Abstract
Background
The study presents the phenotypic and genetic characterization of selected P. salmonis isolates from Atlantic salmon and rainbow trout suffering from SRS (salmonid rickettsial septicemia) in Chile and in Canada. The phenotypic characterization of the P. salmonis isolates were based on growth on different agar media (including a newly developed medium), different growth temperatures, antibiotics susceptibility and biochemical tests.
Results
This is the first study differentiating Chilean P. salmonis isolates into two separate genetic groups. Genotyping, based on 16S rRNA-ITS and concatenated housekeeping genes grouped the selected isolates into two clades, constituted by the Chilean strains, while the Canadian isolates form a branch in the phylogenetic tree. The latter consisted of two isolates that were different in both genetic and phenotypic characteristics. The phylogenies and the MLST do not reflect the origin of the isolates with respect to host species. The isolates included were heterogeneous in phenotypic tests.
Conclusions
The genotyping methods developed in this study provided a tool for separation of P. salmonis isolates into distinct clades. The SRS outbreaks in Chile are caused by minimum two different genetic groups of P. salmonis. This heterogeneity should be considered in future development of vaccines against this bacterium in Chile. Two different strains of P. salmonis, in regards to genetic and phenotypic characteristics, can occur in the same contemporary outbreak of SRS.
Electronic supplementary material
The online version of this article (doi:10.1186/s12917-016-0681-0) contains supplementary material, which is available to authorized users.
Keywords: P. salmonis, Phenotyping, Genotyping, 16S rDNA-ITS, Housekeeping genes, Phylogeny, MLST
Background
The Chilean aquaculture industry has constantly faced problems with Piscirickettsia salmonis, which causes salmonid rickettsial septicemia (SRS) [1]. This disease causes high mortalities in several salmonids species Atlantic salmon (Salmo salar), Rainbow trout (Onchorhyncus mykiss), Coho salmon (Onchorhyncus kisutch), and was responsible for 90 % of the total use of antibiotics in Chile in 2014 [2]. P. salmonis has been detected in several countries including Norway, Canada, Scotland, Ireland and Chile [3, 4], but opposed to the situation in Chile, SRS is considered manageable in these countries.
The present knowledge on P. salmonis is restricted in several aspects such as characterization (phenotypical and genetic), geographical distribution, host specificity, presence of natural reservoirs and transmission routes. Although several vaccines are available [5–7] none of them are able to induce complete protection against SRS. This lack of protection suggests that more basic knowledge is needed about the biology of P. salmonis.
P. salmonis is a Gram-negative, predominantly coccoid, aerobic, non-encapsulated, and highly fastidious bacterium of approx. 0.5–1.5 μm diameter [3]. The pathogen was first described as an obligatory intracellular bacterium and has traditionally been cultured on Chinook salmon embryos cells (CHSE-214) and others fish cell lines [8]. In 2008 it was shown that P. salmonis could be grown on artificial cell-free media [9–11]. However, work at our laboratory has shown that not all isolates growth well at these media, hence, we developed a new improved medium for isolation and culturing of P. salmonis strains.
This study present new basic knowledge of P. salmonis isolated from sub-acute, acute and chronic outbreaks of SRS. These isolates are compared to the type strain, LF-89. The new knowledge presented could form a better basis for development of efficient strategies for control and treatment of SRS, and possibly a new basis for future development of vaccines against P. salmonis.
Methods
Collection of P. salmonis
The isolates of P. salmonis were obtained in 2011 and 2012 from Atlantic salmon and Rainbow trout suspected or diagnosed with SRS. The Chilean isolates, were obtained from a pool of homogenized fish tissues (kidney, spleen, liver, brain). The Canadian isolates were collected from the same site during a single outbreak of SRS, and from kidney. All the isolates were sent to the Fish Diseases Research Group at the University of Bergen where they were sub-cultured on CHAB agar [10] and stored at −80 °C for later characterization. An overview of all the isolates included in the study is presented in the Table 1. The geographical origins of the isolates are presented in Fig. 1. The P. salmonis type isolate (LF-89) was also included in the study.
Table 1.
Data for all the selected P. salmonis isolates included in the study
| Isolate code | Country | County (Region) | Sampling date | Mortality (%) | Host | Sample tissue | Clinical condition |
|---|---|---|---|---|---|---|---|
| LF-89 | Chile | Puerto Montt (X) | 1989 | na | Coho salmon | kidney | na |
| Ch2-As-I | Chile | Chiloé Sur (X) | 08.08.2012 | 7,8 | Atlantic salmon | k-l-sp-b | acute |
| Ch3-Rt-L | Chile | Calbuco (X) | 03.10.2012 | 6,7 | Rainbow trout | lession | sub-acute |
| Ch4-Rt-L | Chile | Calbuco (X) | 05.10.2012 | 6,7 | Rainbow trout | lession | sub-acute |
| Ch5-As-I | Chile | Chiloé Centro (X) | 18.07.2012 | 5,1 | Atlantic salmon | k-l-b | sub-acute |
| Ch6-Rt-L | Chile | Calbuco (X) | 10.08.2012 | 13,2 | Rainbow trout | lession | sub-acute |
| Ch7-As-L | Chile | Aysén (XI) | na | na | Atlantic salmon | muscle | chronic |
| Ch8-Rt-K | Chile | Chiloé Centro (X) | 17.06.2011 | 1,9 | Rainbow trout | kidney | sub-acute |
| Ch9-As-na | Chile | Chiloé Centro (X) | 27.03.2012 | 2,4 | Atlantic salmon | na | sub-acute |
| Ch10-As-I | Chile | Chiloé Sur (X) | 24.07.2012 | 21,5 | Atlantic salmon | k-l-sp-b | acute |
| Ch11-As-I | Chile | Chiloé Centro (X) | 04.05.2012 | 2,2 | Atlantic salmon | k-l-b | sub-acute |
| Ch12-As-I | Chile | Chiloé Centro (X) | 07.05.2012 | 14,8 | Atlantic salmon | k-l-b | acute |
| Ch13-As-I | Chile | Chiloé Centro (X) | 18.04.2012 | 3,6 | Atlantic salmon | k-l-sp-b | chronic |
| Ch14-As-I | Chile | Chiloé Centro (X) | 13.01.2012 | 14,8 | Atlantic salmon | k-sp | acute |
| Ch15-As-I | Chile | Chiloé Centro (X) | 23.08.2012 | 5,4 | Atlantic salmon | k-l-b-sp | sub-acute |
| Ch16-As-I | Chile | Chiloé Centro (X) | June 2012 | 14,8 | Atlantic salmon | k-l-b | acute |
| Ch17-As-I | Chile | Chiloé Centro (X) | 23.05.2012 | 14,8 | Atlantic salmon | k-l-b | acute |
| Ch18-As-I | Chile | Chiloé Centro (X) | 22.06.2012 | 3,6 | Atlantic salmon | k-l-b-sp | chronic |
| Ca19-As-I | Canada | British Columbia | 11.12.2012 | <0,03 | Atlantic salmon | kidney | chronic |
| Ca20-As-I | Canada | British Columbia | 11.12.2012 | <0,03 | Atlantic salmon | kidney | chronic |
Codes indicates the country of isolation, Chile (Ch), Canada (Ca); number of isolate (1–20); fish species, Atlantic salmon (As), Rainbow trout (Rt), Coho salmon (Cs); and tissue, kidney (K), liver (l), spleen (sp) and brain (b). The samples were obtained from a pool of internal organs. The codes are: I from internal lesions, L from external lesions, and K from kidney. Sub-acute and acute clinical conditions frequently present hemorrhages in the brain, splenomegaly and extensive congestion particularly in the swimbladder. In chronic conditions typical findings are whitish nodules in the liver which becomes brownish and finally progress as granuloma. Mostly in Atlantic salmon and rainbow trout, the dermis present speckle erosions which progress to pustules and finally caverns. It has also seen splenomegaly and hepatomegaly; pericarditis with fibrin deposits in Atlantic salmon and rainbow trout. In Coho salmon the fibrin deposits are located mostly internally in the abdomen. Mortality percentage is described as percentage of dead fish due to SRS, from necropsy findings, in one production cycle. In most of the farms antimicrobials were used for treatment. Na: information not available
Fig. 1.
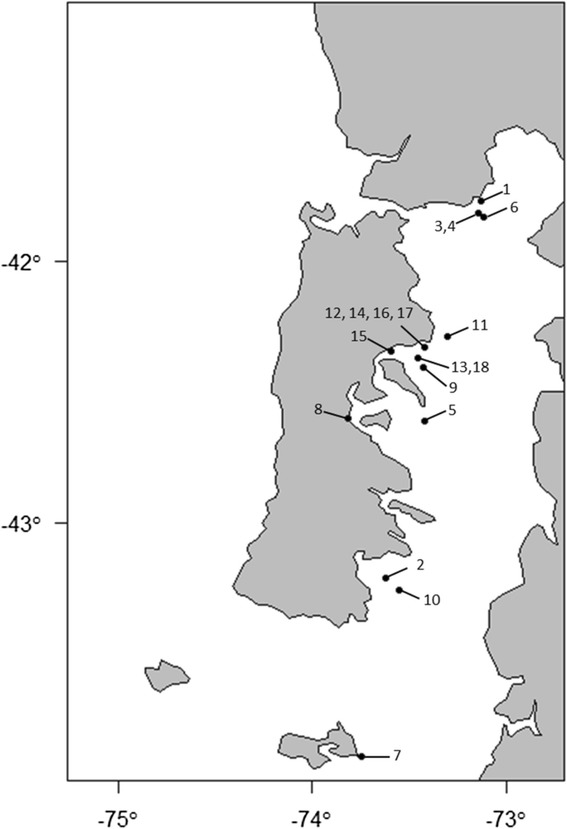
Geographical location of the Chilean farms from were P. salmonis isolates were obtained. The number correspond to isolates: 1 = LF-89, 2 = Ch2-As-I, 3 = Ch3-Rt-L, 4 = Ch4-Rt-L, 5 = Ch5-As-I, 6 = Ch6-Rt-L, 7 = Ch7-As-L, 8 = Ch8-Rt-K, 9 = Ch9-As-na, 10 = Ch10-As-I, 11 = Ch11-As-I, 12 = Ch12-As-I, 13 = Ch13-As-I, 14 = Ch14-As-I, 15 = Ch15-As-I, 16 = Ch16-As-I, 17 = Ch17-As-I, and 18 = Ch18-As-I
This study followed the recommendations of the Norwegian Animal Welfare Act (01.01.2010) and the work was done under the regulations given by the Norwegian Food Safety Authority. The bacterial samples were obtained from the fish after they has been anaesthetized by a blow to the head and killed by instantly decapitation. This procedure complies with the Norwegian fish welfare regulations.
New growth medium
To improve the growth of P. salmonis on solid media, a new optimized SRS blood agar (SRS-BA) was developed. The composition of this agar was 40 g of TSA (BD, Difco); 20 g of Red Sea Salt (RSS) (Red Sea, USA); 50 ml of defibrinated sheep blood (DSB) (Oxoid Limited, UK); 1 g of L-cysteine (Sigma-Aldrich); 5 g of D-glucose (Sigma-Aldrich); 50 ml of fetal bovine serum (FBS) (Thermo Scientific Hyclone, USA); 0.2 mM ferric nitrate (Sigma-Aldrich) and reverse osmosis water (RO) to a final volume of 1000 ml. RSS was chosen to simulate natural sea water composition. RO water, TSA and RSS were autoclaved at 121 °C for 15 min. DSB was added after cooling from autoclaving. Subsequently, the agar was cooled down to 50 °C and L-cysteine, D-glucose, FBS and ferric nitrate were added. The pH was adjusted to 6.8 ± 0.2.
Phenotypic characterization
Colonies > 1 mm were used for morphological characterization and bacteria from these colonies were Gram-stained for the description of cell morphology and other phenotypic characteristics.
Testing of different growth media was performed using two colonies from each isolate streaked on the following media: SRS-BA, Austral-TSHem [11], CHAB [10], CHAB with 0.2 mM Fe, blood agar (BA), BA with 2 % NaCl, marine agar (MA) and tryptone-yeast extract-salts with glucose agar (FLPA) [12] and incubated at 19 °C for 14 days. The temperature range for bacterial growth measured as number of colonies and colony size was tested using three colonies streaked out on SRS-BA and incubated at 8, 11, 16, 19, 22, and 25 °C. The growth was recorded at days 3, 7, 10 and 14 in both tests.
Test of the susceptibility to antimicrobial drugs was performed by the agar diffusion method using tablets as described by Justesen et al. [13], with some modifications. 19 °C was used for incubation, as this is within the common optimum temperature range of all isolates. The inhibition zone was measured after 10 days of incubation, to ensure sufficient growth. The antimicrobial sensitivity was tested for all the isolates against: streptomycin (10 μg); oxytetracycline (30 μg); penicillin (1 unit); ceftazidime (30 μg); ampicillin (2 μg); and florfenicol (30 μg). The inhibition zone was measured, in millimeters, from the tablet border towards to all the extension of the inhibition area.
Indole and oxidase tests were performed with BBL™ DrySlide™ (BD BBL™, U.S.A) kits. The cefinase test using BBL™ Cefinase™ Paper Disc (BD BBL™, U.S.A) was used to determine the production of β-lactamases. Catalase was detected by adding a drop of hydrogen peroxide 3 % (Sigma-Aldrich, Germany) to a microscope slide before transferring a small amount of bacteria onto the H2O2 solution. The API ZYM galleries (BioMérieux, U.S.A) for the detection of bacterial enzymes were used according to the manufacturer’s protocol, except that the incubation temperature was set at 19 °C and the incubation time at 24 h. A 6.0 McFarland standard, as recommended by BioMérieux, was made in advance for turbidity comparison. H2S production was done using hydrogen Sulfide Test Strips (Sigma-Aldrich, Switzerland). All the phenotypical and biochemical tests were performed in triplicate, with the exception of the antimicrobial susceptibility analysis, which was done in duplicate.
Genetic characterization
The DNA extraction from pure cultures of all P. salmonis was performed with an E.Z.N.A Tissue DNA kit. The quality of DNA was tested using NanoDrop (Saveen Werner, Life Science) before storing at −20 °C.
PCR, purification and visualization of products by gel electrophoresis, and sequencing were performed as described by Apablaza et al. [14]. Sequencing of the 20 isolates was performed using specific primers for 16S rDNA and ITS, and for the housekeeping genes, HK (dnaK, groEL, tbpB, mltB, ospA, radA, airA, bax, tnpA, elfP). The primers are presented in Table 2. The Vector NTI® v9.0 Software package (Invitrogen) was used for sequence assembling and alignment. Manual adjustments and verification of reading frames for the protein coding genes were conducted in GeneDoc (Karl Nicholas ©2000). The evolutionary analysis and calculation of the best fit model for the 16S rDNA-ITS dataset (2191 base pairs) was conducted in MEGA6 [15]. The maximum likelihood method using the Hasegawa-Kishino-Yano [16] model with discrete Gamma distribution was applied.
Table 2.
Primers used for PCR analysis in the present study
| Target gene | Primer | Direction | Sequence (5´–3´) | Reference |
|---|---|---|---|---|
| 16 s rDNA | Eug B27F | Fwd | AGAGTTTGATCMTGGCTCAG | [25] |
| Eug A1518R | Rev | AAGGAGGTGATCCANCCRCA | [25] | |
| ITS | SRS-ITS/F | Fwd | GTACACACCGCCCGTCACAC | Present study |
| SRS-ITS/R | Rev | CCTCACGGTACTAGTTCACTATCGG | Present study | |
| dnaK | SRS-dnaK/F2 | Fwd | CCGTGTCGTGTGGCGCTAAAA | Present study |
| SRS-dnaK/R2 | Rev | TTGAGATTGAGCCTGCTCCGC | Present study | |
| SRS-dnaK3/F1 | Fwd | CCGCGTGTGATTGAGAGTGC | Present study | |
| SRS-dnaK3/R1 | Rev | CGTCATCACCCCACCCATGG | Present study | |
| groEL | SRS-groEL/F1 | Fwd | CTTCGGTACCGGTTCCCGTC | Present study |
| SRS-groEL/R1 | Rev | TCTTGCAGTTTCTCGCGGTCG | Present study | |
| SRS-groEL/F2 | Fwd | GTGAAGCTCTGGCAACACTCGTC | Present study | |
| SRS-groEL/R2 | Rev | AGGAAGCTCTGCAACCATCGC | Present study | |
| tbpB | SRS-tbpB/F1 | Fwd | AACTGGGCAGGCGTCACTGTT | Present study |
| SRS-tbpB/R1 | Rev | CGGCGCGTCTCTAATGTTCG | Present study | |
| SRS-tbpB2/F2 | Fwd | CCAAGCTGGATCACCGCCAT | Present study | |
| SRS-tbpB2/R2 | Rev | AAAGATAGGCCCAGCCACGC | Present study | |
| mltB | SRS-mltB/F | Fwd | ACCACTCACGCGGCATCTAA | Present study |
| SRS-mltB/R | Rev | ACTCAAATCATACACCGCCATTGCA | Present study | |
| ospA | SRS-ospA/F | Fwd | AGCCGTCAAGAAGTCGGAGCT | Present study |
| SRS-ospA/R | Rev | TGCCAACGACCATCCGCTTG | Present study | |
| radA | SRS-radA/F1 | Fwd | ATCAGTCGCCAGCCTGTTGG | Present study |
| SRS-radA/FR1 | Rev | GTCCTCGTTGCACTGGACGA | Present study | |
| airA | SRS-air/F1 | Fwd | GGGTGCGTCCGGGGATTATG | Present study |
| SRS-rairA/R1 | Rev | TAAGGTGCACGCAGTGGCAT | Present study | |
| bax | SRS-bax/F1 | Fwd | TCAAGGGATCTGGGAAGTGCT | Present study |
| SRS-bax/R1 | Rev | ACCACTGCCTATCTTGCTCAACA | Present study | |
| tnpA | SRS-tnpA/F1 | Fwd | ACCTGTTAAGTTCTCGGCCATT | Present study |
| SRS-tnpA/R1 | Rev | AGCCTTCACAAATGTCAACAAGTGA | Present study | |
| elfP | SRS-elfP/F | Fwd | GCCACKGCTAATTCAGCAA | Present study |
| SRS-elfP/R | Rev | STGGAATGGTCAGCCACYT | Present study |
The software KAKUSAN4 was used for construction of concatenated sequences of the 10 HK genes (8969 base pairs), calculation of substitution rate, and the best fit model for the individual loci and codon positions [17]. The data were exported into an MrBays-block (V. 3.2.2 x86) for analysis. The phylogenetic analysis was performed with Proportional Codon, Proportional model and a mcmc of 40 000 000 generations. The phylogram was constructed using TreeAnotator and viewed in FigTree [18]. A statistics report of percentage identity (PID) and average nucleotide identity (ANI) was made in GeneDoc (Karl Nicholas ©2000).
The multi locus sequence typing (MLST) method was applied as described by Apablaza et al. [14]. Briefly, the different allele types (AT) within the 10 HK were used to create the sequence types (ST). Based on the different STs, a data matrix from the 18 isolates of P. salmonis was exported as a nexus file into PAUP 4.0. A dendrogram was constructed using neighbor-joining (NJ) distance method.
All the nucleotide sequences were deposited in the GenBank database. Accession numbers are presented in Additional file 1: Table S1.
Results
Phenotypic characterization
The colonies grown on SRS-BA were slightly convex, grey-white, shiny, and centrally opaque with translucent, slightly undulating margins. At the microscopic level, the cells were Gram-negative, coccoid shaped, measuring from 0.4 to 1.8 μm in diameter. No distinctive colony or cell morphology differences were observed among the isolates.
The results of the growth at the different media are presented in Table 3. The minority of the isolates were classified as ‘fastidious’ (LF-89, Ch5-As-I, Ch6-Rt-L, and Ca20-As-K) while the rest of the isolates were defined as ‘less fastidious’. Five of the isolates (Ch3-Rt-L, Ch4-Rt-L, Ch5-As-I, Ch6-Rt-L and Ca20-As-I) grew on SRS-BA only. No growth was recorded on MA and FLPA media. The isolate Ch15-G2-As-I produced the highest number of colonies in SRS-BA. Ch14-As-I induced α- (dark green/brown discoloration around colonies) and β-hemolysis (medium translucence under the colonies) on BA with 2 % NaCl. This isolate grew on all the tested media, with exception of MA and FLPA (Fig. 2).
Table 3.
Summary of growth of P. salmonis isolates on different culture media
| Isolate | SRS-BA | CHAB | CHAB w/Fe | Austral-TSHem | BA | BA w/2 % NaCl |
|---|---|---|---|---|---|---|
| Ch11-As-I | +++ | +++ | +++ | ++ | ++ | +++ |
| Ch7-As-L | +++ | +++ | +++ | ++ | ++ | ++ |
| Ch14-As-I | +++ | ++ | ++ | ++ | ++ | +++ |
| Ch9-As-na | +++ | +++ | +++ | ++ | + | + |
| Ch13-As-I | +++ | ++ | ++ | ++ | ++ | +++ |
| Ch17-As-I | ++ | ++ | + | + | ++ | ++ |
| Ch2-As-I | +++ | ++ | ++ | + | ++ | ++ |
| Ch12-As-I | +++ | ++ | ++ | + | + | ++ |
| Ch15-As-I | +++ | + | + | W | + | + |
| Ch10-As-I | ++ | ++ | ++ | + | + | ++ |
| Ch8-Rt-K | +++ | + | + | + | + | + |
| Ch18-As-I | +++ | + | + | + | - | + |
| Ca19-As-I | +++ | ++ | ++ | - | + | + |
| Ch16-As-I | ++ | + | + | - | + | ++ |
| LF-89 | +++ | ++ | ++ | - | + | + |
Symbols: (−), no growth; (+), scant growth; (++), moderate growth; (+++), vigorous growth; and (w), weak growth. Isolates Ch3-Rt-L, Ch4-Rt-L, Ch5-As-I, Ch6-Rt-L, and Ca20-As-I were grew only in SRS-BA
Fig. 2.
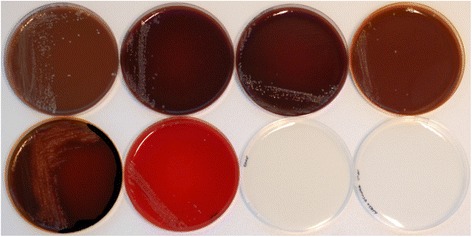
Isolate Ch14-As-I cultured in eight different growth media: from upper left to the right, SRS-BA, CHAB, CHAB supplemented with ferric nitrate; Austral-TSHem; BA w/2 % NaCl, BA, MA, and FLPA
The maximum temperature range for the isolates growth was from 8 to 25 °C on SRS-BA (data not shown). Ch2-As-1, Ch7-As-L, Ch8-Rt-K, Ch12-As-I, Ch18-As-I, and Ca19-As-K had the ability to grow at 25 °C, while the type strain LF-89 did not grow at this temperature. After three days of incubation all isolates showed growth at 16, 19 and 22 °C. The optimum temperature for maximum growth was in the range 19–22 °C for the less fastidious isolates (including Ca19-As-K), and 16 to 19 °C for the remaining strains, including Ca20-As-K (Table 4).
Table 4.
Optimum growth temperature for the P. salmonis isolates
| Isolate | Optimum growing temp (°C) |
|---|---|
| LF-89 | 16–19 |
| Ch2-As-I | 16–19 |
| Ch3-Rt-L | 16–19 |
| Ch4-Rt-L | 16–19 |
| Ch5-As-I | 16–19 |
| Ch6-Rt-L | 16–19 |
| Ca20-As-K | 16–19 |
| Ch7-As-L | 19–22 |
| Ch8-Rt-K | 19–22 |
| Ch9-As-na | 19–22 |
| Ch10-As-I | 19–22 |
| Ch11-As-I | 19–22 |
| Ch12-As-I | 19–22 |
| Ch13-As-I | 19–22 |
| Ch14-As-I | 19–22 |
| Ch15-As-I | 19–22 |
| Ch16-As-I | 19–22 |
| Ch17-As-I | 19–22 |
| Ch18-As-I | 19–22 |
| Ca19-As-K | 19–22 |
The study of the antibiotic sensitivity of the isolates with respect to different antibiotics shows large variation in the inhibition zones. The strain Ch3-Rt-L seems to be resistant to the most of the antibiotics tested (Table 5). All isolates were sensitives to oxytetracycline, while florfenicol yielded the largest inhibition zones with the exception of above mentioned strain. Ch10-As-I had the largest inhibition zones in all antibiotics with the exception of penicillin.
Table 5.
Antibiotic inhibition zones (mm) of the P. salmonis isolates
| Isolate | Streptomycin | Oxytetracycline | Penicilin | Ceftazidime | Ampicilin | Florfenicol |
|---|---|---|---|---|---|---|
| LF-89 | 5 | 23 | 12 | 15,5 | 19,5 | 24 |
| Ch2-As-I | 0,5 | 0,5 | 1,5 | 10 | 3 | 14,5 |
| Ch3-Rt-L | 3,5 | 8,5 | 0,5 | 0,5 | 2,5 | 4 |
| Ch4-Rt-L | 2 | 18 | 6,5 | 17,5 | 14 | 19 |
| Ch5-As-I | 1 | 17 | 12 | 16,5 | 12,5 | 19,5 |
| Ch6-Rt-L | 0 | 16,5 | 13 | 15,5 | 13 | 18 |
| Ch7-As-L | 0 | 10 | 10 | 13,5 | 10,5 | 14,5 |
| Ch8-Rt-K | 1 | 21 | 2 | 13,5 | 13,5 | 24,5 |
| Ch9-As-na | 2 | 25 | 7 | 16 | 9,5 | 24 |
| Ch10-As-I | 32,5 | 27,5 | 1,5 | 18,5 | 22 | 23,5 |
| Ch11-As-I | 1 | 21 | 4 | 5 | 7,5 | 19 |
| Ch12-As-I | 0,5 | 17 | 9,5 | 12,5 | 1,5 | 22 |
| Ch13-As-I | 0,5 | 22 | 2,5 | 10 | 10 | 25 |
| Ch14-As-I | 0 | 22 | 9,5 | 11,5 | 11,5 | 24,5 |
| Ch15-As-I | 1 | 20 | 6 | 12 | 9 | 20,5 |
| Ch16-As-I | 0 | 3,5 | 6,5 | 12,5 | 8 | 17,5 |
| Ch17-As-I | 0,5 | 24,5 | 5,5 | 11 | 14,5 | 23 |
| Ch18-As-I | 2,5 | 20 | 0 | 10 | 1,5 | 15,5 |
| Ca19-As-I | 2 | 17 | 21,5 | 22 | 25 | 24,5 |
| Ca20-As-I | 2,5 | 24 | 13 | 13,5 | 15 | 27,5 |
Indole and oxidase tests were negative for all of the isolates, while catalase and cefinase tests were positive.
In the APY ZYM galleries, the results which differentiate the isolates are shown in Table 6. The tests were negative for all the isolates in the remaining enzymes included in the gallery. The Canadian isolate Ca19-As-I and the Chilean Ch8-Rt-K were the only ones positive for the enzyme esterase lipase and esterase, respectively. In addition, the isolate Ch14-As-I gave a positive reaction for β-galactosidase and α-glucosidase while the rest of the isolates were negative.
Table 6.
P. salmonis differential enzymatic reactions in API ZYM galleries
| Isolate | AP | E | EL | LA | VA | CA | AP | NP |
|---|---|---|---|---|---|---|---|---|
| LF-89 | W | W | W | + | + | W | + | + |
| Ch2-As-I | W | W | W | + | + | − | W | W |
| Ch3-Rt-L | W | W | W | + | + | − | W | + |
| Ch4-Rt-L | W | W | W | + | + | − | W | + |
| Ch5-As-I | W | W | W | + | + | − | W | W |
| Ch6-Rt-L | W | W | W | + | + | W | + | + |
| Ch7-As-L | + | W | W | + | W | W | + | W |
| Ch8-Rt-K | + | + | W | W | W | − | + | W |
| Ch9-As-na | + | W | W | + | + | − | + | W |
| Ch10-As-I | + | W | W | + | + | + | + | + |
| Ch11-As-I | + | W | W | + | + | − | + | W |
| Ch12-As-I | + | W | W | + | + | − | + | + |
| Ch13-As-I | + | W | W | W | W | + | + | W |
| Ch14-As-I | + | W | W | + | + | − | + | + |
| Ch15-As-I | + | W | W | + | + | − | + | + |
| Ch16-As-I | + | W | W | + | + | − | + | W |
| Ch17-As-I | + | W | W | + | + | − | + | + |
| Ch18-As-I | + | W | W | + | + | − | + | + |
| Ca19-As-K | + | W | + | + | + | − | + | + |
| Ca20-As−K | + | W | W | + | + | − | + | + |
W: weak reaction; +: positive reaction; −: negative reaction
Genetic characterization
Phylogenetic analysis of the 18 partial (2198 bp) sequences of the 16S rDNA-ITS genes identified two clades (G1 and G2) as shown in the Fig. 3. The Canadian isolates are distinct and belong to the same branch in the phylogenetic tree. The analysis of the genetic relationships of the concatenated HKs is presented in Fig. 4. After several PCR attempts it was not possible to obtain all the expected PCR products for the isolates Ch13-As-I and Ch9-As-na, therefore they were not includes in the genetic analysis.
Fig. 3.
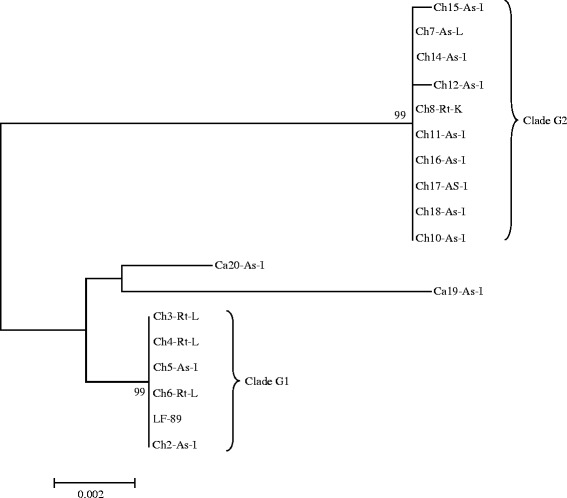
The phylogenetic relationships of the 18 isolates of P. salmonis, based in the analysis of 2198nt within 16S rDNA-ITS genes. The phylogenetic tree was obtained by Mega 6.05 software. The codes for all the isolates are explained in the Table 1
Fig. 4.
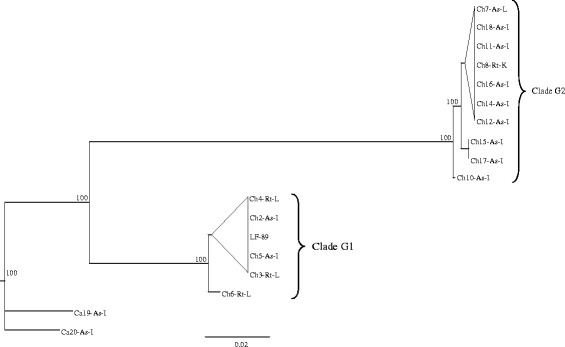
The genetic relationships of the 18 isolates of P. salmonis, based in the analysis of concatenated HK. The phylogenetic three was obtained by the mrBayes software. The codes for all the isolates are explained in the Table 1
Comparison of 10 housekeeping sequences from the isolates, included in the MLST scheme, revealed 438 variable nucleotide positions across 11176 nucleotides (nts) (Table 7). The highest percentage of variable nucleotide positions (5.6 %) was seen in the tnpA locus and the lowest (2.4 %) in mltB. The putative amino acid sequence showed variation in 7.2 positions of a total of 2985 amino acids. The highest percentage of variable amino acid positions (4.4 %) was seen in the airA and bax loci; and the lowest (0.0 %) in elfP. In the analysis of the genetic relationships of P. salmonis isolates, based in the analysis of concatenated HK, it were found two clades with similar grouping shown by the phylogenetic analysis of 16S rDNA-ITS genes. However, a better separation was seen in the clades G1 and G2 (Fig. 4).
Table 7.
Overview of the number of variable nucleotide and putative aminoacids positions in the alignment of 16S rDNA, ITS and the ten housekeeping genes of P. salmonis included
| Gene | N | No. Nucleotides | Variable positions | % | No. Amino acids | Variable positions | % |
|---|---|---|---|---|---|---|---|
| 16S | 18 | 1433 | 14 | 1,0 | - | - | - |
| ITS | 18 | 765 | 34 | 4,4 | - | - | - |
| dnaK | 18 | 1684 | 66 | 3,9 | 560 | 3 | 0,5 |
| groEL | 18 | 1454 | 73 | 5,0 | 484 | 10 | 2,1 |
| tbpB | 18 | 1879 | 95 | 5,1 | 626 | 26 | 4,2 |
| mltB | 18 | 843 | 20 | 2,4 | 280 | 5 | 1,8 |
| ospA | 18 | 364 | 14 | 3,8 | 121 | 3 | 2,5 |
| radA | 18 | 906 | 37 | 4,1 | 301 | 5 | 1,7 |
| airA | 18 | 407 | 21 | 5,2 | 135 | 6 | 4,4 |
| bax | 18 | 612 | 18 | 2,9 | 203 | 9 | 4,4 |
| tnpA | 18 | 356 | 20 | 5,6 | 118 | 5 | 4,2 |
| elfP | 18 | 473 | 26 | 5,5 | 157 | 0 | 0,0 |
| MLST | 216 | 11176 | 438 | 4,1 | 2985 | 7,2 | 2,6 |
Symbols: N: number of P. salmonis sequences
The MLST analyses (Fig. 5) resulted in 12 STs based on the different ATs. The Chilean isolates presented 10 STs and the Canadian, two. The MLST analysis reflects the 16S rDNA-ITS phylogeny, arranging the isolates in three groups (Fig. 5).
Fig. 5.
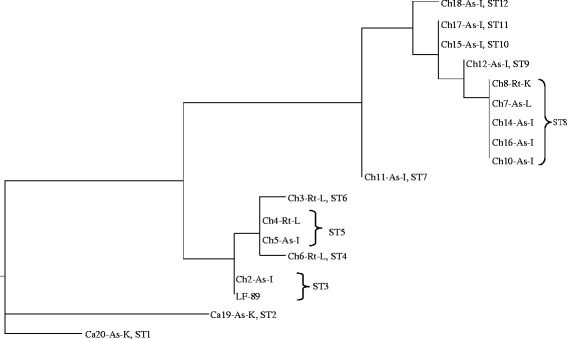
Dendrogram showing the sequence type relations in the multilocus sequence typing scheme (MLST) of the 18 isolates of P. salmonis. The MLST analysis was constructed using 11176nt from the HK dnaK, groEL, trpB, mltB, ospA, radA,alr, bax, tnpA, and elfP. The analysis was performed using the distance method in PAUP 4.0. The codes for all the isolates are explained in the Table 1
Discussion
The present study shows the 18 Chilean P. salmonis isolates as two genetically distinct groups, a separation supported by all three genotyping methods. Clade G1, containing isolates from Puerto Montt area and two isolates from Chiloe; and clade G2, including isolates retrieved from sites along the East coast of Chiloé Island and Melinka Island (Fig. 1). The present study, which does not included European isolates, clearly shows that the 18 Chilean isolates are genetically distinct from the two Canadian isolates of P. salmonis (Figs. 3, 4 and 5). Previous studies have shown genetic variations among Chilean isolates of P. salmonis and that Chilean strains are more genetically related to each other than to European and Canadian isolates [19–21]. However, these phylogenetic studies have only consisted of five or less Chilean isolates [19, 20], and were therefore not able to have sufficient resolution to separate them in phylogenetic clades.
All three genetic methods used in this study are suitable for separation of P. salmonis isolates, and we present the first MLST typing system for P. salmonis. However, more isolates from a wider geographical area are needed for mapping of geographical distribution, reservoirs, possible transmission routes, host specificity, and variation in tissue tropism. Other methods, like variable number of tandem repeats (VNTR), giving a better separation of the isolates, are needed for studies of virulence.
The isolates studied were separated in two groups under the optimum growing temperature tests which correlate to a large extent with the results of genotyping. An optimal cultivation temperature at 16–19 °C was seen for isolates includes in clade G1 isolates confirming the findings of Fryer et al. [22]. Whereas, the less fastidious isolates, included in clade G2, had an optimal temperature between at 19–22 °C, which correlates with data presented by Mikalsen et al. [10].
The Canadian isolates differed both in genetic and phenotypic characteristics, as both were isolated from the same site during a contemporary outbreak SRS, showing that two different strains of P. salmonis can occur during a single outbreak of SRS.
Comparison of the different culture media clearly shows that SRS-BA is the best medium for culturing all the isolates included. Ch14-As-I had the fastest growth and was the only isolate positive for β-galactosidase and α-glucosidase in the API ZYM test. The presences of α-glucosidase and β-galactosidase have also been described to vary for strains of the related intracellular bacterium Francisella philomiragia subsp. philomiragia [23]. β-galactosidase and α-glucosidase participate in metabolic pathways in Gram negative bacteria [24] and might influence the bacterial growth.
Conclusions
This is the first study showing that there are two genetic groups of P. salmonis present in Chile, and that more than one strain of P. salmonis can occur under a contemporary outbreak of SRS. The two Canadian isolates were genetically separate when compared to the 18 Chilean isolates, although belong to the same branch. Other isolates should be included in further genotyping studies on Canadian strains of P. salmonis. The genotyping methods used are suitable for separation of the isolates; however, other molecular tools are most likely needed for separating isolates with different virulence. The phenotypic tests showed variation among the isolates, with the temperature test giving the best separation. In future studies the SRS-BA should be the preferred agar for isolation and culturing of P. salmonis. Studies of geographical distribution and host specificity will have to include more isolates from different fish hosts in all areas with production of salmonids and presence of P. salmonis.
The MLST and the sequences alignments for 16S rRNA-ITS and the concatenated housekeeping genes are made available at the TreeBASE data base (http://purl.org/phylo/treebase/phylows/study/TB2:S18993).
Availability of supporting data
The nucleotide sequences are deposited in the GenBank data Base DOI http://www.ncbi.nlm.nih.gov/genbank/. The accession numbers are presented in the Additional file 1: Table S1.
Acknowledgements
Thanks to the field veterinarians Luis Almonacid, Carlos Bertin and Karin Ruiz (Cermaq Chile), and Kathleen Frisch (Cermaq Canada), for providing detailed description of the clinical findings of SRS in the different host species. This work was financed by a collaboration project between Cermaq Chile, Cermaq Group AS, Norway, and the University of Bergen.
Abbreviations
- ANI
average nucleotide identity
- DSB
defibrinated sheep blood
- HK
housekeeping genes
- ITS
internal transcriber space
- MA
marine agar
- MLST
multi locus of sequence typing
- RO
reverse osmosis water
- RSS
red sea salt
- SRS
salmonid rickettsial septicemia
- SRS-BA
salmonid rickettsial septicemia-blood agar
- TSA
tryptic soy agar
- VNTR
variable number of tandem repeats
Additional file
Accession numbers of the P. salmonis isolates included in the study. (DOCX 20 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AO was the main responsible for the experimental work and data analysis, ØJB identified the HK genes, contributed to the genetic data analysis and writings, DJ performed experimental work, HD coordinated the project, contributed to the experimental design and writings, IS and PF contribute to the experimental design and writings, JM coordinated the project and gave the critical review of the different drafts, PMK contributed with the writing of the manuscript, AN contributed with different parts of the project design and reviewed all the drafts and PA coordinated the laboratory work and was the main responsible for the writing of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Alexander Otterlei, Email: alexander.otterlei@analytic.no.
Øyvind J. Brevik, Email: Oyvind.Brevik@cermaq.com
Daniel Jensen, Email: Daniel.Jensen@student.uib.no.
Henrik Duesund, Email: Henrik.Duesund@cermaq.com.
Ingunn Sommerset, Email: Ingunn.Sommerset@merck.com.
Petter Frost, Email: Petter.Frost@merck.com.
Julio Mendoza, Email: Julio.Mendoza@cermaq.com.
Peter McKenzie, Email: Peter.McKenzie@cermaq.com.
Are Nylund, Email: Nylund@bio.uib.no.
Patricia Apablaza, Email: Patricia.Apablaza@cermaq.com.
References
- 1.Portnoy DS, Hollenbeck CM, Vidal RR, Gold JR. A comparison of neutral and immune genetic variation in Atlantic salmon, Salmo salar L. in Chilean aquaculture facilities. PLoS ONE. 2014;9(6):e99358. doi: 10.1371/journal.pone.0099358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sernapesca. Report of the use of antimicrobials in the national salmoniculture. National Service of Fisheries, Subdivision of Aquaculture, Animal Health Unit. Chile; 2015. (In Spanish).
- 3.Fryer JL, Hedrick RP. Piscirickettsia salmonis: a Gram-negative intracellular bacterial pathogen of fish. J Fish Dis. 2003;26(5):251–62. doi: 10.1046/j.1365-2761.2003.00460.x. [DOI] [PubMed] [Google Scholar]
- 4.Rozas M, Enríquez R. Piscirickettsiosis and Piscirickettsia salmonis in fish: a review. J Fish Dis. 2014;37(3):163–88. doi: 10.1111/jfd.12211. [DOI] [PubMed] [Google Scholar]
- 5.Kuzyk MA, Burian J, Machander D, Dolhaine D, Cameron S, Thornton JC, Kay WW. An efficacious recombinant subunit vaccine against the salmonid rickettsial pathogen Piscirickettsia salmonis. Vaccine. 2001;19(17–19):2337–44. doi: 10.1016/S0264-410X(00)00524-7. [DOI] [PubMed] [Google Scholar]
- 6.Wilhelm V, Huaracan B, Martínez R, Rosemblatt M, Burzio LO, Valenzuela PDT. Cloning and expression of the coding regions of the heat shock roteins HSP10 and HSP16 from Piscirickettsia salmonis. Biol Res. 2003;36(3–4):421–8. doi: 10.4067/s0716-97602003000300013. [DOI] [PubMed] [Google Scholar]
- 7.Tobar JA, Jeréz S, Caruffo M, Bravo C, Contreras F, Bucarey SA, Harel M. Oral vaccination of Atlantic salmon (Salmo salar) against salmonid rickettsial septicaemia. Vaccine. 2011;29(12):2336–40. doi: 10.1016/j.vaccine.2010.12.107. [DOI] [PubMed] [Google Scholar]
- 8.Fryer JL, Lannan CN, Giovannoni SJ, Wood ND. Piscirickettsia salmonis gen. nov., sp. nov., the causative agent of an epizootic disease in salmonid fishes. Int J System Bacteriol. 1992;42(1):120–6. doi: 10.1099/00207713-42-1-120. [DOI] [PubMed] [Google Scholar]
- 9.Mauel MJ, Ware C, Smith PA. Culture of Piscirickettsia salmonis on enriched blood agar. J Vet Diag Inv. 2008;20(2):213–4. doi: 10.1177/104063870802000211. [DOI] [PubMed] [Google Scholar]
- 10.Mikalsen J, Skjaervik O, Wiik-Nielsen J, Wasmuth MA, Colquhoun DJ. Agar culture of Piscirickettsia salmonis, a serious pathogen of farmed salmonid and marine fish. Fems Microbiol Letters. 2008;278(1):43–7. doi: 10.1111/j.1574-6968.2007.00977.x. [DOI] [PubMed] [Google Scholar]
- 11.Yañez AJ, Silva H, Valenzuela K, Pontigo JP, Godoy M, Troncoso J, Romero A, Figueroa J, Cárcamo JG, Avendaño-Herrera R. Two novel blood-free solid media for the culture of the salmonid pathogen Piscirickettsia salmonis. J Fish Dis. 2013;36(6):587–91. doi: 10.1111/jfd.12034. [DOI] [PubMed] [Google Scholar]
- 12.Cepeda C, García-Márquez S, Santos Y. Improved growth of Flavobacterium psychrophilum using a new culture medium. Aquaculture. 2004;238(1–4):75–82. doi: 10.1016/j.aquaculture.2004.05.013. [DOI] [Google Scholar]
- 13.Justesen US, Acar Z, Olsson K, Jensen TG, Kerrn MB, Skov RL, Gahrn-Hansen B. Comparison of Rosco Neo-Sensitabs with Oxoid paper disks in EUCAST disk diffusion antimicrobial susceptibility testing on Mueller-Hinton agar. Eur J Clin Microbiol Infect Dis. 2013;32(5):621–5. doi: 10.1007/s10096-012-1785-5. [DOI] [PubMed] [Google Scholar]
- 14.Apablaza P, Loland AD, Brevik OJ, Ilardi P, Battaglia J, Nylund A. Genetic variation among Flavobacterium psychrophilum isolates from wild and farmed salmonids in Norway and Chile. J Appl Microbiol. 2013;114(4):934–46. doi: 10.1111/jam.12121. [DOI] [PubMed] [Google Scholar]
- 15.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–9. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasegawa M, Kishino H, Yano T. Dating the human-ape split by a molecular clock of mitochondrial DNA. J Mol Evol. 1985;22:160–74. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- 17.Tanabe AS. Kakusan4 and Aminosan. two programs for comparing nonpartitioned, proportional and separate models for combined molecular phylogenetic analyses of multilocus sequence data. Mol Ecol Res. 2011;11(5):914–21. doi: 10.1111/j.1755-0998.2011.03021.x. [DOI] [PubMed] [Google Scholar]
- 18.Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012;29(8):1969–73. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid HI, Griffen AA, Birkbeck TH. Isolates of Piscirickettsia salmonis from Scotland and Ireland show evidence of clonal diversity. Appl Env Microbiol. 2004;70(7):4393–7. doi: 10.1128/AEM.70.7.4393-4397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauel MJ, Giovannoni SJ, Fryer JL. Phylogenetic analysis of Piscirickettsia salmonis by 16S, internal transcribed spacer (ITS) and 23S ribosomal DNA sequencing. Dis Aquatic Org. 1999;35(2):115–23. doi: 10.3354/dao035115. [DOI] [PubMed] [Google Scholar]
- 21.Heath S, Pak S, Marshall S, Prager EM, Orrego C. Monitoring Piscirickettsia salmonis by denaturant gel electrophoresis and competitive PCR. Dis Aquatic Org. 2000;41(1):19–29. doi: 10.3354/dao041019. [DOI] [PubMed] [Google Scholar]
- 22.Fryer JL, Lannan CN, Garces LH, Larenas JJ, Smith PA. Isolation of a rickettsiales-like organism from diseased coho salmon (Oncorhynchus kisutch) in Chile. Fish Pathol. 1990;25(2):107–14. doi: 10.3147/jsfp.25.107. [DOI] [Google Scholar]
- 23.Ottem KF, Nylund A, Karlsbakk E, Friis-Moller A, Kamaishi T. Elevation of Francisella philomiragia subsp noatunensis Mikalsen et al. (2007) to Francisella noatunensis comb. nov. [syn. Francisella piscicida Ottem et al. (2008) syn. nov.] and characterization of Francisella noatunensis subsp. orientalis subsp. nov., two important fish pathogens. J Applied Microbiol. 2009;106(4):1231–43. doi: 10.1111/j.1365-2672.2008.04092.x. [DOI] [PubMed] [Google Scholar]
- 24.Juers DH, Heightman TD, Vasella A, McCarter JD, Mackenzie L, Withers SG, Matthews BW. A structural view of the action of Escherichia coli (lacZ) beta-galactosidase. Biochemistry. 2001;40(49):14781–94. doi: 10.1021/bi011727i. [DOI] [PubMed] [Google Scholar]
- 25.Giovannoni SJ, Rappe MS, Vergin KL, Adair NL. 16S rRNA genes reveal stratified open ocean bacterioplankton populations related to the Green Non-Sulfur bacteria. Proc Natl Acad Sci U S A. 1996;93(15):7979–84. doi: 10.1073/pnas.93.15.7979. [DOI] [PMC free article] [PubMed] [Google Scholar]


