Abstract
Objective
To examine the association of body mass index (BMI), waist circumference (WC) and waist hip ratio (WHR) with sudden cardiac death (SCD) in community dwelling individuals.
Methods
Data from a multicentre, prospective, cohort study of 14 941 men and women (African American, and white), aged 45–64 years, participating in the Atherosclerosis Risk in Communities study was analysed. Obesity measures were assessed at baseline (1987–1989). SCD was adjudicated by a committee.
Results
At enrolment mean±SD age of the participants was 54±6 years (55% female; 26% African American). During 12.6±2.5 years of follow-up, 253 SCD occurred (incidence rate 1.34/100 person-years). The association between obesity and SCD differed by smoking status (interaction p≤0.01). In models adjusting for age, sex, race, study centre and education level, SCD risk was positively associated (p<0.001) with BMI, WC and WHR in non-smokers, but not in smokers. WHR was more strongly associated with SCD in non-smokers than was BMI or WC (HR per SD increment (95% CI) 2.00 (1.65 to 2.42); 1.34 (1.15 to 1.56) and 1.49 (1.28 to 1.74), respectively). After adjustment for potential mediators (hypertension, diabetes, lipid profile, prevalent coronary heart disease, heart failure, and LV hypertrophy), non-smokers in the highest WHR category (>0.95 in women; >1.01 in men) had double the risk of SCD (HR 2.03, 95% CI 1.19 to 3.46; incidence rate 1.43/1000 person-years) versus those with normal WHR.
Conclusions
General obesity is associated with increased risk of SCD in middle-aged, non-smoking individuals, mediated by traditional cardiovascular risk factors. Central obesity, however, is independently associated with SCD by pathways that remain to be elucidated.
INTRODUCTION
Obesity is a global public health problem, the prevalence of which has risen substantially among adults in the vast majority of countries such that there are currently an estimated one billion obese individuals worldwide.1,2 In the USA, between 30–35% of adults are classified as obese (defined as having a body mass index (BMI) in excess of 30 kg/m2) with associated healthcare costs of $147 billion annually.2,3 Obesity is widely acknowledged to be a risk factor for a wide spectrum of cardiovascular, metabolic, neoplastic and musculoskeletal disorders and is estimated to reduce life expectancy by as much as 10 years in those who are severely affected (BMI >40 kg/m2).4–7
From a cardiovascular perspective, obesity is associated with numerous haemodynamic alterations, including increased cardiac output and diastolic filling pressures, which result in LV hypertrophy and dilatation.8–10 Adverse cardiac electrical changes, such as prolongation of the QRS and QT intervals on ECG and increases in QT dispersion and late potentials on signal averaged ECG, are also more common in obese compared with lean individuals.11 These adverse structural and electrical influences on the heart create a substrate that is susceptible to sudden cardiac death (SCD), which accounts for 300 000 deaths in the USA annually.
Data on the relationship between obesity and SCD are sparse and derived largely from community-based autopsy studies12–14 and from a small number of cohort studies,15–18 that often had methodological limitations or issues of generalisability.16,17 To date, the association between SCD and measures of general and abdominal obesity—which may have a different relationship with SCD than that of BMI—has not been explored in detail in the general population. Thus, we examined the nature of the associations between different measures of obesity with SCD in a large, biracial cohort of middle-aged men and women who participated in the Atherosclerosis Risk in Communities (ARIC) study.
METHODS
Study population
The ARIC study is a multicentre prospective cohort study investigating the aetiology of atherosclerotic disease in a middle-aged, biracial population. Participants at baseline (1987–1989) included 15 792 men and women aged 45–64, recruited from four communities in the USA: Forsyth County, North Carolina; Jackson, Mississippi (African Americans only); the northwest suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Participants with baseline BMI recorded as <12 kg/m2 and >60 kg/m2 (n=14), those with race other than African American (n=103), those with missing or unreadable ECGs (n=242), and those with missing covariates (n=492) were excluded from the present analysis, leaving a sample size of 14 941 participants.
The ARIC study protocol was approved by the institutional review board of each participating centre. After obtaining written informed consent, participants underwent a baseline clinical examination in 1987–1989 and were re-examined in 1990–1992, 1993–1995, 1996–1998 and 2011–2013. Follow up was done by annual telephone contact between study visits.
Ascertainment of exposures
The SCD risk factors examined in this analysis were ascertained at the baseline examination. Anthropometric measurements were obtained with the participant wearing light-weight, non-constricting underwear without shoes. Weight was measured to the nearest pound (and converted to kilograms) with a mechanical balance scale (Detecto, model 437) positioned on a firm, level surface. The scale was zeroed daily and calibrated every week or whenever it was moved. Height was measured with the participant standing erect with his/her back against the vertical mounted metal centimetre ruler. BMI was calculated by dividing the weight in kilograms by the square of height in metres. Waist circumference (WC) was measured by wrapping a flexible measuring tape around the waist at the level of the umbilicus while the participant was standing and breathing quietly. Hip circumference was measured at the level of maximal protrusion of the gluteal muscles with the measuring tape kept in horizontal position. Waist-to-hip ratio (WHR) was calculated by WC divided by hip circumference. The inter-technician reliability coefficient for WC was r >0.94.
Questionnaires during study visits were administered to assess self-reported smoking status (current, former, never) and smoking amount. The definitions of other covariates can be found in the attached online supplementary file.
Assessment of study outcome
Participants were contacted annually by phone, and all hospitalisations and deaths in between ARIC baseline examination in 1987–1989 and 30 May 2001 were identified. In addition, deaths among participants were sought in state vital registries and via the National Death Index. If death occurred in the hospital, medical records related to the hospitalisation were obtained. If the death certificate indicated that death occurred out-of-hospital, next of kin interviews and physician, coroner, and autopsy information were sought. To adjudicate SCD, all cases of fatal coronary heart disease (CHD) were reviewed by a committee of physicians, in which SCD was defined as a sudden pulseless condition from a cardiac origin in a previously stable individual. After review of available data, cases were classified as definite SCD, possible SCD, not SCD, or unclassifiable. Definite and possible out-of-hospital SCD were included in this analysis.
Statistical analysis
We hypothesised that risk of SCD would be directly and independently associated with BMI, WC and WHR. BMI was categorised as <18.5, 18.5–24.9 (normal), 25–29.9, 30–34.9, and ≥35 kg/m2. Normal sex-specific values for WC were: <88 cm in women and <102 cm in men; and for WHR were <0.8 in women and <0.95 in men.19 Individuals with baseline WC and WHR measurements that exceeded these values were divided into thirds of roughly equal sample size.
Continuous variables were presented as mean±SD and categorical variables as percentages. Comparison of the baseline characteristics of the SCD and non-SCD groups was performed with t test for continuous variables and χ2 test for categorical variables. The association between measures of obesity and SCD was examined with Cox proportional hazard regression. We tested multiplicative interactions of obesity–SCD association with age, sex, race and smoking status. Due to significant interaction with each of the obesity measures (BMI, p=0.006; WC, p=0.01; WHR, p=0.004), we performed stratified analyses by smoking status (current vs non-current smokers). There was no interaction with age, sex or race. Former smokers were categorised with non-smokers due to a similar association with SCD (data not shown). A trend test was performed by assigning the median value of each category of BMI, WC and WHR to corresponding individuals and treating it as a continuous variable in the model.
In multivariable analyses, a baseline model (model 1) was used with adjustment for age, sex, race, study field centre, and highest level of education attained. A second model (model 2) was examined with inclusion of potential mediators that may explain the influence of obesity on the risk of SCD. These variables included systolic blood pressure, antihypertensive medication use, diabetes mellitus, low density lipoprotein (LDL), high density lipoprotein (HDL), triglycerides, prevalent CHD, prevalent heart failure, resting heart rate, and LV hypertrophy on ECG as defined by the Cornell criteria. If death during follow-up was due to causes other than SCD, the individual was censored at that time. We separately examined non-linear associations between measures of obesity with SCD risk using cubic splines. All analyses were performed using SAS V.9.2 (SAS Institute Inc, Cary, North Carolina, USA).
RESULTS
Baseline characteristics
Baseline characteristics of the study cohort are displayed in table 1. Mean age at enrolment was 54.2±5.8 years (55% female; 26% African American). At baseline, 26% of the participants were current smokers. Prevalent CHD and prevalent heart failure was present in 4.7% and 4% of the study cohort, respectively. Mean BMI, WC and WHR were 27.6±5.3, 96.9±13.9 and 0.93±0.08, respectively.
Table 1.
Baseline characteristics of the ARIC participants with and without SCD
| No SCD (n=14 688) | SCD (n=253) | p Value | |
|---|---|---|---|
| Age (years)* | 54.1 (5.8) | 56.5 (5.7) | <0.0001 |
| Female (%) | 55.6 | 33.2 | <0.0001 |
| African American (%) | 25.4 | 41.5 | <0.0001 |
| High school degree (%) | 77.0 | 58.1 | <0.0001 |
| Current smoker (%) | 25.9 | 40.7 | <0.0001 |
| Prevalent CHD (%) | 4.3 | 31.2 | <0.0001 |
| Prevalent MI (%) | 3.5 | 30.4 | <0.0001 |
| Prevalent heart failure (%) | 4.5 | 10.3 | <0.0001 |
| LVH at baseline ECG (%) | 2.0 | 7.9 | <0.0001 |
| Atrial fibrillation (%) | 0.2 | 0.8 | 0.06 |
| Weight (lbs)* | 172.7 (36.9) | 181.0 (37.1) | 0.0004 |
| Height (cm)* | 168.5 (9.3) | 170.1 (8.4) | 0.0005 |
| BMI (kg/m2)* | 27.6 (5.3) | 28.4 (5.4) | 0.01 |
| Waist circumference (cm)* | 96.8 (13.9) | 101.0 (14.1) | <0.0001 |
| Hip circumference (cm)* | 104.5 (10.3) | 103.9 (10.9) | 0.38 |
| Waist: hip ratio* | 0.92 (0.08) | 0.97 (0.06) | <0.0001 |
| Total cholesterol (mg/dL)* | 214.2 (41.4) | 220.0 (40.8) | 0.03 |
| HDL cholesterol (mg/dL)* | 51.9 (17.0) | 44.9 (13.4) | <0.0001 |
| LDL cholesterol (mg/dL)* | 137.3 (39.3) | 146.5 (38.5) | 0.0002 |
| Triglycerides (mg/dL)* | 124.7 (64.5) | 143.4 (72.1) | <0.0001 |
| Prevalent diabetes mellitus (%) | 10.9 | 32.0 | <0.0001 |
| Prevalent hypertension (%) | 33.5 | 61.7 | <0.0001 |
| Antihypertensive medication (%) | 29.5 | 56.9 | <0.0001 |
| Heart rate on ECG (beats/min)* | 66.6 (10.2) | 68.6 (12.1) | 0.002 |
| Systolic blood pressure (mm Hg) | 120.9 (18.6) | 133.3 (23.6) | <0.0001 |
| β-blockers, (%) | 10.1 | 21.0 | <0.0001 |
| Antiarrhythmic medications (%) | 0.7 | 3.2 | <0.0001 |
Mean (SD).
ARIC, Atherosclerosis Risk in Communities; BMI, body mass index; CHD, coronary heart disease; HDL, high density lipoprotein; LDL, low density lipoprotein; LVH, LV hypertrophy; MI, myocardial infarction; SCD, sudden cardiac death.
Incidence of SCD during follow-up
During a mean follow-up of 12.6±2.5 years there were a total of 253 out-of-hospital SCDs (mean baseline age 56.5 ±5.7 years, 33% female, 42% African American; incidence rate 1.34/1000 person-years of follow-up). Participants who died suddenly had a greater baseline BMI (28.4±5.4 kg/m2 vs 27.6 ±5.3 kg/m2; p=0.01), WC (101.0±14.1 vs 96.8±13.9 cm; p<0.0001) and WHR (0.97±0.06 vs 0.92±0.08; p<0.0001) than those without SCD (table 1). Participants with SCD also had a greater prevalence of CHD risk factors than those without SCD.
Association between measures of obesity and SCD in non-smokers
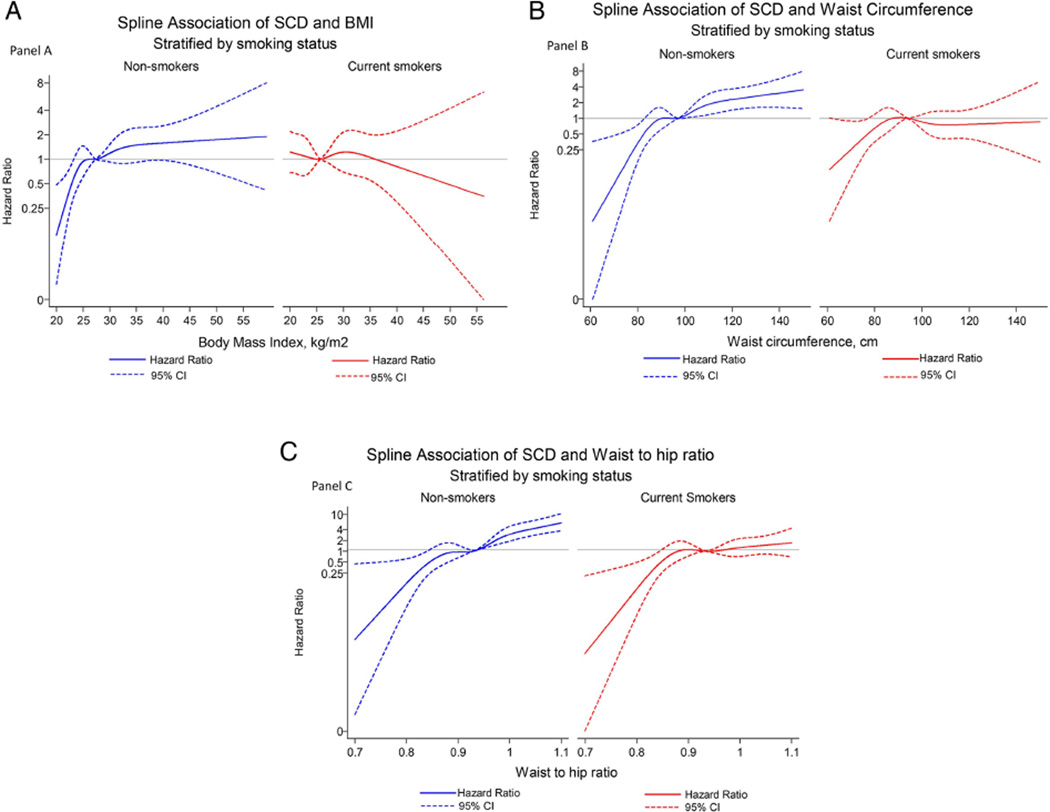
There were continuous and positive associations between each of the measures of obesity with risk of SCD among non-smokers in models that were adjusted for age, sex, race, education level and study centre (figure 1). A 1SD increment in BMI, WC and WHR was associated with 34% (95% CI 15% to 56%; p=0.0002), 49% (28% to 74%; p<0.0001) and 100% (65% to 142%; p<0.0001) increased risk of SCD respectively. After adjusting for possible mediators, the relationships were largely attenuated for BMI and WC but to a lesser extent for WHR: a 1SD increment in BMI, WC and WHR was associated with 3% (95% CI −13% to 27%; p=0.74), 15% (−3% to 37%; p<0.11) and 59% (27% to 100%; p<0.0001) increase in risk of SCD respectively.
Figure 1.
Cubic spline model for risk of sudden cardiac death (SCD) in relation to body mass index (BMI) (A), waist circumference (B) and waist hip (C), stratified by smoking status and adjusted for age, sex and race, field centre and education level.
In the categorical analysis, individuals who were centrally or generally obese had substantially increased risk of SCD compared with their leaner counterparts (tables 2–4). In the most obese individuals (BMI >35 kg/m2; WHR ≥0.95 (women), ≥1.01 (men)) the risk of SCD was between two to three times greater than those individuals within the normal range of values. Incidence rate of SCD was 1.55/1000 person-years in participants with BMI >35 kg/m2 and 1.43/1000 person-years in those within the highest WHR category (tables 2–4).
Table 2.
Incidence rate and HR of SCD in relation to BMI categories in ARIC, stratified by smoking status
| BMI categories | ||||||
|---|---|---|---|---|---|---|
| <18.5 | 18.5–24.9 | 25–29.9 | 30–34.9 | 35+ | P for trend | |
| Non-smokers (n=11 036) | ||||||
| N | 44 | 3282 | 4463 | 2130 | 1117 | |
| SCD cases | 0 | 21 | 67 | 36 | 26 | |
| Person-years | 533 | 42 394 | 56 973 | 26 796 | 14 006 | |
| SCD incidence* | 0 | 0.41 | 0.75 | 0.87 | 1.55 | |
| Model 1 HR† | 0 | 1 (ref) | 1.73 (1.06 to 2.84) | 1.94 (1.12 to 3.33) | 3.36 (1.85 to 6.07) | 0.0001 |
| Model 2 HR‡ | 0 | 1 (ref) | 1.27 (0.77 to 2.10) | 1.06 (0.60 to 1.87) | 1.46 (0.78 to 2.76) | 0.41 |
| Current smokers (n=3905) | ||||||
| N | 88 | 1607 | 1424 | 580 | 206 | |
| SCD cases | 3 | 43 | 38 | 17 | 2 | |
| Person-years | 970 | 19 186 | 17 382 | 7133 | 2486 | |
| SCD incidence* | 1.92 | 1.37 | 1.19 | 1.35 | 0.49 | |
| Model 1 HR† | 1.39 (0.43 to 4.50) | 1 (ref) | 0.86 (0.55 to 1.33) | 0.94 (0.53 to 1.65) | 0.34 (0.08 to 1.41) | 0.25 |
| Model 2 HR‡ | 1.82 (0.55 to 6.03) | 1 (ref) | 0.66 (0.42 to 1.04) | 0.60 (0.33 to 1.09) | 0.16 (0.04 to 0.69) | 0.003 |
Per 1000 person-year. Adjusted for age, sex, and race.
Model 1: Cox proportional hazard model adjusted for age, sex and race, field centre, education level.
Model 2: Model 1+ adjustment for SBP, antihypertensive medication use, diabetes, LDLc, HDLc, triglycerides, prevalent CHD, prevalent heart failure, resting heart rate on ECG and LVH at baseline ECG.ARIC, Atherosclerosis Risk in Communities; BMI, body mass index; CHD, coronary heart disease; HDLc, high density lipoprotein cholesterol; LDLc, low density lipoprotein cholesterol; LVH, LV hypertrophy; SBP, systolic blood pressure; SCD, sudden cardiac death.
Table 4.
Incidence rate and HR of SCD in relation to waist to hip ratio categories in ARIC, stratified by smoking status
| Waist to hip ratio categories* | |||||
|---|---|---|---|---|---|
| Reference | High category 1 | High category 2 | High category 3 | P for trend | |
| Non-smokers | |||||
| N | 2743 | 2773 | 3009 | 2511 | |
| SCD cases | 25 | 19 | 46 | 60 | |
| Person-years | 35 193 | 35 707 | 38 614 | 31 186 | |
| SCD incidence† | 0.37 | 0.44 | 0.87 | 1.43 | |
| Model 1 HR‡ | 1 (ref) | 1.15 (0.63 to 2.11) | 2.29 (1.39 to 3.77) | 3.54 (2.15 to 5.81) | <0.0001 |
| Model 2 HR§ | 1 (ref) | 1.00 (0.54 to 1.84) | 1.47 (0.88 to 2.46) | 2.03 (1.19 to 3.46) | 0.003 |
| Current smokers | |||||
| N | 995 | 947 | 1031 | 932 | |
| SCD cases | 26 | 15 | 36 | 26 | |
| Person-years | 11 896 | 11 660 | 12 547 | 11 056 | |
| SCD incidence† | 1.34 | 1.28 | 2.64 | 2.07 | |
| Model 1 HR‡ | 1 (ref) | 0.96 (0.50 to 1.85) | 1.96 (1.15 to 3.33) | 1.48 (0.83 to 2.63) | 0.04 |
| Model 2 HR§ | 1 (ref) | 0.71 (0.36 to 1.37) | 1.27 (0.73 to 2.21) | 0.76 (0.41 to 1.42) | 0.78 |
Reference: <0.80 (women); < 0.95 (men).
High category 1: 0.80–0.87 (women); 0.95–0.974 (men).
High category 2: 0.88–0.94 (women); 0.975–1.009 (men).
High category 3: 0.95–1.29 (women); 1.01–1.39 (men).
Per 1000 person-year. Adjusted for age, sex, and race.
Model 1: Cox proportional hazard model adjusted for age, sex and race, field centre, education level.
Model 2: Model 1+ adjustment for SBP, antihypertensive medication use, diabetes, LDLc, HDLc, triglycerides, prevalent CHD, prevalent heart failure, resting heart rate on ECG and LVH at baseline ECG.ARIC, Atherosclerosis Risk in Communities; CHD, coronary heart disease; HDLc, high density lipoprotein cholesterol; LDLc, low density lipoprotein cholesterol; LVH, LV hypertrophy; SBP, systolic blood pressure; SCD, sudden cardiac death.
The association between obesity and SCD among non-smokers was largely mediated through diabetes mellitus and hypertension (see online supplementary tables S1–S3).
Association between measures of obesity and SCD in smokers
In current smokers the relationships between measures of obesity and SCD were inconsistent (figure 1). In the minimally adjusted model there was no significant association between BMI (Ptrend=0.25) or WC (Ptrend=0.53) with risk of SCD (tables 2–4). For WHR, there was evidence of a positive association with SCD (Ptrend=0.04). Upon adjustment for intermediary variables the relationship was no longer apparent (Ptrend=0.78).
Sensitivity analyses
Sensitivity analyses considering non-sudden deaths as a competing risk for SCD did not show a significant change in the HRs for the association between obesity measures and SCD (see online supplementary tables S4–S6). The association between obesity and all-cause mortality was similar to the association between obesity and SCD (data not shown).
DISCUSSION
In this large, prospective, cohort study, routine measures of obesity were significantly associated with increased risk of SCD among non-smoking, middle-aged individuals in the community. The association between each of the three measures of obesity, namely BMI, WC and WHR, with SCD were continuous, indicating that the relationship exists across the continuum of the body size spectrum. However, the impact of BMI and WC on risk of SCD appeared to be mediated through their negative effects on other traditional cardiovascular risk factors—namely hypertension, diabetes mellitus and prevalent CHD. Only for WHR was there evidence of a direct association with SCD—even after adjusting for variables on the causal pathway, individuals in the top category of WHR had double the risk of mortality from SCD compared with those in the lowest WHR group.
Although previous cohort studies such as Framingham and the Nurses’ Health Study have previously reported an increased risk of SCD associated with greater age-adjusted weight,15 general obesity,16 and central obesity,17 in agreement with the current study, this is the first report that has directly compared the strength and nature of the relationships between different measures of obesity with SCD in a biracial population of men and women.
Fat mass and distribution can vary considerably among individuals with a similar BMI. Thus, WHR has been used as an additional measure of body fat distribution and provides an index of both subcutaneous and intra-abdominal adipose tissue. In previous reports, abdominal obesity was suggested to be a stronger independent risk factor for myocardial infarction, stroke and premature death than BMI.20 Although not reproduced in some other studies,21 this concept is based largely on the rationale that visceral fat tissue is associated with a range of metabolic abnormalities such as glucose intolerance, reduced insulin sensitivity and adverse lipid profiles. Abdominal fat also has a greater influence on inflammation than fat stored in other areas of the body, which might be one of the mechanisms responsible from its association with adverse cardiovascular outcomes and SCD. Indeed, elevated white blood cell count, a general measure of inflammation, is associated with increased risk of SCD.17,22 Recently, the inflammatory markers interleukin 6 and C reactive protein were also found to be associated with SCD in older individuals.23 The observation that the effect of obesity on SCD was chiefly mediated through its effects on cardiovascular risk factors and CHD in our study is consistent with these potential mechanisms.
The association between obesity and SCD could also be mediated via the haemodynamic and electrical effects of obesity on the cardiovascular system.8,24 Obese individuals have increased preload and reduced systemic vascular resistance. Collectively, these haemodynamic changes lead to higher cardiac output and cardiac work, eventually causing LV hypertrophy and dilatation.8 Hypertension, which commonly occurs with obesity, further promotes LV hypertrophy by increasing after-load. LV hypertrophy is a risk factor for SCD. In the Framingham Heart Study, the presence of LV hypertrophy on echocardiography was associated with >3-fold increase in the risk of SCD.25 Furthermore, regression of LV hypertrophy with antihypertensive therapy was shown to reduce the risk of SCD among hypertensive individuals.26 Heart failure and mechanical stretch of the ventricular myocardium could be the mediators connecting the haemodynamic effects of obesity with SCD.27
Cardiac electrical abnormalities associated with obesity include frequent and complex premature ventricular complexes, which were 30 times more frequent in obese patients with LV hypertrophy than age, sex, height and blood pressure matched lean subjects.28 Obesity has also been associated with a modest prolongation of corrected QT interval on ECG.11 Prolongation of the QT interval—a measure of myocardial repolarisation—has been associated with mortality and SCD.29 Furthermore, the prevalence and number of abnormal late potentials, measured by signal-averaged ECG, were increased with obesity.12 Finally, obesity has been associated with autonomic imbalance, demonstrated by reduced heart rate variability and increased risk of SCD.12 These potential mechanisms support the hypothesis that factors other than prevalent CHD could be mediating the relationship between obesity and SCD. Indeed, a recent large autopsy series showed that obesity-related cardiomyopathy was the biggest cause of non-ischaemic SCD in Finland.13
The lack of an association between BMI and WC with risk of SCD among current smokers may be a chance finding due to the relatively small number of SCD cases among current smokers in the ARIC population (only two SCD events among current smokers with BMI >35). It may also reflect what has been termed the ‘obesity paradox’, whereby an inverse association between obesity and mortality in diseases such as heart failure and CHD is driven by unintentional weight loss in patients with severe comorbidities.9,24 Alternatively, being overweight or mildly obese might allow patients with existing comorbidities to weather acute illnesses.9,24
Strengths and limitations
The most notable strength of this investigation is its large, prospective and community-based source population of men and women who were subjected to systematic examination and follow-up. Thus the predictor variables were carefully measured, and the outcome variable was adjudicated by a committee using standardised criteria. Limitations include the age distribution of the study cohort. By design the ARIC study included middle-aged adults. Thus, these findings should also be examined in cohorts of older adults. Second, our analysis only included the obesity measurements that were made at the baseline visit. We did not examine the influence of the changes in body habitus during follow-up. Third, except for the participants in Jackson, Mississippi, echocardiograms were not routinely obtained in the ARIC study. Thus, LVEF, which is the mainstay of current SCD risk stratification, could not be considered in our analysis. This study does not identify the mechanism of the association between obesity and SCD and whether it is mediated by CHD. Although our analyses were adjusted for prevalent CHD, presence of subclinical CHD cannot be excluded. Further, the association between measures of obesity and SCD attenuated significantly when adjusted for potential cardiovascular mediators. Future studies to examine this issue are warranted.
CONCLUSIONS
This analysis extends our current knowledge regarding the hazards of excess bodyweight on risk of SCD. Among non-smoking middle-aged adults living in the community, general obesity, measured by BMI, was indirectly associated with SCD through its adverse effects on blood pressure, lipids, diabetes mellitus and CHD. In comparison, abdominal obesity, measured by WHR, appears to be directly associated with increased risk of SCD by mechanisms that remain to be elucidated.
Supplementary Material
Table 3.
Incidence rate and HR of SCD in relation to waist circumference categories in ARIC, stratified by smoking status
| Waist circumference categories* | |||||
|---|---|---|---|---|---|
| Reference | High category 1 | High category 2 | High category 3 | P for trend | |
| Non-smokers | |||||
| N | 4968 | 1988 | 2044 | 2036 | |
| SCD cases | 58 | 22 | 24 | 46 | |
| Person-years | 63 865 | 25 359 | 26 000 | 25 477 | |
| SCD incidence† | 0.56 | 0.68 | 0.71 | 1.08 | |
| Model 1 HR‡ | 1 (ref) | 1.22 (0.74 to 2.01) | 1.25 (0.76 to 2.03) | 2.35 (1.56 to 3.54) | 0.0002 |
| Model 2 HR§ | 1 (ref) | 0.89 (0.54 to 1.48) | 0.89 (0.54 to 1.47) | 1.27 (0.81 to 2.00) | 0.36 |
| Current smokers | |||||
| N | 2101 | 650 | 636 | 518 | |
| SCD cases | 59 | 19 | 15 | 10 | |
| Person-years | 25 125 | 7989 | 7797 | 6246 | |
| SCD incidence† | 1.81 | 2.35 | 1.83 | 1.19 | |
| Model 1 HR‡ | 1 (ref) | 1.25 (0.73 to 2.13) | 0.99 (0.55 to 1.77) | 0.75 (0.38 to 1.49) | 0.53 |
| Model 2 HR§ | 1 (ref) | 1.05 (0.60 to 1.83) | 0.65 (0.35 to 1.20) | 0.42 (0.20 to 0.85) | 0.01 |
Reference: <88 cm (women); <102 cm (men).
High category 1: 88–95 cm (women); 102–105 cm (men).
High category 2: 96–106 cm (women); 106–111 cm (men).
High category 3: 107–168 cm (women); 112–178 cm (men).
Per 1000 person-year. Adjusted for age, sex, and race.
Model 1: Cox proportional hazard model adjusted for age, sex and race, field centre, education level.
Model 2: Model 1+ adjustment for SBP, antihypertensive medication use, diabetes, LDLc, HDLc, triglycerides, prevalent CHD, prevalent heart failure, resting heart rate on ECG and LVH at baseline ECG.ARIC, Atherosclerosis Risk in Communities; CHD, coronary heart disease; HDLc, high density lipoprotein cholesterol; LDLc, low density lipoprotein cholesterol; LVH, LV hypertrophy; SBP, systolic blood pressure; SCD, sudden cardiac death.
Key messages.
What is already known on this subject?
Obesity has wide-ranging haemodynamic, arrhythmic and structural influences on the cardiovascular system and is acknowledged to be a risk factor for cardiovascular, metabolic and other disorders affecting the heart such as coronary heart disease, hypertension, diabetes mellitus and obstructive sleep apnoea. There is suggestive evidence that obesity characterised as age-adjusted weight, body mass index (BMI) and waist circumference (WC) is associated with sudden cardiac death (SCD). However, detailed analysis comparing the general and abdominal measures of obesity with SCD is lacking. Further, whether the association between obesity and SCD is mediated by the aforementioned cardiovascular and metabolic disorders or is independent of them is unknown.
What might this study add?
Our study, performed in a large, biracial, community-based cohort, shows that obesity, measured as BMI, WC and waist to hip ratio (WHR), is associated with SCD in non-smokers but not in smokers. Of the three obesity measures, WHR had the strongest association with SCD. As opposed to BMI and WC, the association between WHR and SCD was independent of coronary heart disease, diabetes mellitus, hypertension and other potential confounders.
How might this impact on clinical practice?
The association between obesity and SCD in the general population strengthens the evidence on the adverse cardiovascular effects of obesity. These data might allow clinicians, patients and policymakers to take a more aggressive approach against obesity. Our findings might also result in more widespread measurement of WHR as a stronger risk factor of SCD than BMI.
Acknowledgments
The authors thank the ARIC participants and staff for their important contributions.
Funding The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Competing interests AA received support from the National Heart, Lung, and Blood Institute (RC1 HL099452) and the American Heart Association (09SDG2280087).
Footnotes
This paper was presented as an abstract at the 2013 American Heart Association Scientific Sessions.
Contributors Study concept and design: SA, RRH, AA and ARF. Acquisition of data: AA and ARF. Analysis and interpretation of data: SA, RRH, FLL, LYC, NS, DS, RD, SK, AA and ARF. Drafting of the manuscript: SA and RRH. Critical revision of the manuscript for important intellectual content: SA, RRH, FLL, LYC, NS, DS, RD, SK, AA and ARF. Statistical analysis: FLL. Obtained funding: AA and ARF. Final approval: SA, RRH, FLL, LYC, NS, DS, RD, SK, AA and ARF.
Ethics approval Institutional Review Boards of all centres participating in the ARIC study.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement AA and ARF have full access to all the data in the ARIC study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Patient consent Obtained.
REFERENCES
- 1.Poirier P, Giles TD, Bray GA, et al. American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 2.Finucane MM, Stevens GA, Cowan MJ, et al. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index). National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkelstein EA, Trogdon JC, Cohen JW, et al. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 4.Whitlock G, Lewington S, Sherliker P, et al. Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biggs ML, Mukamal KJ, Luchsinger JA, et al. Association between adiposity in midlife and older age and risk of diabetes in older adults. JAMA. 2010;303:2504–2512. doi: 10.1001/jama.2010.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kizer JR, Biggs ML, Ix JH, et al. Measures of adiposity and future risk of ischemic stroke and coronary heart disease in older men and women. Am J Epidemiol. 2011;173:10–25. doi: 10.1093/aje/kwq311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huxley R, Mendis S, Zheleznyakov E, et al. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk—a review of the literature. Eur J Clin Nutr. 2010;64:16–22. doi: 10.1038/ejcn.2009.68. [DOI] [PubMed] [Google Scholar]
- 8.Owan T, Litwin SE. Is there a cardiomyopathy of obesity? Curr Heart Fail Rep. 2007;4:221–228. doi: 10.1007/s11897-007-0016-3. [DOI] [PubMed] [Google Scholar]
- 9.Lavie CJ, Alpert MA, Arena R, et al. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. doi: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–236. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Fraley MA, Birchem JA, Senkottaiyan N, et al. Obesity and the electrocardiogram. Obes Rev. 2005;6:275–281. doi: 10.1111/j.1467-789X.2005.00199.x. [DOI] [PubMed] [Google Scholar]
- 12.Adabag AS, Peterson G, Apple FS, et al. Etiology of sudden death in the community: results of anatomical, metabolic, and genetic evaluation. Am Heart J. 2010;159:33–39. doi: 10.1016/j.ahj.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hookana E, Junttila MJ, Puurunen VP, et al. Causes of nonischemic sudden cardiac death in the current era. Heart Rhythm. 2011;8:1570–1575. doi: 10.1016/j.hrthm.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 14.Noheria A, Teodorescu C, Uy-Evanado A, et al. Distinctive profile of sudden cardiac arrest in middle-aged vs. older adults: a community-based study. Int J Cardiol. 2013;168:3495–3499. doi: 10.1016/j.ijcard.2013.04.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannel WB, Doyle JT, McNamara PM, et al. Precursors of sudden coronary death. Factors related to the incidence of sudden death. Circulation. 1975;51:606–613. doi: 10.1161/01.cir.51.4.606. [DOI] [PubMed] [Google Scholar]
- 16.Albert CM, Chae CU, Grodstein F, et al. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 17.Bertoia ML, Allison MA, Manson JE, et al. Risk factors for sudden cardiac death in post-menopausal women. J Am Coll Cardiol. 2012;60:2674–2682. doi: 10.1016/j.jacc.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 18.Empana JP, Ducimetiere P, Charles MA, et al. Sagittal abdominal diameter and risk of sudden death in asymptomatic middle-aged men: the Paris Prospective Study I. Circulation. 2004;110:2781–2785. doi: 10.1161/01.CIR.0000146395.64065.BA. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Geneva: 2008. Dec 8–11, Waist circumference and waist-hip ratio: report of a WHO expert consultation; pp. 1–39. ISBN:978 92 4 150149 1. [Google Scholar]
- 20.Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case–control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 21.Wormser D, Kaptoge S, Di Angelantonio E, et al. Emerging Risk Factors Collaboration. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kucharska-Newton AM, Couper DJ, Pankow JS, et al. Hemostasis, inflammation, and fatal and nonfatal coronary heart disease: long-term follow-up of the atherosclerosis risk in communities (ARIC) cohort. Arterioscler Thromb Vasc Biol. 2009;29:2182–2190. doi: 10.1161/ATVBAHA.109.192740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussein AA, Gottdiener JS, Bartz TM, et al. Inflammation and sudden cardiac death in a community-based population of older adults: the Cardiovascular Health Study. Heart Rhythm. 2013;10:1425–1432. doi: 10.1016/j.hrthm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 25.Haider AW, Larson MG, Benjamin EJ, et al. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32:1454–1459. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 26.Wachtell K, Okin PM, Olsen MH, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: the LIFE Study. Circulation. 2007;116:700–705. doi: 10.1161/CIRCULATIONAHA.106.666594. [DOI] [PubMed] [Google Scholar]
- 27.Adabag AS, Therneau TM, Gersh BJ, et al. Sudden death after myocardial infarction. JAMA. 2008;300:2022–2029. doi: 10.1001/jama.2008.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messerli FH, Nunez BD, Ventura HO, et al. Overweight and sudden death. Increased ventricular ectopy in cardiopathy of obesity. Arch Intern Med. 1987;147:1725–1728. doi: 10.1001/archinte.147.10.1725. [DOI] [PubMed] [Google Scholar]
- 29.Chugh SS, Reinier K, Singh T, et al. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119:663–670. doi: 10.1161/CIRCULATIONAHA.108.797035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.