibition and extinction of freezing and fear-potentiated startle following electrolytic lesions of medical prefrontal cortex in rats. Behavioral Neuroscience. 1997;111:712–726. doi: 10.1037//0735-7044.111.4.712. [DOI] [PubMed] [Google Scholar]
- Hitchcock J, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behavioral Neuroscience. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Wu JQ, Boettcher H. D-Cycloserine as an augmentation strategy for cognitive behavioral therapy of anxiety disorders. Biology of Mood & Anxiety disorders. 2013;3:11. doi: 10.1186/2045-5380-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood WF, Compton RP, Monahan JB. D-cycloserine: a ligand for the N-methyl-D-aspartate coupled glycine receptor has partial agonist characteristics. Neuroscience Letters. 1989;98:91–95. doi: 10.1016/0304-3940(89)90379-0. [DOI] [PubMed] [Google Scholar]
- Hussain A, Weisaeth L, Heir T. Psychiatric disorders and functional impairment among disaster victims after exposure to a natural disaster: a population based study. Journal of Affective Disorders. 2011;128:135–141. doi: 10.1016/j.jad.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Jiao X, Pang KC, Beck KD, Minor TR, Servatius RJ. Avoidance perseveration during extinction training in Wistar-Kyoto rats: an interaction of innate vulnerability and stressor intensity. Behavioural Brain Research. 2011;221:98–107. doi: 10.1016/j.bbr.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SX, Feig LA. Long-term potentiation in the CA1 hippocampus induced by NR2A subunit containing NMDA glutamate receptors is mediated by Ras-GRF2/Erk map kinase signaling. PloS One. 2010 doi: 10.1371/journal.pone.0011732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan NN, Hoge CW, Tobler SK, Wells J, Dydek GJ, Egerton WE. Mental health impact of 9/11 Pentagon attack: validation of a rapid assessment tool. American Journal of Preventive Medicine. 2004;26:284–293. doi: 10.1016/j.amepre.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learning and Memory. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behavioural Brain Research. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learning and Memory. 2008;15:657–666. doi: 10.1101/lm.1080108. [DOI] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learning and Memory. 2009;16:520–529. doi: 10.1101/lm.1474609. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behavioral Neuroscience. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. Journal of Neuroscience. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litz BT, Salters-Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, Hofmann SG. A randomized placebo-controlled trial of D-cycloserine and exposure therapy for posttraumatic stress disorder. Journal of Psychiatric Research. 2012;46:1184–1190. doi: 10.1016/j.jpsychires.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacology & Therapeutics. 2003;97:55–85. doi: 10.1016/s0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Lozovaya NA, Grebenyuk SE, Tsintsadze T, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst EPSC in rat hippocampus. The Journal of Physiology. 2004;558:451–463. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. Journal of Neuroscience. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. Journal of Neuroscience. 2003;23:4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. Journal of Neuroscience. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley JD, Stewart AL, Webber ES, Cromwell HC, Servatius RJ, Pang KC. Wistar-Kyoto rats as an animal model of anxiety vulnerability: support for a hypervigilance hypothesis. Behavioural Brain Research. 2009;204:162–168. doi: 10.1016/j.bbr.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the mediodorsal thalamus and prefrontal cortex: a fluorescence retrograde transport study in the rat. Journal of Comparative Neurology. 1987;262:46–58. doi: 10.1002/cne.902620105. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: it’s not what you thought it was. The American Psychologist. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behavioral Neuroscience. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Moscarello Moscarello J, Ledoux JE. Active Avoidance Learning Requires Prefrontal Supression of Amygdala-Mediated Defensive Reactions. Journal of Neuroscience. 2013;33:3815–3823. doi: 10.1523/JNEUROSCI.2596-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell ML, Elliott P, Lau W, Creamer M. PTSD symptom trajectories: from early to chronic response. Behaviour Research and Therapy. 1995;45:601–606. doi: 10.1016/j.brat.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Pang KC, Jiao X, Sinha S, Beck KD, Servatius RJ. Damage of GABAergic neurons in the medial septum impairs spatial working memory and extinction of active avoidance: effects on proactive interference. Hippocampus. 2011;21:835–846. doi: 10.1002/hipo.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon MC, Gould GG, Garcia A, Phillips L, Cook MC, Miller SA, Mason PA, Morilak DA. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience. 2002;115:229–242. doi: 10.1016/s0306-4522(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Pare WP. Stress ulcer and open-field behavior of spontaneously hypertensive, normotensive, and Wistar rats. The Pavlovian Journal of Biological Science. 1989;24:54–57. doi: 10.1007/BF02964537. [DOI] [PubMed] [Google Scholar]
- Pare WP, Redei E. Depressive behavior and stress ulcer in Wistar Kyoto rats. Journal of Physiology, Paris. 1993;87:229–238. doi: 10.1016/0928-4257(93)90010-q. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience. 1993;57:1077–1090. doi: 10.1016/0306-4522(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Pare WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiology & Behavior. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Hua F, Yi Z, Zhou A, Ge L, Stephenson FA, Wenthold RJ. Organization of NMDA receptors at extrasynaptic locations. Neuroscience. 2010;167:68–87. doi: 10.1016/j.neuroscience.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. Journal of Neuroscience. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32:471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Levin G, Maroun M. Stress and amygdala suppression of metaplasticity in the medial prefrontal cortex. Cerebral Cortex. 2010;20:2433–2441. doi: 10.1093/cercor/bhp311. [DOI] [PubMed] [Google Scholar]
- Rittenhouse PA, Lopez-Rubalcava C, Stanwood GD, Lucki I. Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology. 2002;27:303–318. doi: 10.1016/s0306-4530(01)00052-x. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. Journal of Neuroscience. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaud E, Billard JM. D-cycloserine facilitates synaptic plasticity but impairs glutamatergic neurotransmission in rat hippocampal slices. British Journal of Pharmacology. 2003;140:1051–1056. doi: 10.1038/sj.bjp.0705541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley HL, Martin KF, Marsden CA. Determination of in vivo amino acid neurotransmitters by high performance liquid chromatography with o-phthalaldehyde-sulphite derivatisation. Journal of Neuroscience Methods. 1995;57:93–99. doi: 10.1016/0165-0270(94)00132-z. [DOI] [PubMed] [Google Scholar]
- Sah P, Westbrook RF. Behavioural Neuroscience: The circuit of fear. Nature. 2008;454:589–590. doi: 10.1038/454589a. [DOI] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDAindependent to NMDA-dependent memory. Journal of Neuroscience. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servatius RJ, Jiao X, Beck KD, Pang KC, Minor TR. Rapid avoidance acquisition in Wistar-Kyoto rats. Behavioural Brain Research. 2001;192:191–197. doi: 10.1016/j.bbr.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Sheinin A, Shavit S, Benveniste M. Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology. 2001;41:151–158. doi: 10.1016/s0028-3908(01)00073-9. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Myers DA. Strain differences in anxiety-like behavior: association with corticotropinreleasing factor. Behavioural Brain Research. 2008;186:239–245. doi: 10.1016/j.bbr.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Golfels F, Finck C, Halisch A, Rath D, Plag J, Strohle A. D-cycloserine does not improve but might slightly speed up the outcome of in-vivo exposure therapy in patients with severe agoraphobia and panic disorder in a randomized double blind clinical trial. Journal of Psychiatric Research. 2011;45:1042–1047. doi: 10.1016/j.jpsychires.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2Bcontaining NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Stopper CM, Zahm DS, Floresco SB. Separate prefrontal-subcortical circuits mediate different components of risk-based decision making. Journal of Neuroscience. 2012;32:2886–2899. doi: 10.1523/JNEUROSCI.5625-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. The Journal of Physiology. 1998;507:13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Merlo LJ, Bengtson M, Murphy TK, Lewis MH, Yang MC, Jacob ML, Larson M, Hirsh A, Fernandez M, Geffken GR, Goodman WK. D-cycloserine does not enhance exposure-response prevention therapy in obsessive-compulsive disorder. International Clinical Psychopharmacology. 2007;22:230–237. doi: 10.1097/YIC.0b013e32819f8480. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt SM, Pare WP, Yang J. Effect of repeated novel stressors on depressive behavior and brain norepinephrine receptor system in Sprague-Dawley and Wistar Kyoto (WKY) rats. Brain Research. 1994;649:27–35. doi: 10.1016/0006-8993(94)91045-6. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. Journal of Neuroscience. 1994;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouimba RM, Maroun M. Learning-induced changes in the mPFC-BLA connection after fear conditioning, extinction, and reinstatement of fear. Neuropsychopharmacology. 2011;36:2276–2285. doi: 10.1038/npp.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. Journal of Neuroscience. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Cen C, Li C, Cao S, Wang N, Zhou Z, Liu X, Xu Y, Tian N, Zhang Y, Wang J, Wang L, Wang Y. Deactivation of excitatory neurons in the prelimbic cortex via Cdk5 promotes pain sensitivity and anxiety. Nature Communications. 2015;6:7760. doi: 10.1038/ncomms8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Hart J, Richardson R. Effects of D-cycloserine on extinction of learned fear to an olfactory cue. Neurobiology of Learning and Memory. 2007;87:476–482. doi: 10.1016/j.nlm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Wieland S, Boren JL, Consroe PF, Martin A. Stock differences in the susceptibility of rats to learned helplessness training. Life Sciences. 1986;39:937–944. doi: 10.1016/0024-3205(86)90376-0. [DOI] [PubMed] [Google Scholar]
- Will CC, Aird F, Redei EE. Selectively bred Wistar-Kyoto rats: an animal model of depression and hyperresponsiveness to antidepressants. Molecular Psychiatry. 2003;8:925–932. doi: 10.1038/sj.mp.4001345. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil, a novel NMDA receptor antagonist: site and mechanism of action. Current Drug Targets. 2001;2:285–298. doi: 10.2174/1389450013348489. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology. 2008;33:2108–2116. doi: 10.1038/sj.npp.1301605. [DOI] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]