Abstract
One concept used in traditional Persian medicine (TPM) for multidrug therapy is that of the convoy drug (Mobadregh). According to TPM texts, convoy drugs are substances (or drugs), which facilitate the access of drugs or foods to the whole body or to specific organs. This study reviewed some convoy drugs presented in TPM, their biological effects, and their probable interactions with main drugs, considering the increased absorption through inhibition of P-glycoprotein (P-gp) efflux function, bioavailability-enhancing effects, and decreased metabolism of the main drug using electronic databases including PubMed, Scopus, ScienceDirect, and Google Scholar in November and December, 2013. Recent studies have proven the beneficial effects of Crocus sativus L. (saffron) and camphor on the heart and brain, the cerebral therapeutic effects of Asarum europaeum (hazelwort), the hepatoprotective effects of Cichorium intybus (chicory), and Apium graveolens (celery) seeds, and the diuretic effects of Cinnamomum zeylanicum (cinnamon), and Cucumis melo (melon) seeds. The effects of vinegar in targeting the liver and brain have also been demonstrated. An evaluation of the results demonstrated that the suggested convoy drugs, including Piper nigrum (black pepper), Piper longum (long pepper), red wine, Camellia sinensis (tea), hazelwort, Mentha longifolia (pennyroyal), Anethum graveolens (dill), Foeniculum vulgare (fennel), cinnamon, and Sassafras albidum (sassafras) can increase the bioavailability of coadministered drugs by inhibition of P-gp or cytochrome P450s (CYP450s) or both of them. This evidence could be a good basis for the use of these agents as convoys in TPM.
Keywords: Cytochrome P450s, medicinal convoy plant, P-glycoprotein (P-gp), traditional Persian medicine
INTRODUCTION
In traditional Persian medicine (TPM), lifestyle modification is more important than treatment. Similarly, single-drug therapy is more desirable than multiple-drug therapy because it has fewer toxic side effects. Based on this theory, medical scientists have tried to interpret the conditions and causes for multiple drug therapy. One concept used in TPM for multidrug therapy is the use of convoy drugs (Mobadregh). According to TPM texts, convoy drugs are a class of modifiers that penetrate well and rapidly into the whole body or into particular organs. Moreover, convoys are substances (or drugs) that facilitate access to the whole body or to specific organs for drugs or food.[1,2,3,4,5,6,7,8,9]
Recent studies have proven that the simultaneous administration of two medicaments (drug-drug, herb-drug, and herb-herb) can result in clinically significant drug interactions. The medicaments may interact with each other in their absorption and metabolism and so can impact their bioavailability and exhibit synergistic pharmacological activity.[10]
This study reviewed some convoy drugs presented in TPM, their biological effects, and their probable interactions with the main drug considering increased absorption through the inhibition of P-glycoprotein (P-gp) efflux function, the bioavailability enhancement effect, and decreasing the metabolism of the main drug.
MATERIALS AND METHODS
This paper reviews some medicinal convoy plants mentioned in the most famous TPM books, such as Qanon (“Canon of Medicine”), Kholasatol Hekmah (“Survey of Knowledge”), Zakhire Kharazmshahi (“Kharazmid Reservoir”), ExirAzam (“Great Panacea”), and Makhzan al Advieh (“Drug Reservoir”). Electronic databases including PubMed, Scopus, ScienceDirect, and Google Scholar were searched between November and December, 2013 for each of the plants plus “biological effect,” “interaction,” “herb-herb interaction,” “herb-drug interaction,” “cytochrome P450,” “P-glycoprotein,” and “phase II enzyme” as key words.
RESULTS AND DISCUSSION
Convoying drugs to the heart and brain
Saffron
Some of the convoy medicinal plants and their target organs were shown in Table 1. Convoying drugs to the heart, brain, and other organs is a unique ability of saffron mentioned in TPM texts. It has been noted that when saffron is combined with a camphor tablet, it guides the camphor to the target organ. The moment the camphor tablet arrives at the heart, the saffron separates from it and its function is finished.[5]
Table 1.
Some medicinal convoy plants and their target organ(s)
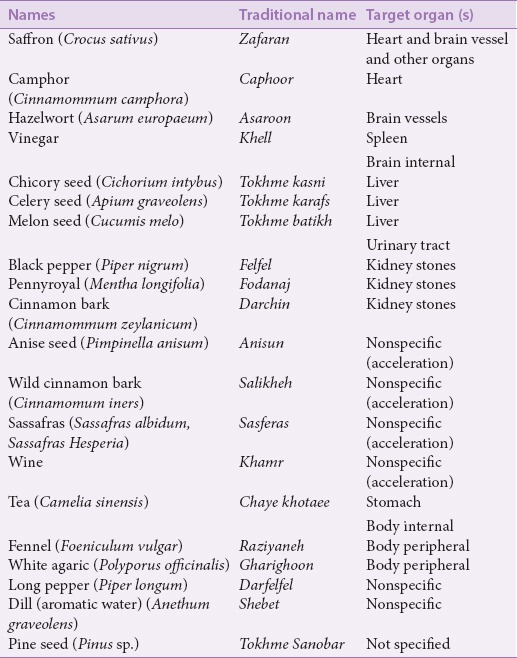
Recent studies have established the effects of saffron aqueous-ethanolic extract in reducing heart rate and the contractility of isolated guinea pig hearts via the inhibition of calcium channels.[11] The role of saffron in protecting ischemic hearts has also been proven by biochemical and histopathological findings.[12]
Saffron and hazelwort can be combined with scammony (Convolvolus scammonia) and agaric (Polyporus officinalis) to convoy them to the brain and its veins where they perform their functions properly.[4] The beneficial influences of saffron extracts and its major component crocin that have been confirmed include treating mild to moderate depression; improving learning and memory; radical scavenging; and its anticonvulsant, neuroprotectant, antioxidant, and antitumor properties; it also increases retinal blood flow.[11,13,14]
Camphor
Belz et al. (2002) proved the efficacy of camphor and crataegus berry combination (CCC) in the symptomatic therapy of orthostatic hypotension.[15] Moreover, CCC caused an immediate and short-term increase in blood pressure and improved mental performance in aged women. Possible physiological mediating mechanisms are hemodynamic modification, sympathetic stimulation, cerebral metabolism enhancement, and direct influences on neural activation.[16]
(+)-camphor becomes hydroxylated to borneol in the body mediated by cytochrome P450 (CYP450). Borneol, a bicyclic monoterpene, has been used in China for the treatment of cardiovascular and cerebrovascular diseases as a component in multidrug prescriptions.[17] Borneol is principally distributed in abundant blood-supply tissues after intravenous and intranasal administrations in mice. Brain tissue indicated the highest target coefficient and target efficiency after intranasal administration.[18] Distribution indices of borneol in rats with cerebral ischemia-reperfusion (stroke) significantly increased after oral administration in comparison to sham-operated rats.[19] Due to the affinity of saffron and camphor for the heart, they have a more specific effect on the heart in therapeutic doses, while in lower doses they can guide cardiac medicaments toward the heart. In these cases, when saffron and camphor convey the main drug to the heart, their function is finished.[4]
Hazelwort
According to TPM texts, hazelwort can transfer the power of scammony and agaric to the brain and its veins, where they do their function correctly.[4,8] Asarone is a compound in the rhizome of Asarum europaeum L. and Acorus calamus L. that is responsible for the therapeutic effects and specific odor of these herbs. Asarum is used in phytotherapy and the food and beverage industries. The roots of these plants contain two isomers, α-asarone and β-asarone.[20] β-asarone easily passes through the blood-brain-barrier (BBB) and shows significant pharmacological effects on the cardiovascular and central nervous systems, while α-asarone shows neuroprotective, hypocholestrolemic, hypolipidemic, and antiepileptic properties.[21]
Convoying effects of vinegar on the spleen and brain
Vinegar
Vinegar is a tenuous, rapidly penetrating supplier of the power and faculties of medicines to the spleen and brain. It has an affinity for the spleen, so it is able to convoy drugs to it.[8,22] For example, the use of vinegar is suggested for convoying and accompanying rose oil to the brain ventricles in treating brain and meninges inflammation.[23]
Recent studies showed that vinegar can induce a liver- and brain-targeting effect by increasing the distribution of the main drug in the liver and brain while simultaneously reducing its distribution in other organs.[24,25,26]
Convoying drugs to the liver
Chicory seeds
Chicory seed is tenuous; it opens obstructions of and convoys drugs to the liver.[8] Recent studies have confirmed the benefits of chicory and celery seeds in preventing toxic effects in the liver. In a study by Ahmed et al. (2003), different fractions of ethanol extract of chicory seeds showed antihepatotoxic effects, while the methanol fraction containing phenolic compounds was more effective than other fractions.[27] A new guaianolidese sesquiterpene glycoside, cichotyboside, isolated from the seeds of Cichorium intybus L., exhibited significant antihepatotoxic effects similar to those of the standard drug silymarin against CCl4-induced toxicity in rats.[28] The aqueous extract of chicory seeds showed beneficial short-term and long-term effects in diabetic rats. Furthermore, the seed extract improved fatty liver caused by diabetes, oleic acid, and steatohepatitis.[29,30]
Celery seeds
Celery seed is a potent antiblocking (mofateh) agent of liver and spleen obstructions. Because of its antiblocking and discutient (mohalel) properties, it can be tenuous and penetrating and can convoy drugs to the liver.[8]
Their hepatoprotective effect,[31] inactivation of toxic metabolites in liver,[32] antihepatocarcinogenesis,[33] antiinflammatory, antioxidant, cyclooxygenase- and topoisomerase-inhibitory activity,[34] and antihypertensive properties[35] have been observed in celery seed extracts.
Convoying drugs to the urinary tract, kidney, and bladder
Melon seed
Melon (Curcumis melo) seed can convoy drugs to the liver and the urinary tract.[8] Two species of the genus Cucumis (Curcumis melo and Curcumis trigonus) were shown to significantly increase urinary volume (UV). They also displayed increases in urinary chloride excretion due to the extract interfering with absorption in the renal tubule.[36] Isomultiflorenol as a major compound and its isomer Δ7-isomer multiflorenol were identified from triterpene alcohol fractions of the unsaponifiable part of Curcumis melo seed lipid.[37] Two pentacyclic triterpenoids, 16α-hydroxy-3-ketoisomultiflorene and 3β- hydroxyl-16-ketoisomultiflorene, isolated from Antidesma menasu, showed diuretic activity in experimental animals.[38]
Convoying litholytic drugs
Drug components that allow litholytic main drugs to penetrate to the location of kidney stones faster contain pepper, pennyroyal, and cinnamon.[5] In addition to their convoying abilities, these drugs are effective in moving stones. Some studies have proven the diuretic and nephroprotective properties of cinnamon bark.[39]
Recent studies have proven the beneficial effects of saffron and camphor in the heart and brain, cerebral therapeutic effects of hazelwort, hepatoprotective effects of chicory and celery seeds, and diuretic effects of cinnamon and melon seed. This evidence could explain the use of these agents as convoy agents in TPM. Based on TPM sources, a convoy agent must have a therapeutic effect on the target organ(s). If it is used in an amount lower than its therapeutic dose and lower than the amount of other components in a formulation, it can direct the main drug to the target organ without showing any therapeutic effect. However, the pharmacokinetic interactions and tissue-targeting effects of these agents need further investigation.[4,5]
Bioavailability enhancement effects
Several herbal compounds including quercetin, genistein, naringin, sinomenine, borneol, and nitrile glycoside as well as herbal medicines such as Piper longum and its active ingredient piperine, Glycyrrhiza glabra (glycyrrhizin), Carum carvi, and Cumminum cyminum have exhibited the capability of enhancing bioavailability.[11,40,41,42,43]
Furthermore, curcumin has been reported to have inhibited the activity of breast cancer resistance protein (BCRP1) in mice. Recently, it was reported that monoterpenoids containing the extract of Zanthoxyli Fructus can inhibit the P-gp–mediated efflux of digoxin. Another study screened the inhibitory activities of different terpenoids on P-gp–mediated efflux in human multidrug resistance-associated protein-1(MDR1)–expressing cell lines. It was found that the inhibitory activities of (R)-(+)-citronellal, (S)-(−)-β-citronellol, α-terpinene, terpinolene, (−)-β-pinene, abietic acid, ophiobolin A, cucurbitacin I, and glycyrrhetic acid on the P-gp–mediated efflux of digoxin were greater than 50%.[44,45,46] In addition, the potential inhibitory effect of glycyrrhetic acid and abietic acid on MRP2- or BCRP-mediated membrane transport has been reported.[47]
Based on the literature reviewed [Table 2], furanocoumarins, including psoralen derivatives in celery seed,[48,49,50] alkaloids such as piperine in black pepper,[51,52] polyphenolic compounds and catechins in green tea,[53] borneol as a metabolite of camphor and as a component of the essential oil of pennyroyal,[17,54] are responsible for inhibiting P-gp and increasing the bioavailability of the coadministered main drug. P-gp is a well-known transporter that acts as a gatekeeper protein for xenobiotics at the luminal membrane of brain capillary endothelial cells (BCEC). Additionally, it has been proven that active efflux mechanisms at the BBB limit the brain penetration of xenobiotics. P-gp–restricted penetration into the brain has led to several assays to eliminate this barrier by using specific P-gp inhibitors.[55] Among the introduced convoying drugs for the brain, α-asarone and β-asarone existing in hazelwort may increase the permeability of drugs into the brain by inhibiting P-gp at the BCEC, thereby increasing the drug concentration in the brain.[21]
Table 2.
Effects of some medicinal convoy plants on CYP450s, phase II enzymes, and efflux transporters
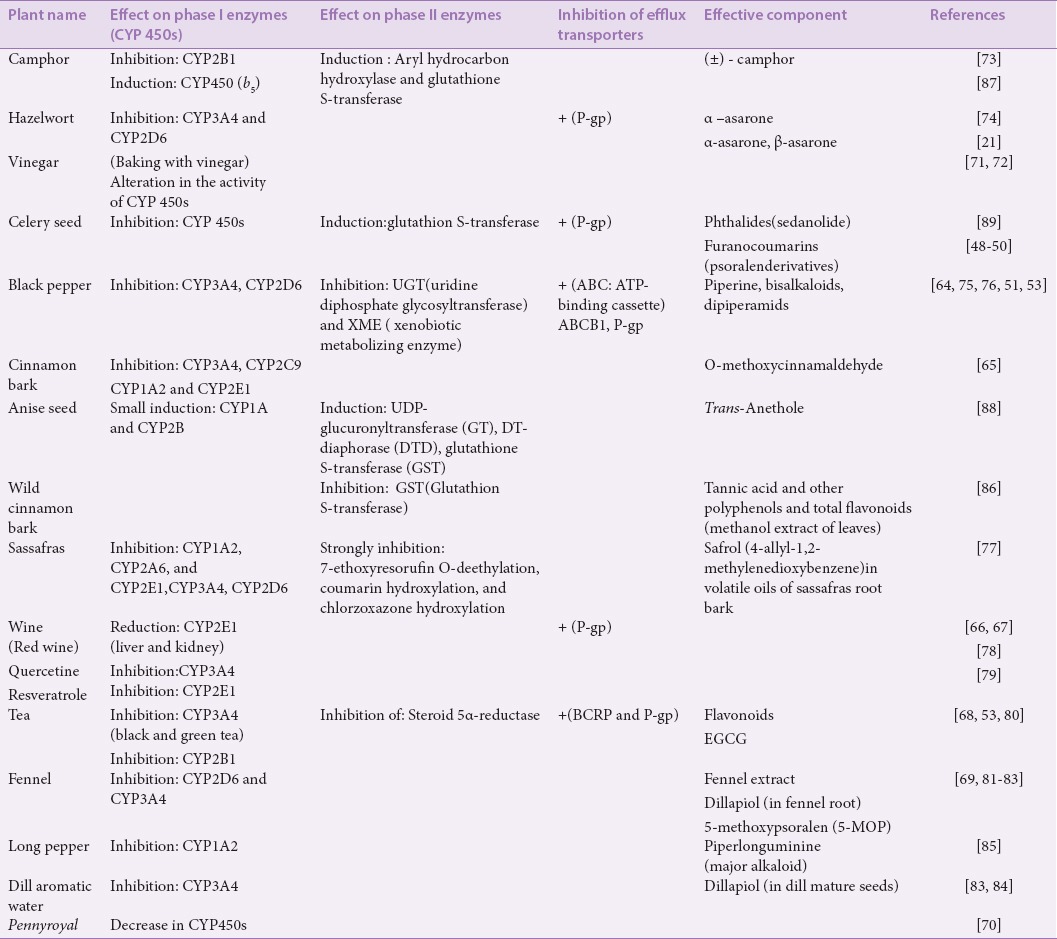
Cytochrome P450s’ inhibitory effects
Herbal medicines such as St. John's wort (Hypericum perforatum), garlic (Allium sativum), piperine (from Piper sp.), ginseng (Ginseng sp.), gingko (Gingko biloba), soybean (Glycine max), alfalfa (Medicago sativa), and grapefruit juice display clinical interactions when coadministered with drugs.[56]
Some reports have demonstrated the effects of several components isolated from medicinal herbs on drug metabolizing-enzymes CYP450, uridine 5’-diphospho (UDP)-glucuronosyltransferase, and glutathione S-transferase (GST). Some natural compounds, such as flavonoids, anthocyanins and their metabolites, furanocoumarins, pipermethystine from kava, and some dietary polyphenols, can inhibit or induce these metabolic enzymes and alter the therapeutic effects of coadministered drugs.[57,58,59,60]
A number of flavonoids have been shown to modulate the CYP450 system, including the inhibition or induction of these enzymes by various mechanisms. Among the inhibitors, five polyphenols (quercetin, phloretin, chrysin, apigenin, and acacetin) exhibited strong inhibitory activity (100% inhibition) against CYP3A4. Apigenin from among the inhibitors of flavone compounds displayed a significant inhibition on CYP3A4. Accordingly, oral coadministrations of flavonoids can improve the therapeutic effects of drugs with low bioavailability.[59,61,62]
The results presented in Table 2 show that from among the medicinal plants introduced as convoys in TPM, extracts from celery, black pepper, cinnamon, red wine, tea, fennel, and pennyroyal have inhibitory effects on CYP450s, with the maximum inhibition being related to CYP3A4, CYP2E1, CYP2D6, and CYP1A2, respectively.[48,63,64,65,66,67,68,69] Cytochrome P3A (CYP3A) is the most abundant and clinically significant family of CYP450 enzymes and includes CYP3A3, CYP3A4, CYP3A5, and CYP3A7. CYP3A4 is responsible for the metabolism of 30% of drugs.[70] Recent studies have also shown that baking a medicinal herb with vinegar may change or alter its activity on CYP450s.[71,72]
The natural compounds identified in convoying medicines that exhibited strong to moderate inhibitory effects on CYP450s are (±)camphor found in the essential oils of several useful plants,[73] α-asarone from hazelwort,[74] piperine, and bisalkaloids, dipiperamides from black pepper and long pepper,[51,52,64,75,76] O-methoxycinnamaldehyde (OMCA) from cinnamon,[64] saffrol from sassafras,[77] quercetin and resveratrol from red wine,[78,79] flavonoids from tea,[80] 5-methoxypsoralen (5-MOP) from fennel,[81] dillapiole from root of fennel and dill mature seeds,[81,82,83,84] and piperlonguminine from long pepper.[85] As the results mentioned above indicate, CYP3A4 is the most inhibited cytochrome, followed by CYP2D6, CYP2E1, CYP1A2, and CYP2B1. These natural compounds belong to phenylpropanoid compounds (dillapiol, safrol, OMCA, α-asarone), furanocoumarins, especially psoralen derivatives (O-MOP), which are characteristically natural components for the Umbelliferae family, flavonoids (quercetin), piper alkaloids and their derivatives, and monoterpenoids (camphor).
The results also indicated that the tannic acid-rich extract of wild cinnamon leaves, flavonoids from tea, and piperine from black and long pepper can inhibit phase II conjugation and monooxygenase enzymes such as GST, uridine diphosphate glycosyltransferase (UGT), and xenobiotic metabolizing enzyme (XME).[77,80,86]
Among the mentioned convoy drugs, some exhibited induction effects on phase I and II metabolic enzymes. The oral administration of camphor increased the activity of CYP450s and phase II metabolizing enzymes such as GST. Moreover, vinegar induced CYP2E1, CYP2D6, and CYP3A4.[72,87] Natural compounds from convoy drugs also showed induction effects such as transanethol and eugenol from anise seed, which strongly induced UDP-glucuronyltransferase, DT-diaphorase (DTD), and GST, as well as a small induction effect on CYP1A and CYP2B.[88] Phthalides from celery seeds also exhibited induction activity on GST in target organs.[89]
CONCLUSION
According to the beneficial effects of saffron and camphor on the heart and brain, cerebral therapeutic effects of hazelwort, hepatoprotective effects of chicory and celery seeds, and diuretic effects of cinnamon and melon seed, we can clarify the use of these herbs as convoy agents to the specific organs based on TPM experiences. In addition, vinegar can induce a liver- and brain-targeting effect by increasing the distribution of the main drug in the liver and the brain. The evaluation of the results demonstrated that the suggested convoy drugs, including black and long pepper, red wine, tea, hazelwort, pennyroyal, and dill, can increase the bioavailability of coadministered drugs by two mechanisms, the inhibition of P-gp and CYP450s. Fennel, cinnamon, sassafras, and camphor can increase the bioavailability of other drugs when orally consumed by inhibiting their metabolism. Although camphor, vinegar, celery, and anise seed have inductive effects on the metabolism of orally coadministered drugs, they may accompany other drugs to the site of action with different mechanisms such as targeting or synergistic effects or other unknown mechanisms that need to be studied.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Seyede Nargess Sadati
Seyede Nargess Sadati, Assistant Professor of Pharmacognosy, Department of Traditional Pharmacy, School of Traditional Iranian Medicine, Tehran University of Medical Sciences.

Mohammad Reza Shams Ardekani
Mohammad Reza Shams Ardekani, Professor of Pharmacognosy, Department of Pharmacognosy and Persian Medicine and Pharmacy Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences.

Nastaran Ebadi
Nastaran Ebadi, Pharm D., Ph.D candidate of Traditional Pharmacy, School of Traditional Iranian Medicine, Tehran University of Medical Sciences.

Maryam Yakhchali
Maryam Yakhchali, Pharm D., Ph.D candidate of Traditional Pharmacy, School of Traditional Iranian Medicine, Tehran University of Medical Sciences.

Azadeh Raees Dana
Azadeh Raees Dana, Pharm D., Ph.D candidate of Traditional Pharmacy, School of Traditional Iranian Medicine, Tehran University of Medical Sciences.

Fatemeh Masoomi
Fatemeh Masoomi, Ph.D of Traditional Pharmacy, School of Traditional Iranian Medicine, Tehran University of Medical Sciences.

Mahnaz Khanavi
Mahnaz Khanavi, Associate Profesor of Pharmacognosy, Department of Pharmacognosy and Persian Medicine and Pharmacy Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences.

Farid Ramezany
Farid Ramezany, Ph.D of Traditional Pharmacy, School of Traditional Iranian Medicine, Tehran University of Medical Sciences.
REFERENCES
- 1.Heravi MY. Qum: Jalal al din Press; 2008. The Sea of Jewels (Bahr ol Javaher) p. 339. [Google Scholar]
- 2.Nazem Jahan MA. Vol. 1. Tehran: Research Institute for Islamic and Complementary Medicine; 2008. Grat Panacea (Exir Azam) p. 312. [Google Scholar]
- 3.Aghili Khorasani MH. Tehran: Research Institute for Islamic and Complementary Medicine; 2008. Grand Formulary (Gharabadin kabir) p. 511. [Google Scholar]
- 4.Aghili Khorasani MH. Vol. 2. Qom: Esmailian Press; 2006. Survey of Knowledge (Kholasat ol Hekmah) p. 287. [Google Scholar]
- 5.Avecina H. Vol. 5. Tehran: Soroosh Press; 2009. Canon of Medicine (Qanun) p. 141. [Google Scholar]
- 6.Mohammad Arzani MM. Vol. 2. Qum: Jalal Al Din Press; 2008. Akbari's Medicine (Tebe Akbari) p. 1324. [Google Scholar]
- 7.Jorjani MH. Vol. 3. Qom: Institute of Natural Medicine Resurgence; 2012. Kharazmid Reservoir (Zakhire Kharazmshahi) p. 368. [Google Scholar]
- 8.Aghili Khorasani MH. Tehran: Tehran University of Medical Sciences Press; 2009. Drugs Resevoir (Makhzan ol Advieh) p. 52. [Google Scholar]
- 9.Alqarshi (Ibn Nafis) AA. Vol. 17. Tehran: Research Institute for Islamic and Complementary Medicine; 2008. Comprehensive Medical Technology (Al Shamel fi Sana’at) p. 727. [Google Scholar]
- 10.Posadzki P, Watson L, Ernst E. Herb-drug interactions: An overview of systematic reviews. Br J Clin Pharmacol. 2013;75:603–18. doi: 10.1111/j.1365-2125.2012.04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boskabady MH, Shafei MN, Shakiba A, Sefidi HS. Effect of aqueous-ethanol extract from Crocus sativus (saffron) on guinea-pig isolated heart. Phytother Res. 2008;22:330–4. doi: 10.1002/ptr.2317. [DOI] [PubMed] [Google Scholar]
- 12.Joukar S, Najafipour H, Khaksari M, Sepehri G, Shahrokhi N, Dabiri S, et al. The effect of saffron consumption on biochemical and histopathological heart indices of rats with myocardial infarction. Cardiovasc Toxicol. 2010;10:66–71. doi: 10.1007/s12012-010-9063-1. [DOI] [PubMed] [Google Scholar]
- 13.Javadi B, Sahebkar A, Emami SA. A survey on saffron in major islamic traditional medicine books. Iran J Basic Med Sci. 2013;16:1–11. [PMC free article] [PubMed] [Google Scholar]
- 14.Xuan B, Zhou YH, Li N, Min ZD, Chiou GC. Effects of crocin analogs on ocular blood flow and retinal function. J Ocul Pharmacol Ther. 1999;15:143–52. doi: 10.1089/jop.1999.15.143. [DOI] [PubMed] [Google Scholar]
- 15.Belz GG, Butzer R, Gaus W, Loew D. Camphor-crataegus berry extract combination dose-dependently reduces tilt induced fall in blood pressure in orthostatic hypotension. Phytomedicine. 2002;9:581–8. doi: 10.1078/094471102321616382. [DOI] [PubMed] [Google Scholar]
- 16.Werner NS, Duschek S, Schandry R. D-camphor-crataegus berry extract combination increases blood pressure and cognitive functioning in the elderly - a randomized, placebo controlled double blind study. Phytomedicine. 2009;16:1077–82. doi: 10.1016/j.phymed.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Zuccarini P. Camphor: Risks and benefits of a widely used natural product. J Appl Sci Environ Manage. 2009;13:69–74. [Google Scholar]
- 18.Zhao JY, Du SY, LU Y, Wu HC, Li HY. Study on tissue distribution of borneol in mice by intravenous and intranasal administrations. Zhongguo Zhong Yao Za Zhi. 2013;38:1071–4. [PubMed] [Google Scholar]
- 19.Xu P, Li Y, Du SY, Lu Y, Bai J, Guo QL. Comparative pharmacokinetics of borneol in cerebralischemia-reperfusion and sham-operated rats. J Zhejiang Univ Sci B. 2014;15:84–91. doi: 10.1631/jzus.B1300141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oprean R, Tamas M, Roman L. Comparison of GC-MS and TLC techniques for asarone isomers determination. J Pharm Biomed Anal. 1998;18:227–34. doi: 10.1016/s0731-7085(98)00161-7. [DOI] [PubMed] [Google Scholar]
- 21.Meng X, Liao S, Wang X, Wang S, Zhao X, Jia P, et al. Reversing P-glycoprotein-mediated multidrug resistance in vitro by α-asarone and β-asarone, bioactive cis-trans isomers from Acorus tatarinowii. Biotechnol Lett. 2014;36:685–91. doi: 10.1007/s10529-013-1419-8. [DOI] [PubMed] [Google Scholar]
- 22.Jorjani MH. Qom: Institute of Natural Medicine Resurgence; 2012. Kharazmid Reservoir (Zakhire Kharazmshahi) p. 409. [Google Scholar]
- 23.Nazem Jahan MA. Vol. 1. Tehran: Research Institute for Islamic and Complementary Medicine; 2008. Great Panacea (Exir Azam) p. 111. [Google Scholar]
- 24.Zhao RZ, Yuana D, Liub SJ, Chena YJ, Liua LJ, Zhaoa Y. Liver targeting effect of vinegar-baked Radix Bupleuri on rhein in rats. J Ethnopharmacol. 2010;132:421–8. doi: 10.1016/j.jep.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Zhao R, Liu S, Mao S, Wang Y. Study on liver targeting effect of vinegar-baked Radix Bupleurion resveratrol in mice. J Ethnopharmacol. 2009;126:415–20. doi: 10.1016/j.jep.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Dou Z, Li K, Wang P, Cao L. Effect of wine and vinegar processing of rhizoma corydalis on the tissue distribution of tetrahydropalmatine, protopine and dehydrocorydaline in rats. Molecules. 2012;17:951–70. doi: 10.3390/molecules17010951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmeda B, Al-Howiriny TA, Siddiqui AB. Antihepatotoxic activity of seeds of cichorium intybus. J Ethnopharmacol. 2003;87:237–40. doi: 10.1016/s0378-8741(03)00145-4. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed B, Khan S, Masood MH, Siddique AH. Anti-hepatotoxic activity of cichotyboside, a sesquiterpene glycoside from the seeds of Cichorium intybus. J Asian Nat Prod Res. 2008;10:223–31. doi: 10.1080/10286020701590764. [DOI] [PubMed] [Google Scholar]
- 29.Ghamarian A, Abdollahi M, Su X, Amiri A, Ahadi A, Nowrouzi A. Effect of chicory seed extract on glucosetolerance test (GTT) and metabolic profile in early and late stage diabetic rats. Daru. 2012;20:56. doi: 10.1186/2008-2231-20-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziamajidi N, Khaghani S, Hassanzadeh G, Vardasbi S, Ahmadian S, Nowrouzi A, et al. Amelioration by chicory seed extract of diabetes- and oleic acid-induced non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) via modulation of PPARα and SREBP-1. Food ChemToxicol. 2013;58:198–209. doi: 10.1016/j.fct.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed B, Alam T, Varshney M, Khan SA. Hepatoprotective activity of two plants belonging to the Apiaceaeand the Euphorbiaceae family. J Ethnopharmacol. 2002;79:313–6. doi: 10.1016/s0378-8741(01)00392-0. [DOI] [PubMed] [Google Scholar]
- 32.El-Shinnawy NA. The therapeutic applications of celery oil seed extract on the plasticizer di(2-ethylhexyl) phthalate toxicity. Toxicol Ind Health. 2015;31:355–66. doi: 10.1177/0748233713475515. [DOI] [PubMed] [Google Scholar]
- 33.Sultana S, Ahmed S, Jahangir T, Sharma S. Inhibitory effect of celery seeds extract on chemically inducedhepatocarcinogenesis: Modulation of cell proliferation, metabolismand altered hepatic foci development. Cancer Lett. 2005;221:11–20. doi: 10.1016/j.canlet.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 34.Momin RA, Nair MG. Antioxidant, cyclooxygenase and topoisomerase inhibitory compounds from Apium graveolens Linn. seeds. Phytomedicine. 2002;9:312–8. doi: 10.1078/0944-7113-00131. [DOI] [PubMed] [Google Scholar]
- 35.Moghadam MH, Imenshahidi M, Mohajeri SA. Antihypertensive effect of celery seed on rat blood pressure in chronic administration. J Med Food. 2013;16:558–63. doi: 10.1089/jmf.2012.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright CI, Van-Buren L, Kroner CI, Koning MM. Herbal medicines as diuretics: A review of the scientific evidence. J Ethnopharmacol. 2007;114:1–31. doi: 10.1016/j.jep.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Itoh T, Shigemoto T, Shimizu N, Tamura T, Matsumoto T. Triterpene alcobols in the seeds of two cucumis species of cucurbitaceae. Phytochemistry. 1982;21:2414–5. [Google Scholar]
- 38.Rizvi SH, Shoeb A, Kapil RS, Popli SP. Two new diuretic triterpenoids from Antidesma menasu. Phytochemistry. 1980;19:2409–10. [Google Scholar]
- 39.Afzal M, Khan NA, Ghufran A, Iqbal A, Inamuddin M. Diuretic and nephroprotective effect of Jawarish Zarooni Sada-a polyherbal unani formulation. J Ethnopharmacol. 2004;91:219–23. doi: 10.1016/j.jep.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 40.Kesarwani K, Gupta R, Mukerjee A. Bioavailability enhancers of herbal origin: An overview. Asian Pac J Trop Biomed. 2013;3:53–66. doi: 10.1016/S2221-1691(13)60060-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patil S, Dash RP, Anandjiwala S, Nivsarkar M. Simultaneous quantification of berberine and lysergol by HPLC-UV: Evidence that lysergol enhances the oral bioavailability of berberine in rats. Biomed Chromatogr. 2012;26:1170–5. doi: 10.1002/bmc.2674. [DOI] [PubMed] [Google Scholar]
- 42.Lai XJ, Zhang L, Li JS, Liu HQ, Liu XH, Di LQ, et al. Comparative pharmacokinetic and bioavailability studies of three salvianolic acids after the administration of Salvia miltiorrhizae alone or with synthetical borneol in rats. Fitoterapia. 2011;82:883–8. doi: 10.1016/j.fitote.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 43.Yang S, Zhang K, Lin X, Miao Y, Meng L, Chen W, et al. Pharmacokinetic comparisons of single herb extract of Fufang Danshen preparation with different combinations of its constituent herbs in rats. J Pharm Biomed Anal. 2012;67(68):77–85. doi: 10.1016/j.jpba.2012.03.058. [DOI] [PubMed] [Google Scholar]
- 44.Liu JY, Lee KF, Sze CW, Tong Y, Tang SC, Ng TB, et al. Intestinal absorption and bioavailability of traditional Chinese medicines: A review of recent experimental progress and implication for quality control. J Pharm Pharmacol. 2013;65:621–33. doi: 10.1111/j.2042-7158.2012.01608.x. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida N, Koizumi M, Adachi I, Kawakami J. Inhibition of P-glycoprotein-mediated transport by terpenoids contained in herbal medicines and natural products. Food Chem Toxicol. 2006;44:2033–9. doi: 10.1016/j.fct.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Lee CA, O’Connor MA, Ritchie TK, Galetin A, Cook JA, Ragueneau-Majlessi I, et al. Breast cancer resistance protein (ABCG2) in clinical pharmacokinetics and drug interactions: Practical recommendations for clinical victim and perpetrator drug-drug interaction study design. Drug Metab Dispos. 2015;43:490–509. doi: 10.1124/dmd.114.062174. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida N, Takada T, Yamamura Y, Adachi I, Suzuki H, Kawakami J. Inhibitory effects of terpenoids on multidrug resistance-associated protein 2- and breast cancer resistance protein-mediated transport. Drug Metab Dispos. 2008;36:1206–11. doi: 10.1124/dmd.107.019513. [DOI] [PubMed] [Google Scholar]
- 48.Jakovljevic V, Raskovic A, Popovic M, Sabo J. The effect of celery and parsley juices on pharmacodynamic activity of drugs involving cytochrome P450 in their metabolism. Eur J Drug Metab Pharmacokinet. 2002;27:153–6. doi: 10.1007/BF03190450. [DOI] [PubMed] [Google Scholar]
- 49.Ahluwalia VK, Boyd DR, Jain KA, Sharma ND. Furanocoumarin glucosides from the seeds of Apium graveolens. Phytochemistry. 1988;27:1181. [Google Scholar]
- 50.Dahan A, Altman H. Food-drug interaction: Grapefruit juice augments drug bioavailability-mechanism, extent and relevance. Eur J Clin Nutr. 2004;58:1–9. doi: 10.1038/sj.ejcn.1601736. [DOI] [PubMed] [Google Scholar]
- 51.Nowack R. Review Article: Cytochrome P450 enzyme, and transport protein mediated herb-drug interactions in renal transplant patients: Grapefruit juice, St John's Wort - and beyond! Nephrology (Carlton) 2008;13:337–47. doi: 10.1111/j.1440-1797.2008.00940.x. [DOI] [PubMed] [Google Scholar]
- 52.Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002;302:645–50. doi: 10.1124/jpet.102.034728. [DOI] [PubMed] [Google Scholar]
- 53.Farabegoli F, Papi A, Bartolini G, Ostan R, Orlandi M. (-)-Epigallocatechin-3-gallate downregulates Pg-P and BCRP in a tamoxifen resistant MCF-7 cell line. Phytomedicine. 2010;17:356–62. doi: 10.1016/j.phymed.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Mkaddem M, Bouajila J, Ennajar M, Lebrihi A, Mathieu F, Romdhane A. Chemical composition and antimicrobial and antioxidant activities of Mentha (longifolia L. and viridis) essential oils. J Food Sci. 2009;74:M358–63. doi: 10.1111/j.1750-3841.2009.01272.x. [DOI] [PubMed] [Google Scholar]
- 55.Kusuhara H, Sugiyama Y. Active efflux across the blood-brain barrier: Role of the solute carrier family. NeuroRX. 2005;2:73–85. doi: 10.1602/neurorx.2.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saxena A, Tripathi KP, Roy S, Khan F, Sharma A. Pharmacovigilance: Effects of herbal components on human drugs interactions involving cytochrome P450. Bioinformation. 2008;3:198–204. doi: 10.6026/97320630003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dreiseitel A, Schreier P, Oehme A, Locher S, Hajak G, Sand PG. Anthocyanins and their metabolites are weak inhibitors of cytochrome P450 3A4. Mol Nutr Food Res. 2008;52:1428–33. doi: 10.1002/mnfr.200800043. [DOI] [PubMed] [Google Scholar]
- 58.Manthey JA, Myung K, Merten-Talcott SU, Butterweck V, Derendorf H, Widmer W. The isolation of minor-occurring furanocoumarins in grapefruit and analysis of their inhibition of CYP3A4 and p-glycoprotein transport of talinolol from caco-2 cells. Proc Fla State Hort Soc. 2006;119:361–6. [Google Scholar]
- 59.Kimura Y, Ito H, Ohnishi R, Hatano T. Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity. Food Chem Toxicol. 2010;48:429–35. doi: 10.1016/j.fct.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 60.Lim ST, Dragull K, Tang CS, Bittenbender HC, Efird JT, Nerurkar PV. Effects of kava alkaloid, pipermethystine, and kavalactones on oxidative stress and cytochrome P450 in F-344 rats. Toxicol Sci. 2007;97:214–21. doi: 10.1093/toxsci/kfm035. [DOI] [PubMed] [Google Scholar]
- 61.Zheng J, Zhou HH. Effects of the flavonoids on cytochrome P-450 CYP1, 2E1, 3A4 and 19. Yao Xue Xue Bao. 2007;42:8–12. [PubMed] [Google Scholar]
- 62.Wang HJ, Pao LH, Hsiong CH, Shih TY, Lee MS, Hu OY. Dietary flavonoids modulate CYP2c to improve drug oral bioavailability and their qualitative/quantitative structure-activity relationship. AAPS J. 2014;16:258–68. doi: 10.1208/s12248-013-9549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsukamoto S, Cha BC, Ohta T. Dipiperamides A, B, and C: Bisalkaloids from the white pepper Piper nigrum inhibiting CYP3A4 activity. Tetrahedron. 2002;58:1667–71. [Google Scholar]
- 64.Hasegawa A, Yoshino M, Nakamura H, Ishii I, Watanabe T, Kiuchi M, et al. Identification of inhibitory component in--Cinnamon--o-methylcinnamaldehyde inhibits CYP1A2 and CYP2E1- Drug Metab Pharmacokinet. 2003;17:229–36. doi: 10.2133/dmpk.17.229. [DOI] [PubMed] [Google Scholar]
- 65.Orellana M, Varela N, Guajardo V, Araya J, Rodrigo R. Modulation of rat liver cytochrome P450 activity by prolonged red wine consumption. Comp Biochem Physiol C Toxicol Pharmacol. 2002;131:161–6. doi: 10.1016/s1532-0456(01)00292-7. [DOI] [PubMed] [Google Scholar]
- 66.Orellana M, Araya J, Guajardo V, Rodrigo R. Modulation of cytochrome P450 activity in the kidney of rats following long-term red wine exposure. Comp Biochem Physiol C Toxicol Pharmacol. 2002;132:399–405. doi: 10.1016/s1532-0456(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 67.Nishikawa M, Ariyoshi N, Kotani A, Ishii I, Nakamura H, Nakasa H, et al. Effects of continuous ingestion of green tea or grape seed extracts on the pharmacokinetics of midazolam. Drug Metab Pharmakokinet. 2004;19:280–9. doi: 10.2133/dmpk.19.280. [DOI] [PubMed] [Google Scholar]
- 68.Langhammer AJ, Nilsen OG. In vitro inhibition of human CYP1A2, CYP2D6, and CYP3A4 by six herbs commonly used in pregnancy. Phytother Res. 2014;28:603–10. doi: 10.1002/ptr.5037. [DOI] [PubMed] [Google Scholar]
- 69.Mimica-Dukic N, Popovic M, Jakovljevic V, Szabo A, Gašic O. Pharmacological studies of Mentha longifolia phenolic extracts. ii. hepatoprotective activity. Pharm Biol. 1999;37:221–4. [Google Scholar]
- 70.Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacology and Therapeutics. 2013;138(1):103–41. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 71.Cheng Y, Huang Y, Tian Y, Xu L, Liu GQ, Zahng ZJ. Assessment of the effects of Radix bupleuri and vinegar-baked Radix Bupleuri on cytochrome 450 activity by a six-drug cocktail approach. Chin J Nat Med. 2013;11:302–8. doi: 10.1016/S1875-5364(13)60033-3. [DOI] [PubMed] [Google Scholar]
- 72.Su T, Mao C, Yin F, Yu Z, Lin Y, Song Y, et al. Effects of unprocessed versus vinegar-processed Schisandra chinensis on the activity and mRNA expression of CYP1A2, CYP2E1 and CYP3A4 enzymes in rats. J Ethnopharmacol. 2013;146:734–43. doi: 10.1016/j.jep.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 73.De-Oliveira AC, Ribeiro-Pinto LF, Paumgartten JR. In vitro inhibition of CYP2B1 monooxygenase by beta-myrcene and other monoterpenoid compounds. Toxicol Lett. 1997;92:39–46. doi: 10.1016/s0378-4274(97)00034-9. [DOI] [PubMed] [Google Scholar]
- 74.Pandit S, Mukherjee PK, Ponnusankar S, Venkatesh M, Srikanth N. Metabolism mediated interaction of α-asarone and Acorus calamus with CYP3A4 and CYP2D6. Fitoterapia. 2011;82:369–74. doi: 10.1016/j.fitote.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 75.Subehan, Usia T, Kadota S, Tezuka Y. Mechanism-based inhibition of human liver microsomal cytochrome P450 2D6 (CYP2D6) by alkamides of Piper nigrum. Planta Med. 2006;72:527–32. doi: 10.1055/s-2006-931558. [DOI] [PubMed] [Google Scholar]
- 76.Gurley BJ, Fifer Ek, Gardner Z. Pharmacokinetic herb-drug interactions (part 2): Drug interactions involving popular botanical dietary supplements and their clinical relevance. Planta Med. 2012;78:1490–514. doi: 10.1055/s-0031-1298331. [DOI] [PubMed] [Google Scholar]
- 77.Ueng YF, Hsieh CH, Don MJ. Inhibition of human cytochrome P450 enzymes by the natural hepatotoxin safrole. Food Chem Toxicol. 2005;43:707–12. doi: 10.1016/j.fct.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 78.Randhawa GK, Kullar JS, Rajkumar Bioenhancers from mother nature and their applicability in modern medicine. Int J Appl Basic Med Res. 2011;1:5–10. doi: 10.4103/2229-516X.81972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chan WK, Delucchi AB. Resveratrol, a red wine constituent, is a mechanism-based inactivator of cytochrome P450 3A4. Life Sci. 2000;67:3103–12. doi: 10.1016/s0024-3205(00)00888-2. [DOI] [PubMed] [Google Scholar]
- 80.Donovan JL, Chavin KD, Devane CI, Taylor RM, Wang JS, Ruan Y, et al. Green tea (Camellia sinensis) extract does not alter cytochrome p450 3A4 or 2D6 activity in healthy volunteers. Drug Metab Dispos. 2004;32:906–8. doi: 10.1124/dmd.104.000083. [DOI] [PubMed] [Google Scholar]
- 81.Subehan, Zaidi SF, Kadota S, Tezuka Y. Inhibition on Human Liver Cytochrome P450 3A4 by Constituents of Fennel (Foeniculum vulgare): Identification and characterization of a mechanism-based inactivator. J Agric Food Chem. 2007;55:10162–7. doi: 10.1021/jf0713253. [DOI] [PubMed] [Google Scholar]
- 82.Nowack R, Andrassy J, Fischereder M, Unger M. Effects of dietary factors on drug transportand metabolism: The impact on dosage guidelines in transplant patients. Clin Pharmacol Ther. 2009;85:439–43. doi: 10.1038/clpt.2008.303. [DOI] [PubMed] [Google Scholar]
- 83.Budzinski JW, Foster BC, Vandenhoek S, Amason JT. An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine. 2000;7:273–82. doi: 10.1016/S0944-7113(00)80044-6. [DOI] [PubMed] [Google Scholar]
- 84.Jianu C, Misca C, Pop G, Rusu LC, Ardelean L, Teodora Gruia AT. Chemical composition and antimicrobial activity of essential oils obtained from dill (Anethum graveolens L.) grown in western Romania. Rev Chim. 2012;63:641–5. [Google Scholar]
- 85.Song M, Hwang JY, Lee MY, Jee JG, Lee YM, Bae JS, et al. In vitro inhibitory effect of piperlonguminine isolated from Piper longum on human cytochrome P450 1A2. Arch Pharm Res. 2014;37:1063–8. doi: 10.1007/s12272-013-0281-5. [DOI] [PubMed] [Google Scholar]
- 86.Tan MS, Ab Halim MR, Ismail S, Mustaffa F, Ali NI, Mahmud R. Inhibitory effect of selected Malaysian herbal plants on Glutathione S-transferase activity. Int J Pharm. 2011;7:349–55. [Google Scholar]
- 87.Banerjee S, Welsch CW, Rao AR. Modulatory influence of camphor on the activities of hepatic carcinogen metabolizing enzymes and the levels of hepatic and extrahepatic reduced glutathione in mice. Cancer Lett. 1995;88:163–9. doi: 10.1016/0304-3835(94)03633-t. [DOI] [PubMed] [Google Scholar]
- 88.Rompelberg CJ, Verhagen H, van Bladeren PJ. Effects of the naturally occurring alkenylbenzenes eugenol and trans-anetholeon drug-metabolizing enzymes in the rat liver. Food Chem Toxicol. 1993;31:637–45. doi: 10.1016/0278-6915(93)90046-2. [DOI] [PubMed] [Google Scholar]
- 89.Zheng G, Kenney P, Zhang J, Lam LK. Chemoprevention of benzo[a]pyrene-induced forestomach cancer in mice by natural phthalides from celery seed oil. Nutr Cancer. 1993;19:77–86. doi: 10.1080/01635589309514238. [DOI] [PubMed] [Google Scholar]


