Abstract
Recently there has been increasing interest in plants and plant-derived compounds as raw food and medicinal agents. In Ayurveda, an Indian system of traditional medicine, a wide spectrum of medicinal properties of Pterocarpus santalinus is described. Many important bioactive phytocompounds have been extracted and identified from the heartwood of P. santalinus. Bioactive compounds typically occur in small amounts and have more subtle effects than nutrients. These bioactive compounds influence cellular activities that modify the risk of disease rather than prevent deficiency diseases. A wide array of biological activities and potential health benefits of P. santalinus have been reported, including antioxidative, antidiabetic, antimicrobial, anticancer, and anti-inflammatory properties, and protective effects on the liver, gastric mucosa, and nervous system. All these protective effects were attributed to bioactive compounds present in P. santalinus. The major bioactive compounds present in the heartwood of P. santalinus are santalin A and B, savinin, calocedrin, pterolinus K and L, and pterostilbenes. The bioactive compounds have potentially important health benefits: These compounds can act as antioxidants, enzyme inhibitors and inducers, inhibitors of receptor activities, and inducers and inhibitors of gene expression, among other actions. The present review aims to understand the pharmacological effects of P. santalinus on health and disease with “up-to-date” discussion.
Keywords: Antioxidant activity, oxidative stress, phytocompounds, Pterocarpus santalinus
INTRODUCTION
Plants as natural products are a valuable source of bioactive compounds and have been used for medicinal purposes in all over the world. Recently, scientific attention to oriental medicine has increased in context of the discovery of novel drugs for treating various diseases including cancer and diabetes.[1,2] The World Health Organization (WHO) endorses the evaluation of the potential benefits of plants as effective therapeutic agents, especially in areas where there is a lack of safe modern drugs.[3] One-third of total drugs (35%) in the USA and 80% of drugs used in fast-developing countries such as China and India are derivatives of phytoextracts.[4,5] India has a rich heritage of medicinal plants of wide diversity, which are used by the local population and traditional healers for the treatment of several diseases. One such plant is Pterocarpus santalinus, which is widely used for the treatment of various ailments due to its extensive medicinal properties.
P. santalinus is a small-to-medium–sized deciduous tree belonging to the Fabaceae family. It is widely distributed in the tropical regions of the world, especially in India, Sri Lanka, Taiwan, and China.[6] Earlier reviews explored the phytochemistry, pharmacology, and ethnomedicinal values of P. santalinus.[7,8] Bioactive compounds present in the plant's heartwood have been shown to have a wide range of biological activities, suggesting the potential of P. santalinus for the treatment of various diseases.[7,9] In vitro and in vivo studies showed that the heartwood and bark have exhibited antidiabetic, antimicrobial, anti-inflammatory, and hepatoprotective activities [Figure 1].[9,10,11] In Ayurveda, an Indian system of traditional medicine, it is mentioned that the heartwood of the plant is used as external application for treating inflammation, diabetes, headache, skin diseases, and jaundice, and in wound-healing.[12,13] This review summarizes the current literature on the compositional profile and pharmacological activities of important phytocompounds present in the heartwood and various extracts of the plant.
Figure 1.
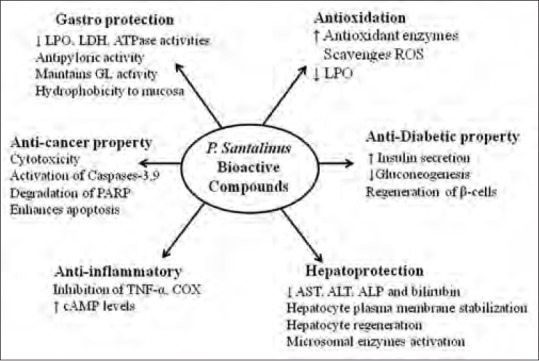
Schematic representation of potential benefits of P. santalinus
Bioactive compounds present in heartwood of P. santalinus
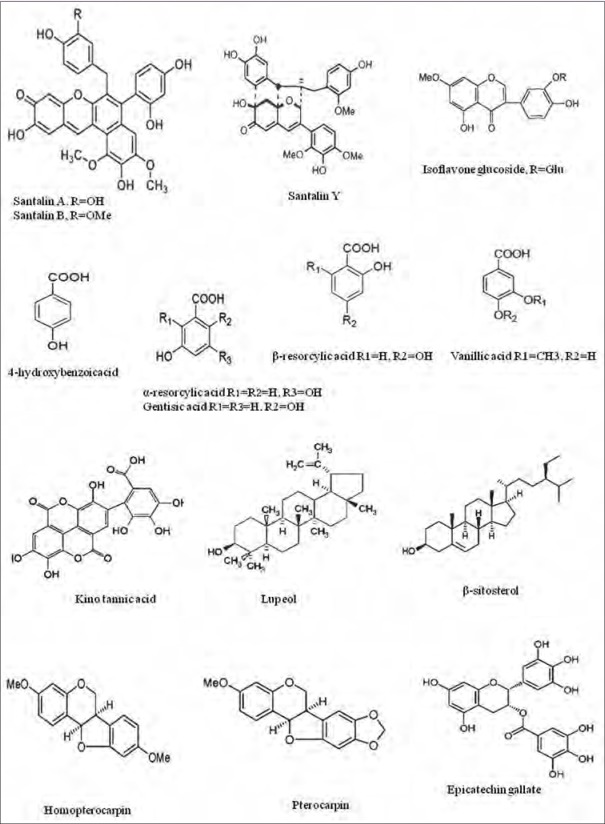
Phytochemical analysis of the plant showed the presence of carbohydrates, flavonoids, terpenoids, phenolic compounds, alkaloids, saponins, tannins, and glycosides.[11,14] In addition, the available literature demonstrated the presence of several specific components in heartwood powder—in particular, pterocarpol; santalin A, B, and Y; pterocarptriol; isopterocarpalone; pterocarpodiolones with β-eudesmol; cryptomeridiol,[15] several nonspecific compounds such as isoflavones; isoflavonoid glucosides; triterpenes; sesquiterpenes, and related phenolic compounds such as β-sitosterol; lupeol; epicatechin; lignans; and pterostilbenes [Figure 2 and Table 1].[16,17,18,19,20,21] Pterostilbene, a structural analog of resveratrol, is more stable than resveratrol. Pterostilbene (3,5-dimethoxy-4′-hydroxy-trans-stilbene) isolated from the heartwood showed anti-inflammatory, antioxidant, and anticancer properties.[22,23] Nonflavonoid polyphenols contain different subgroup compounds such as simple phenols, benzoic acids, hydrolysable tannins, cinnamic acid, acetophenones, phenylacetic acid, lignans, coumarins, benzophenones, xanthones, and stilbenes.[24] Thirty naturally occurring phenolic acids, in particular hydroxyl and polyhydroxybenzoic acids, have been reported to have biological activities, among them 3-hydroxybenzoic acid, gentisic acid, α and β resorcylic acid, and vanillic acid.[25] In addition, aurone glycosides OH-1 methyl-3′,4′,5′-trimethoxy aurone, 4′-O-rhamnoside, 6,4′-dihydroxy aurone, 4′-O neohesperidoside and isoflavone glycoside, 4′,5-dihydroxy-7-methylisoflavone, and 3-O-beta-D-glucoside are also present in P. santalinus.[16,17] Six closely related sesquiterpenes isolated from the heartwood of P. santalinus using different solvents such as petroleum, benzene, and chloroform are isopterocarpalone, pterocarptriol, pterocarpdiolone, and the known β-eudesmol, pterocarpol, and cryptomeridiol.[15,16] Dehydromelanoxin-6, melanoxin-7, melanoxoin-14, S-30-hydroxy-4, 40-dimethoxydalbergione-15, and melannein-16 were the known compounds present in heartwood of P. santalinus and identified with the comparison of nuclear magnetic resonance and mass spectrometry data of the established literature.[26]
Figure 2.
Phytocompounds present in P. santalinus heartwood
Table 1.
Class of phytocompounds and related compounds present in heartwood of P. santalinus

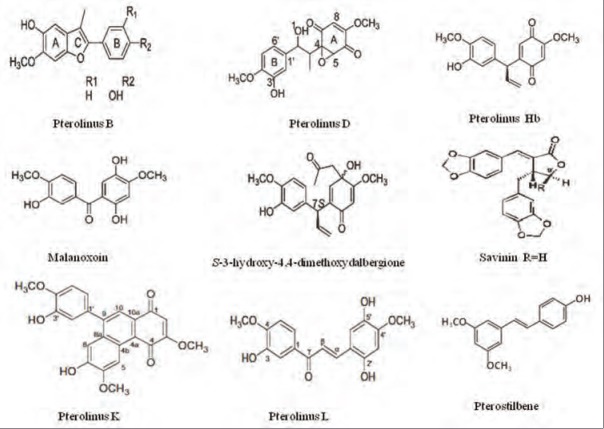
Stilbenes represent a small class of secondary plant metabolites derived from the phenylpropanoid pathway. Stilbenes are phenolic compounds displaying two aromatic rings linked by an ethane bridge, structurally characterized by the presence of a 1,2-diarylethene nucleus with hydroxyls substituted on the aromatic rings.[27] One specific stilbene, namely, pterostilbene was reported in P. santalinus heartwood.[23,28] Pterostilbene, a methyl ether of resveratrol, has recently attracted much attention, as a growing number of reports describe promising pharmacological properties such as anti-inflammatory, antioxidant, and anticancer properties.[29] Lignan compounds can be divided into several different types, such as the dibenzyl butyrolactones (arctigenin and savinin), furofurans (pinoresinol and eudesmin), and dibenzylbutanes (secoisolariciresinol).[30] Lignans are cis- o-hydroxycinnamic acid and are dimers (2 C6-C3 units) resulting from tail–tail linkage of 2 coniferyl or sinapyl alcohol units.[31] Specific lignans, namely, savinin, calocedrin, and eudesmin were reported in the heartwood extract of P. santalinus.[20] Wu et al., isolated two bioactive components from heartwood of P. santalinus, namely one new phenanthrenedione compound pterolinus K and another new chalcone compound pterolinus L [Figure 3], which acted as lead compounds for the development of anti-inflammatory agents.[32] Studies also showed that P. santalinus heartwood contains ferric ion (Fe+3), Ni+2, and Ba+2 elements.[33] The bark ethanolic extract of P. santalinus also showed the presence of aspartic acid, glutamic acid, alanine, serine, glycine, threonine, lysine, asparagine, histidine, arginine, cystine, glutamine, proline, phenylalanine, and leucine amino acids.[34]
Figure 3.
Phytocompounds present in P. santalinus heartwood showing cytotoxic activity against cancer cells
In vitro studies
Radical-scavenging and antioxidant activity
Free radicals are electrically charged molecules, and an excessive generation of free radicals is linked to many human diseases. Reactive oxygen species (ROS) including hydroxyl radicals cause oxidation of lipids, proteins, and DNA; damage the structure and function of cells; and lead to the development of diseases. Animal studies have shown that dietary phytochemical antioxidants are capable of removing free radicals. Among them, phenolic and polyphenolic compounds, such as flavonoids in edible plants, exhibit potent antioxidant activities.[29] The free radical-scavenging activity of extracts of the leaves of P. santalinus has been evaluated using in vitro studies. The methanolic extract of the leaves exhibited radical-scavenging activity for 1,1-diphenyl-2-picrylhydrazyl (DPPH), nitric oxide, and hydrogen peroxide.[13,35] Studies by Kumar demonstrated Fe3+-reducing capacity and DPPH radical-scavenging activity in the methanolic extract of heartwood. The reductive capabilities and scavenging activity were found to increase with increasing concentrations and were compared with butylated hydroxyanisole (BHA).[36] Reports also revealed that pterostilbene exhibited strong in vitro antioxidant activity against free radicals such as DPPH, 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonate) (ABTS), hydroxyl, superoxide, and hydrogen peroxide.[37] Very limited reports are available related to the scavenging activities of extracts isolated by using different solvents and isolated active compounds. Further elaborate and confirmative studies using the heartwood of P. santalinus are needed.
Antibacterial activity
Infectious diseases are a major cause of global morbidity and mortality. They account for roughly 50% of all deaths in tropical countries.[38] Chemical and synthetic drugs that are used to treat microbial infections are associated with multiple side effects. Human pathogens have developed drug resistance after the routine human consumption of these drugs, and it is a big challenge for researchers to develop safe and effective medication for the treatment of infectious diseases. Several research groups have studied the antibacterial and radical-scavenging activities of plant extracts, and they proved that the extracts showed effective antibacterial and radical-scavenging activities with no side effects.[39,40] The antibacterial activity of leaf and bark extract of P. santalinus was tested against Gram-positive bacteria such as Staphylococcus aureus ATCC 25933, Staphylococcus epidermidis MTCC 3615, Bacillus subtilis MTCC4 41, Enterococcus faecalis ATCC2912, Escherichia coli ATCC 25922, and Pseudomonas aeruginosa ATCC 27853. The bark extract has shown greater inhibition activity than the leaf extract.[35,41] The antibacterial activities of 21 timber-yielding plants were tested against uropathogenic bacteria, and P. santalinus was among the most inhibitory-activity–exhibiting plants.[42] A comparative study was done to find out the antibacterial activities of the methanol and aqueous leaf extracts of P. santalinus. The methanolic extract has shown greater inhibitory activity than the aqueous extract.[43] Silver nanoparticles prepared from the leaf extract of P. santalinus exhibited antibacterial potential against Gram-positive and Gram-negative strains.[44] Other reports also have shown that lignans present in P. santalinus could have antibacterial properties.[45] Its anti-Helicobacter pylori effect was tested by culturing H. pylori on gastric epithelial cells in the presence/absence of P. santalinus extract. A reduction in the activity of urease, normal appearance of gastric epithelial cells under electron microscopic examination, and decrease in lipid peroxidation (LPO) and lactate dehydrogenase (LDH) activities suggested an antipyloric effect.[46] In vitro antimicrobial activities of heartwood extracts need confirmation against various pathological microbes and standardization in that context.
In vivo studies
Anti-inflammatory effect
Inflammation plays a crucial role in the initiation and progression of pathological conditions. The majority of phytomedicines exhibit therapeutic efficacy by exerting anti-inflammation and cytotoxic activities. It is well known that T lymphocytes enhance chronic inflammatory conditions by activating the inflammatory cells, such as mast cells, eosinophils, neutrophils, and macrophages, resulting in massive production of chemical mediators and pro-inflammatory cytokines.[20,47] The anti-inflammatory activities of lignan compounds isolated from the heartwood of P. santalinus have been studied in RAW264.7 cells and splenocytes. Tumor necrosis factor (TNF)-α is one of the pro-inflammatory cytokines secreted in chronic inflammatory and allergic diseases. TNF-α production in lipopolysacharide-stimulated RAW264.7 cells was inhibited at a dose of 25 µg/mL by savinin, a lignan compound. The molecular mechanism of inhibitory action of the compound is due to its structural similarity with that of a butyrolactone ring and its polarity on the C-9 position.[20] Specific lignans, namely, savinin, calocedrin, and eudesmin were reported in the heartwood extract of P. santalinus. These compounds were found to inhibit TNF-α and also showed antiproliferative effect with an IC50 value at 40 µg/mL.[10] Studies by Kumar have shown significant inhibitory activity of the heartwood extract (500 µg/mL) against carrageenan-induced inflammation in paw edema.[37] Five new benzofurans, six neoflavonoids, and pterolinus isolated from heartwood, including pterolinus B, showed potent anti-inflammatory activity with an IC50 value at 0.19 µg/mL.[26] Phenanthrenedione (pterolinus K) and chalcone (pterolinus L) compounds isolated from the heartwood of P. santalinus showed notable inhibition of superoxide anion generation and elastase release by human neutrophils in response to formyl-L-methionyl-L-leucyl-L-phenylalanine (FMLP)/cytochalasin B (CB) with IC50 values at 0.99 µM and 0.94 µM, respectively.[32] However, other biological activities and anti-inflammatory mechanism(s) exhibited by lignans and savinins require further studies.
Effect on diabetes
Traditionally made heartwood cups for drinking water have been used as a treatment for diabetes.[48,49] Diabetes mellitus (DM) is a metabolic syndrome that consists of ineffective insulin regulation leading to derangements in carbohydrate metabolism.[50] Rao et al. found that the ethanolic fraction of P. santalinus showed hypoglycemic activity at a dose of 0.25 g/kg body weight/day.[51] The active fraction of ethylacetate: methanol (9:1) of bark ethanolic extract has shown antihyperglycemic activity at a dose of 150 mg/kg body weight by improving insulin secretion and alterations in the carbohydrate metabolism.[11,52] Coadministration (250 mg/kg body weight) of heartwood aqueous extract along with vitamin E to streptozotocin-induced diabetic rats caused significant lowering of blood sugar at a dose of 250 mg/kg body weight/day.[3] The molecular mechanisms involved in the treatment of diabetes by individual as well as whole extracts of either the bark or heartwood of P. santalinus need to be thoroughly investigated. Several researchers paid much attention in studying the effects of P. santalinus against diabetes using classical methods. However, future studies are required to explore the antidiabetic activity using isolated bioactive compounds at the molecular level.
Effect on cancer
Natural phenolic compounds act on multiple targets in pathways and mechanisms related to carcinogenesis, tumor cell proliferation and death, inflammation, metastatic spread, angiogenesis, and radiation resistance. Chemoprevention with food phytochemicals is meanwhile regarded as one of the most important strategies for cancer control. Phenolic compounds from medicinal herbs include phenolic acids, flavonoids, stilbenes, lignans, and others.[53] Bioactive compounds isolated from P. santalinus exhibit cytotoxic and anticancer properties. Treatment of cervical adenocarcinoma HeLa cells with a methanolic extract of P. santalinus resulted in growth inhibition and induction of apoptosis with the IC50 value 40 µg/mL. The methanolic extract of P. santalinus induced apoptosis through the mitochondrial pathway involving cytochrome-c release from mitochondria, the activation of caspase-9 and caspase-3, and degradation of peroxisome-activated receptor protein (PARP).[10] Benzofuran compounds isolated from heartwood showed cytotoxicity against Ca9-22 cancer cells with an IC50 value 0.46 µg/mL.[26] The inhibitory activity of savinin on T cell proliferation was the highest from the methylene chloride extract (25 µg/mL) in comparison with the other solvents ethyl acetate, n-butanol, and water.[10] The therapeutic efficacy of pterostilbene was tested against breast cancer, lung cancer, colon cancer, prostate cancer, and pancreatic cancer.[54] Pterolinus K and pterolinus L isolated from heartwood have shown significant anticancer property with IC50 values at 10.86 µM, 9.81 µM, and 17–8.2 µM, against cancer cell lines HepG2, Hep3B, and A549, respectively.[32] Future studies are required that apply isolated compounds against various cancers and essential signalling pathways involved in the pathogenesis.
Hepatoprotective mechanisms
The use of natural remedies for the treatment of liver diseases has a long history, beginning with Ayurvedic treatment and spreading to the Chinese, European, and other systems of traditional medicine. The pharmacological validation of each hepatoprotective plant should include evaluation of its efficacy against liver diseases induced by various agents.[55] P. santalinus bark and heartwood are rich in flavonoids and protect the liver against chemical-induced toxicity. Studies showed that aqueous (45 mg/mL) and ethanol (30 mg/mL) bark extracts of P. santalinus restored CCl4-induced liver damage in rats.[9] The therapeutic efficacy of heartwood extract was tested against D-galactosamine–induced liver damage in albino rats by administering at a dose of 400 mg/kg body weight/day.[6] In silico docking studies revealed that potent compounds pterocarpol and cryptomeridiol, present in P. santalinus heartwood, targeted the HBx proteins of hepatitis B virus, and were thus reported as strong drug candidates.[56] Itoh et al. reported that P. santalinus suppresses hepatic fibrosis in chronic liver injury.[57,58] Though it has many medicinal properties against various ailments, it has never been tested against alcohol-induced hepatotoxicity. In addition, researchers must elucidate the underlying protective molecular mechanisms of isolated compounds against liver-related diseases.
Gastroprotective mechanisms
Gastric mucosal injury results as a consequence of various conditions and activities, including alcohol intake, refluxed bile salts, stress, aging, H. pylori infection, and most of the nonsteroidal anti-inflammatory drugs. The ethanol extract of P. santalinus was evaluated for gastroprotection against ibuprofen-induced ulcers in albino rats. The extract (150 mg/kg body weight/day) showed a significant reduction in gastric lesions, increase in activities of antioxidant enzymes, and decrease in membrane-bound adenylpyrophosphatase (ATPase) activities, and the ability to maintain the functional integrity of cell membranes.[14] The ethanolic extract of heartwood restored the activities of tricarboxylic acid cycle (TCA) enzymes, prevented mitochondrial dysfunction by providing mitochondrial membrane integrity including a hydrophobic nature to the gastric mucosa, and reversed the damage caused by ulcerogens at a dose of 200 mg/kg body weight/day.[59] The plant extract's ability against various chemical agents that disturb the gut microbial flora, which would be useful in physiology and also protect the intestinal mucosa, has not been studied.
Hypolipidemic activity
Generally, phenolic phytocompounds exhibit antioxidative and hypolipidemic activities and thereby protect the liver and heart against a wide variety of pathological conditions, including hyperlipidemia, by inhibiting lipid synthesis.[60] Modification of lipids is an integral part of the initiation and progression of atherosclerosis, in part by its effects on lipid profile. Circulatory very-low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) are considered to be powerful markers and risk factors for cardiovascular disease (CVD). The lipid-lowering activity of the bark extract (150 mg/kg body weight/day) was studied in diabetic rats.[11] Reduction in total cholesterol and lipoproteins and increase in high-density lipoprotein-cholesterol (HDL-C) was observed in diabetic rats treated with an aqueous extract of heartwood (250 mg/kg body weight/day) in combination with vitamin E.[3] A chloroform extract of heartwood (400 mg/kg body weight/day) significantly lowered the serum total cholesterol and triglycerides in D-galactosamine–intoxicated rats.[6] Pterostilbene has also been reported to have hypolipidemic properties comparable to clinically used fibrate lipid-lowering drugs.[61] Daniel et al. reported that pterostilbene increased LDL and reduced blood pressure in hypercholesterolemic human volunteers.[62] The main etiologic factor in atherogenesis is oxidation of LDL. Reports showed that the 3-hydroxybenzoic acid, gentisic acid, α- and β-resorcylic acid, and vanillic acid present in heartwood of P. santalinus have potential inhibitory properties against LDL oxidation.[24] A plant extract is a source of numerous complex compounds not tested against protein factors, which can regulate the expression of various transcription factors involved in enhanced lipid synthesis.[63]
Angiogenesis and wound-healing activity
The process of generating new blood vessels from preexisting ones is called angiogenesis. The process of angiogenesis is controlled by positive and negative regulators. Chorioallantoic membrane (CAM) assay is one of the most well-established and commonly used in vitro models for evaluating angiogenesis and vasculogenesis. Jadhav et al. demonstrated that an acetone extract of P. santalinus (1 mg/mL)stimulated angiogenesis more significantly than alcohol and benzene extracts. The CAM angiogenic effect was mediated by the proliferation of endothelial cells without any toxicity.[63] Oral administration (2 g/kg or 1 g/kg body weight) and intraperitoneal injection (500 mg/kg body weight or 1 g/kg body weight) was made to study the toxicity of the heartwood extract in rat models.[64] The plant also has a wound-healing property, and this was demonstrated by Biswas et al. in normal and diabetic wound rat models, and concluded that the ointment made from the plant is effective in treating acute wounds.[64] The angiogenic activity of the plant may be attributed to the phytoconstituents present in the extract. The wound-healing property represents the biochemical and biomechanical properties of the plant. Although the biological activities of specific compounds were not established and it is suggested that bioactive compounds present in the extract might act by stimulating growth factors or signal cascade systems, further confirmative studies are needed.
Other pharmacological uses
The heartwood extract showed strong analgesic action in mice by inhibiting acetic acid-induced writhing by increasing the latency period.[37] Phenolic acids, in particular hydroxyl and polyhydroxybenzoic acids, 3-hydroxybenzoic acid, gentisic acid, α- and β-resorcylic acid, and vanillic acid present in the heartwood of P. santalinus have been reported to have antifungal, antimutagenic, anti-inflammatory,[24] and nematicidal activity,[65] and a thyroid peroxidise inhibitor effect.[66] Many other reports also have shown that lignans possess antiviral, antiallodynic, and antimutagenic properties.[33,45,67] Aurone glycosides isolated from heartwood have been reported to exhibit leishmanicidal activity against Leishmania donovani, Leishmania enrietti, and Leishmania major.[68] Additionally, it has been reported to inhibit in vitro erythrocytic stages of Plasmodium falciparum strains.[69] The Indian traditional medicinal system, Ayurveda, suggested that P. santalinus could be used to treat various medical complications such as hemorrhage, dysentery, eye diseases, and mental aberrations, and to act as an aphrodisiac and diaphoretic.[70] It is used in treating leprosy, bone fracture, scorpion sting, hiccough, and snakebite.[13] P. santalinus is highly valued for its heavy, dark claret-red sanders, which yields 16% of the red coloring matter santalin, used as a coloring agent in pharmaceutical preparations and foodstuff.[7]
Perspectives on future research
From the compositional point of view, the phytocompounds present in the heartwood of P. santalinus are very complex compounds. Many compounds that were identified are general preliminary compounds, but each preliminary compound is a mixture of many more compounds, which are not as yet identified as therapeutic agents. Further research is necessary to provide a detailed characterization of the compounds present in the preliminary compounds. Limited studies have suggested the radical-scavenging and beneficial effects of the heartwood of P. santalinus, and the molecular biological mechanisms of the biological activities and physiological effects need further study; the bioavailability and metabolism of the compounds have not been reported. Further studies on these aspects would provide useful data for substantiating the physiological effects of these compounds. A limited area of research has explored this plant as a therapeutic agent, and there is a need to extend studies against a wide variety of pathological conditions. Most of the biological activities and physiological effects of the plant extracts have been studied in vitro or in animal models. Clinical and human intervention studies are very limited; therefore the biological and physiological effects of the isolated compounds of the heartwood are also worth investigation.
CONCLUSIONS
P. santalinus has significant hepatoprotective, antioxidant, antimicrobial, and anti-inflammatory activities. The phytocompounds present in P. santalinus alsoshowedantidiabetic, hypolipidemic, anticancer, gastroprotective, and wound-healing properties. The therapeutic properties of phytocompounds present in P. santalinus extract appear to have a concerted mode of action and conferred protection against various disease complications. Finally, the current review provides the evidence to enable other researchers to use P. santalinus as an efficacious natural drug. Further preclinical and clinical studies for adequate evaluation of the safety and therapeutic efficacy of P. santalinus are recommended.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Damodara Reddy Vaddi
Damodara Reddy Vaddi, DST-Young Scientist, Oil Technological Research Institute.

Saradamma Bulle
Saradamma Bulle, Research scholar, Sri Krishnadevaraya University.

Hymavathi Reddyvari
Hymavathi Reddyvari, Research scholar, Sri Krishnadevaraya University.

Varadacharyulu Nallanchakravarthula
Varadacharyulu Nallanchakravarthula, Professor in Biochemistry, Sri Krishnadevaraya University.
REFERENCES
- 1.Cheng YL, Lee SC, Lin SZ, Chang WL, Chen YL, Tsai NM, et al. Anti-proliferative activity of Bupleurum scrozonerifolium in A549 human lung cancer cells in-vitro and in-vivo. Cancer Lett. 2005;222:183–93. doi: 10.1016/j.canlet.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Tan ML, Suaiman SF, Najimuddin N, Smian MR, Muhammad TS. Methanolic extract of Pereskia bleo (Kunth) DC. (Cactaceae) induces apoptosis in breast carcinoma, T47-D cell line. J Ethnopharmacol. 2005;96:287–94. doi: 10.1016/j.jep.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Halim ME, Misra A. The effect of aqueous extract of Pterocarus santalinus heartwood and vitamin E supplementation in streptozotocin-induced diabetic rats. J Med Plants Res. 2011;5:398–409. [Google Scholar]
- 4.Ramawat KG, Goyal S. The Indian herbal drugs scenario in global perspectives. Bioact Molec Med Plant. 2008;18:325–47. [Google Scholar]
- 5.Ajay Kumar M, Bansal P, Kumar S. Plants- herbal wealth as a potential source of ayurvedic drugs. Asian J Trad Med. 2009;4:152–70. [Google Scholar]
- 6.Dhanabal P, Kannan SE, Bhojraj S. Protective and therapeutic effects of the Indian medicinal plant Pterocarpus santalinus on D-galactosamine-induced liver damage. Asian J Trad Med. 2007;2:51–7. [Google Scholar]
- 7.Arunakumara KK, Walpola BC, Subasinghe S, Yoon M. Pterocarpus santalinus Linn. f. (Roth handgun): A Review of Its Botany, uses, Phytochemistry and Pharmacology. J Korean Soc Appl Biol Chem. 2011;54:495–500. [Google Scholar]
- 8.Navada KK, Vittal RR. Ethnomedicinal value of Pterocarpus santalinus (Linn. f.), a Fabaceae member. Orient Pharm Exp Med. 2014;14:313–7. [Google Scholar]
- 9.Manjunatha BK. Hepatoprotective activity of Pterocarpus santalinus L. f., an endangered plant. Indian J Pharmacol. 2006;38:25–8. [Google Scholar]
- 10.Kwon HJ, Hong YK, Kim KH, Han CH, Cho SH, Choi JS, et al. Methanolic extract of Pterocarpus santalinus induces apoptosis in Hela cells. J Ethnopharmacol. 2006;105:229–34. doi: 10.1016/j.jep.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Kondeti VK, Rao BK, Maddirala DR, Thur SK, Fatima SS, Kasetti RB, et al. Effect of Pterocarpus santalinus bark, on blood glucose, serum lipids, plasma insulin and hepatic carbohydrate metabolic enzymes in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2010;48:1281–7. doi: 10.1016/j.fct.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Rastogi RP, Mehrotra BN. I. New Delhi: Central Drug Institute Lucknow and Publication and Information Directorate; 1991. Compendium Indian Medicinal Plants. 1960-1969; p. 437. [Google Scholar]
- 13.Arokiyaraj S, Martin S, Perinbam K, Marie AP, Beatrice V. Free radical scavenging activity and HPTLC finger print Pterocarpus santalinus L.-an in vitro study. Indian J Sci Technol. 2008;1:1–3. [Google Scholar]
- 14.Narayan S, Devi RS, Srinivasan P, Shyamala Devi CS. Pterocarpus santalinus: A traditional herbal drug as a protectant against ibuprofen induced gastric ulcers. Phytother Res. 2005;19:958–62. doi: 10.1002/ptr.1764. [DOI] [PubMed] [Google Scholar]
- 15.Yoganarasimhan SN. Bangalore: Cyber Media; 2000. Medicinal Plants of India, Tamil Nadu; p. 63. [Google Scholar]
- 16.Kumar N, Seshadri TR. Triterpenoids of Pterocarpus santalinus constitution of a new leupenediol. Phytochem. 1975;14:521–3. [Google Scholar]
- 17.Krishnaveni KS, Srinivasa Rao JV. A new isoflavone glucoside from Pterocarpus santalinus. Asian Nat Prod Res. 2000;2:219–23. doi: 10.1080/10286020008039914. [DOI] [PubMed] [Google Scholar]
- 18.Krishnaveni KS, Srinivasa Rao JV. A new triterpene from callus of Pterocarpus santalinus. Fitoter. 2000;24:167–71. doi: 10.1016/s0367-326x(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 19.Krishnaveni KS, Srinivasa Rao JV. An isoflavone from Pterocarpus santalinus. Phytochem. 2000;53:605–6. doi: 10.1016/s0031-9422(99)00526-9. [DOI] [PubMed] [Google Scholar]
- 20.Cho JY, Park J, Kim PS, Yoo ES, Baik KU, Park MH. Savinin, a lignin from Pterocarpus santalinus inhibits tumor necrosis factor-alpha production and T-cell proliferation. Biol Pharm Bull. 2001;24:167–74. doi: 10.1248/bpb.24.167. [DOI] [PubMed] [Google Scholar]
- 21.Kesari AN, Gupta RK, Watal G. Two aurone glycosides from heartwood of Pterocarpus santalinus. Phytochemistry. 2004;65:3125–9. doi: 10.1016/j.phytochem.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Satheesh MA, Pari L. Effect of pterostilbene on lipids and lipid profiles in streptozotocin-nicotinamide induced type 2 diabetes mellitus. J Appl Biomed. 2008;6:31–7. [Google Scholar]
- 23.Remsberg CM, Yanez JA, Ohgami Y, Vega-Villa KR, Rimando AM, Davies NM. Pharmacometrics of pterostilbene: Preclinical pharmacokinetics and metabolism, anticancer, anti-inflammatory, antioxidant and analgesic activity. Phytother Res. 2008;22:169–79. doi: 10.1002/ptr.2277. [DOI] [PubMed] [Google Scholar]
- 24.Ashidate K, Kawamura M, Mimura D, Tohda H, Miyazaki S, Teramoto T, et al. Gentisic acid, an aspirin metabolite, inhibits oxidation of low-density lipoprotein and the formation of cholesterol ester hydroperoxides in human plasma. Eur J Pharmacol. 2005;513:173–9. doi: 10.1016/j.ejphar.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Khadem S, Marles RJ. Monocyclic phenolic acids; hydroxy- and polyhydroxybenzoic acids: Occurrence and recent bioactivity studies. Molecules. 2010;15:7985–8005. doi: 10.3390/molecules15117985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu SF, Chang FR, Wang SY, Hwang TL, Lee CL, Chen SL, et al. Anti-inflammatory and cytotoxic neoflavonoids and benzofurans from Pterocarpus santalinus. J Nat Prod. 2011;74:989–96. doi: 10.1021/np100871g. [DOI] [PubMed] [Google Scholar]
- 27.Fressco P, Borges F, Diniz C, Marques MP. New insights on the anticancer properties of dietary polyphenols. Med Res Rev. 2006;26:747–66. doi: 10.1002/med.20060. [DOI] [PubMed] [Google Scholar]
- 28.Spath E, Schlager J. Constituents of red sandalwood. II. Constitution of pterostilbene. Ber Dtsch Chem Ges. 1940;73:881. [Google Scholar]
- 29.McCormack D, McFadden D. Pterostilbene and cancer: Current review. J Surg Res. 2012;173:e53–61. doi: 10.1016/j.jss.2011.09.054. [DOI] [PubMed] [Google Scholar]
- 30.Ayres DC, Loike JD. Cambridge, United Kingdom: Cambridge University Press; 1990. Lignans: Chemical, Biological and Clinical Properties. [Google Scholar]
- 31.Cai YZ, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–84. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu SF, Hwang TL, Chen SL, Wu CC, Ohkoshi E, Lee KH, et al. Bioactive components from the heartwood of Pterocarpus santalinus. Bioorg Med Chem Lett. 2011;21:5630–2. doi: 10.1016/j.bmcl.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 33.Reddy L, Reddy G, Reddy RS, Reddy S, Reddy J. Characterization of Red sandalwood by ICP-MS Analysis, Optical absorption and EPR spectroscopic methods. Radiat Eff Defect Solids. 2006;161:537–47. [Google Scholar]
- 34.Devi RK, Basha SK. Comparative amino acid profiling of pterocarpus santalinus and boswelia ovalifoliolata barks of Tirumala Hills, Eastern Ghats, Andhra Pradesh. Int J Basic App Chem Sci. 2012;2:102–6. [Google Scholar]
- 35.Krishnamoorthy P, Stella J, Mohamed AJ, Anand M. Radical scavenging and antibacterial evaluation of Pterocarpus santalinus leaf in-vitro study. Int J Pharm Sci Res. 2011;2:1204–8. [Google Scholar]
- 36.Kumar D. Anti-inflammatory, analgesic, and antioxidant activities of methanolic wood extract of Pterocarpus santalinus L. J Pharmacol Pharmacother. 2011;2:200–2. doi: 10.4103/0976-500X.83293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Achary JD, Ghaskadbi SS. Protective effect of pterostilbene against free radical mediated oxidative damage. BMC Complement Altern Med. 2013;13:238. doi: 10.1186/1472-6882-13-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. New York: Oxford University Press; 2006. Global Burden of Disease and Risk Factors. [PubMed] [Google Scholar]
- 39.Faremi TY, Suru SM, Fafunso MA, Obioha UE. Hepato-protective potentials of Phyllanthus amarus against ethanol-induced oxidative stress in rats. Food Cheml Toxicol. 2008;46:2658–64. doi: 10.1016/j.fct.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 40.Briskin DP. Medicinal plants and phytomedicines. Linking plant Biochemistry and Physiology to Human Health plant. Plant Physiol. 2000;124:507–14. doi: 10.1104/pp.124.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manjunatha BK. Antibacterial activity of Pterocarpus santalinus. Indian J Pharm Sci. 2006;68:115–6. [Google Scholar]
- 42.Mishra MP, Padhy RN. In vitro antibacterial efficacy of 21 Indian timber-yielding plants against multidrug-resistant bacteria causing urinary tract infection. Osong Public Health Res Perspect. 2013;4:347–57. doi: 10.1016/j.phrp.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dey SK, Chattopadhay S, Masanta NC. Antimicrobial activities of some medicinal plants of red and laterite zone of West Bengal, India. WJPPS. 2014;3:719–34. [Google Scholar]
- 44.Gopinath K, Gowri S, Arumugam A. Phytosynthesis of silver nanoparticles using Pterocarpus santalinus leaf extract and their antibacterial properties. J Nanostruct Chem. 2013;3:68–85. [Google Scholar]
- 45.Yamauchi S, Hayashi Y, Nakashima Y, Kirikihira T, Yamada K, Masuda T. Effect of benzylic oxygen on the antioxidant activity of phenolic lignans. J Nat Prod. 2005;68:1459–70. doi: 10.1021/np050089s. [DOI] [PubMed] [Google Scholar]
- 46.Narayan S, Veeraraghavan M, Devi CS. Pterocarpus santalinus: AnIn Vitro study on its anti-Helicobacter pylori effect. Phytother Res. 2007;21:190–3. doi: 10.1002/ptr.2047. [DOI] [PubMed] [Google Scholar]
- 47.Panayi GS, Lanchbury JS, Kingsley GH. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992;35:729–35. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- 48.Nagaraju N, Rao KN. Folk-medicine for diabetes from Rayalaseema of Andhra Pradesh. Anc Sci Life. 1989;9:31–5. [PMC free article] [PubMed] [Google Scholar]
- 49.Nagaraju N, Prasad M, Gopalakrishna G, Rao KN. Blood sugar lowering effect of Pterocarpus santalinus (Red Sanders) wood extract in different rat models. Int J Pharmacogn. 1991;29:141–4. [Google Scholar]
- 50.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet. 2011;378:169–81. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- 51.Kameswara Rao B, Giri R, Kesavulu MM, Apparao C. Effect of oral administration of bark extracts of Pterocarpus santalinus on blood glucose level in experimental animals. J Ethnopharmacol. 2001;74:69–74. doi: 10.1016/s0378-8741(00)00344-5. [DOI] [PubMed] [Google Scholar]
- 52.Rao KB, Rajasekhar MD, Sreelatha A, Apparao C. Treatment of Diabetes Mellitus: Plant Drugs vs. Oral Hypoglycaemic Agents and Insulin. In: Govil J.N., Singh V.K., Khalil Ahmad, editors. Recent Progress in medicinal Plants. Biopharmaceuticals. Vol. 14. LLC, Texas, USA: Studium Press; 2006. pp. 279–96. [Google Scholar]
- 53.Huang WY, Cai YZ, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr Cancer. 2010;62:1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- 54.Mena S, Rodríguez ML, Ponsoda X, Estrela JM, Jäättela M, Ortega AL. Pterostilbene-induced tumor cytotoxicity: A lysosomal membrane permeabilization-dependent mechanism. PLoS One. 2012;7:e44524. doi: 10.1371/journal.pone.0044524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding RB, Tian K, Huang LL, He CW, Jiang Y, Wang YT, et al. Herbal medicines for the prevention of alcoholic liver diseases: A review. J Ethnopharmacol. 2012;144:457–65. doi: 10.1016/j.jep.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 56.Manjunatha BK, Amit R, Priyadarshini P, Paul K. Lead findings from Pterocarpus santalinus with hepatoprotective potentials through in silico methods. Int J Pharma Sci and Res. 2010;7:265–70. [Google Scholar]
- 57.Itoh A, Isoda K, Kondoh M, Kawase M, Kobayashi M, Tamesada M, et al. Hepatoprotective Effect of Syringic Acid and Vanillic Acid on Concanavalin A-Induced Liver Injury. Biol Pharm Bull. 2009;32:1215–9. doi: 10.1248/bpb.32.1215. [DOI] [PubMed] [Google Scholar]
- 58.Itoh A, Isoda K, Kondoh M, Kawase M, Watari A, Kobayashi M, et al. Hepatoprotective effect of syringic acid and vanillic acid on CCl4-induced liver injury. Biol Pharm Bull. 2010;33:983–7. doi: 10.1248/bpb.33.983. [DOI] [PubMed] [Google Scholar]
- 59.Narayana S, Devi RS, Devi CS. Role of Pterocarpus santalinus against mitochondrial dysfunction and membrane lipid changes induced by ulcerogens in rat gastric mucosa. Chem Biol Interact. 2007;170:67–75. doi: 10.1016/j.cbi.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Suanarunsawat T, Boonnak T, Na Ayutthaya WD, Thirawarapan S. Anti-hyperlipidemic and cardioprotective effects of Ocimum sanctum L. fixed oil in rats fed a high fat diet. J Basic Clin Physiol Pharmacol. 2010;21:387–400. doi: 10.1515/jbcpp.2010.21.4.387. [DOI] [PubMed] [Google Scholar]
- 61.Rimando AM, Nagmani R, Feller DR, Yokoyama W. Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor alpha-isoform, lowers plasma lipoproteins and cholesterol in hypercholesterolemic hamsters. J Agric Food Chem. 2005;53:3403–7. doi: 10.1021/jf0580364. [DOI] [PubMed] [Google Scholar]
- 62.Riche DM, Riche KD, Blackshear CT, McEwen CL, Sherman JJ, Wofford MR, et al. Pterostilbene on metabolic parameters: A randomized, double-blind, and placebo-controlled trial. Evid Based Complement Alternat Med 2014. 2014:459165. doi: 10.1155/2014/459165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jadhav J, Mane A, Kanase A. Stimulatory effect of Pterocarpus santalinus on vasculogensis in chick chorioallantoic membrane (CAM) J Pharmacy Res. 2012;5:208–11. [Google Scholar]
- 64.Biswas TK, Maity LN, Mukherjee B. Wound healing potential of Pterocarpus santalinus Linn: A Pharmacological evaluation. Int J Low Extrem Wounds. 2004;3:143–50. doi: 10.1177/1534734604268385. [DOI] [PubMed] [Google Scholar]
- 65.Sultana N, Akhter M, Khatoon Z. Nematicidal natural products from the aerial parts of Rubus niveus. Nat Prod Res. 2010;24:407–15. doi: 10.1080/14786410802696429. [DOI] [PubMed] [Google Scholar]
- 66.Chattopadhyay SK, Kumar TR, Maulik PR, Srivastava S, Garga A. Absolute configuration and anticancer activity of taxiresinol and related lignans of Taxas wallichiana. Bioorg Med Chem. 2003;11:4945–8. doi: 10.1016/j.bmc.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 67.Kayser O, Kiderlen AF, Folkens U, Kolodziej H. In vitro leishmanicidal activity of aurones. Planta Med. 1999;65:316–9. doi: 10.1055/s-1999-13993. [DOI] [PubMed] [Google Scholar]
- 68.Kayser O, Kiderlen AF, Brun B. In vitro activity of aurones against plasmodium falciparum strains K1 and NF54. Planta Med. 2001;67:718–21. doi: 10.1055/s-2001-18356. [DOI] [PubMed] [Google Scholar]
- 69.Haschek WM, Wallig MA, Rousseaux C. Fundamentals of Toxicologic Pathology. San Diego, CA, USA: Academic Press; 2010. Nomenclature: Terminology for morphologic alterations; pp. 67–80. [Google Scholar]
- 70.Kirtikar KR, Basu BD. 2nd ed. Vol. 1. India: Bishen Singh Mahendra Pal Singh; 1980. Indian Medicinal Plants; p. 314. [Google Scholar]