Abstract
Background:
Carob - Ceratonia siliqua L., commonly known as St John's-bread or locust bean, family Fabaceae - is one of the most useful native Mediterranean trees. There is no data about the chromatography methods performed by high performance liquid chromatography (HPLC) for determining polyphenols in Egyptian carob pods.
Objective:
To establish a sensitive and specific liquid chromatography–electrospray ionization (ESI)-tandem mass spectrometry (MSn) methodology for the identification of the major constituents in Egyptian carob extract.
Materials and Methods:
HPLC with diode array detector and ESI-mass spectrometry (MS) was developed for the identification and quantification of phenolic acids, flavonoid glycosides, and aglycones in the methanolic extract of Egyptian C. siliqua. The MS and MSn data together with HPLC retention time of phenolic components allowed structural characterization of these compounds. Peak integration of ions in the MS scans had been used in the quantification technique.
Results:
A total of 36 compounds were tentatively identified. Twenty-six compounds were identified in the negative mode corresponding to 85.4% of plant dry weight, while ten compounds were identified in the positive mode representing 16.1% of plant dry weight, with the prevalence of flavonoids (75.4% of plant dry weight) predominantly represented by two methylapigenin-O-pentoside isomers (20.9 and 13.7% of plant dry weight).
Conclusion:
The identification of various compounds present in carob pods opens a new door to an increased understanding of the different health benefits brought about by the consumption of carob and its products.
SUMMARY
This research proposed a good example for the rapid identification of major constituents in complex systems such as herbs using sensitive, accurate and specific method coupling HPLC with DAD and MS, which facilitate the clarification of phytochemical composition of herbal medicine for better understanding of their nature and biological activities.
Abbreviation used: HPLC: High performance liquid chromatography, DAD: Diode array detector, MS: Mass spectrometry, TR: Retention time.
Keywords: Carob pods, Ceratonia siliqua L., liquid chromatography–electrospray ionization-tandem mass spectrometry, methylapigenin-O-pentoside
INTRODUCTION
With gaining popularity of herbal remedies worldwide, the need for assuring safety and efficacy of these products increases as well. By nature they are complex matrices, comprising a multitude of compounds, which are prone to variation due to environmental factors and manufacturing conditions. Furthermore, many traditional preparations compose of multiple herbs, so that only highly selective, sensitive, and versatile analytical techniques will be suitable for quality control purposes. By hyphenating high performance liquid chromatography (HPLC) and mass spectrometry (LC-MS) these high demands are fulfilled, providing the user with a multitude of technical options and applications.[1]
Carob - Ceratonia siliqua L., commonly known as St John's-bread or locust bean, family Fabaceae - is one of the most useful native Mediterranean trees. In producing countries, the pods have traditionally been used as animal and human food, and currently, the main use is the seed for gum extraction. In some countries, e.g. Egypt, carob syrup is a popular drink obtained by extracting carob kibbles with water. Carob powder consists of 46% sugar, 7% protein, and small amounts of numerous minerals and vitamins and is thus quite nutritious.[2] Apart from carbohydrates, high amounts of dietary fiber and polyphenols are characteristic of this Mediterranean food. Dietary fiber itself or a diet rich in dietary fiber is known to exert a variety of physiological effects, including improved digestion and attenuation of blood cholesterol and glucose levels. Dietary fiber probably also has chemopreventive potential against certain cancers, in particular, those of the gastrointestinal tract. Carob contains additional health-promoting constituents such as phytochemicals, among which the polyphenols which can exhibit antioxidative, antimutagenic, anticarcinogenic, antiproliferative, or antiestrogenic activity. Polyphenols are a very heterogeneous group that includes simple phenolic acids, cinnamic acid and its derivatives, flavonoids, isoflavones, lignans, anthocyanins, and tannins. Therefore, when included in the daily diet, these compounds may play an important role in the prevention and reduction of cancer and heart disease.[3]
There is few data about the chromatography methods performed by HPLC for determining polyphenols in carob pods which revealed the presence of condensed tannins (proanthocyanidins), composed of flavan-3-ol groups and their galloyl esters, gallic acid, (+)-catechin, (−)-epicatechingallate, (−)-epigallocatechingallate, and quercetin glycosides.[4,5,6] Much less interest was devoted to Egyptian carob. From this standpoint, the purpose of this research was to identify and quantify phenolic constituents present in the Egyptian carob pods. A sensitive, accurate, and specific method coupling HPLC with diode array detector (DAD) and electrospray ionization (ESI) MS was developed for the identification and quantification of phenolic acids, flavonoid glycosides, and aglycones in the methanolic extract of Egyptian C. siliqua. The subsequent structure characterization was carried out by a tandem mass spectrometric (MSn) method. Fragmentation behavior of investigated compounds was determined using ion trap MS in both negative and positive mode. Negative ion mode was used because previous studies suggested that negative mode was more sensitive than positive mode.[7] Positive mode was also applied leading to the identification of fewer constituents. The fragmentation pattern of each compound offers the ability to identify the related unknown compounds. The MS and MSn data together with HPLC retention time (TR) of phenolic components allowed structural characterization of these compounds.
The lack of reference standards for the phenolic constituents makes it necessary to calculate the amounts present according to the peak area. Peak integration of ions in the MS scans had been used in the quantification technique for decades by analytical chemists because theoretically the peak intensity of any ion is proportional to its abundance.[8,9] The area inscribed by the peak is proportional to the amount of components separated in the chromatographic system. The relative percentage of each constituent was determined from the relative peak area and expressed as percent of dry plant weight.
MATERIALS AND METHODS
Chromatographic system and mass spectrometry conditions
Analyses were performed using a Hewlett-Packard 1100 (Waldbronn, Germany) composed of a quaternary pump with an online degasser, a thermostatic column compartment, a photo DAD, an autosampler, and Agilent 1100 Chem Station software. The HPLC separation was performed on Eclipse XDB C18 column (50 mm × 2.1 mm, 1.8 µm, Agilent Company, USA). Mobile phase consisted of two solvents, (A) methanol and (B) 0.2% formic acid. Separation of compounds was carried out with gradient elution profile: 0 min, A: B 10:90; 36 min, A: B 100:0; and 40 min, A: B 100:0. Chromatography was performed at 30°C with a flow-rate of 0.2 ml/min. Ultraviolet (UV) traces were measured at 290, 254, and 350 nm, and UV spectra (DAD) were recorded between 190 and 900 nm.
The HPLC–MS system consisted of ESI interfaced Bruker Daltonik Esquire-LC ion trap MS (Bremen, Germany) and an Agilent HP1100 HPLC system equipped with an autosampler and a UV-visible absorbance detector. The ionization parameters were as follows: Positive ion mode; capillary voltage 4000 V, end plate voltage − 500 V; nebulizing gas of nitrogen at 35.0 p.s.i.; and drying gas of 10 l/min nitrogen at 350°C. Mass analyzer scanned from 15 to 1000 u. The MS–MS spectra were recorded in auto-MS–MS mode. The fragmentation amplitude was set to 1.0 V. MS2 data were acquired in both negative and positive mode.
Extract preparation
Carob pods were collected from the region of Giza, Egypt, 2014 and kindly authenticated by Prof. Dr. Abd Al Haleem A. Mohamed (Plant Taxonomy and Flora Research Department, Ministry of Agriculture, Giza, Egypt) for whom the authors are thankful. Voucher specimen was deposited at the Department of Pharmacognosy, Faculty of Pharmacy, Beni-Suef University, Beni-Suef, Egypt. The plant material was air smashed into powder. One gram of accurately weight carob powder was extracted with 20 mL of methanol at 60°C, on a sonicator for 30 min, three times. Filtrated extracts were combined, evaporated to dryness under reduced pressure, dispersed in 15 mL methanol, passed through 0.45 μm filters, and stored at 4°C.
RESULTS
The use of LC/MS allowed the identification and quantification of 36 compounds in the Egyptian carob pods methanolic extract. Peaks assigned were based on their TR and MS fragmentation patterns. The TR, ESI-MSn data and relative percentage of each constituent expressed as percent of dry plant weight were summarized in [Tables 1 and 2] in both negative and positive modes, respectively. The chemical structures of some fragments were presented in [Figure 1].
Table 1.
Compounds identified in the Egyptian carob extract by LC-MSn in negative-ion mode
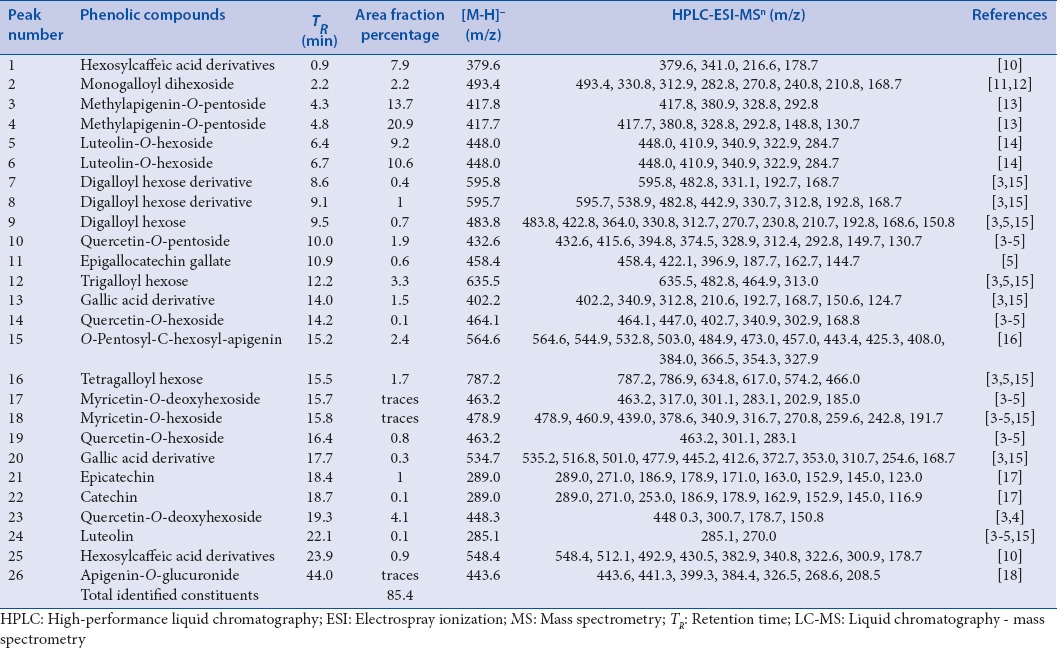
Table 2.
Compounds identified in the Egyptian carob extract by LC-MSn in positive-ion mode
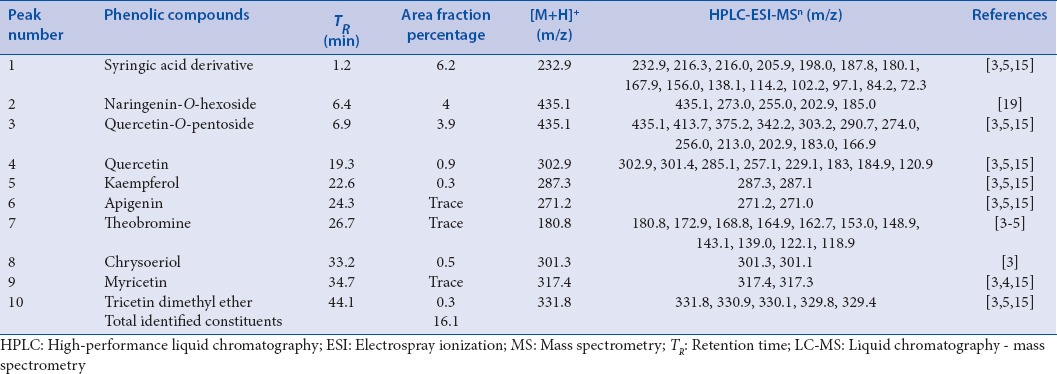
Figure 1.
Chemical structure of some fragments present in Egyptian carob pods extract
DISCUSSION
Structure characterization of major constituents by tandem mass spectrometry
Structure characterization of the hydroxycinnamoyl derivatives by tandem mass spectrometry
Two hydroxycinnamoyl derivatives were observed, especially caffeic acid derivatives, that were tentatively identified according to their characteristic ion at m/z 179 ([caffeic acid-H]−) observed in their MS2 spectra. Peak 1, 25 presented a deprotonated fragment at m/z 341 u corresponding to [hexosyl caffeic acid moiety-H]− and similar fragmentation pattern, with the loss of 162 u (hexosyl moiety), yielding a peak at m/z 179 u [caffeic acid-H]−, being assigned as hexosyl caffeic acid derivatives.[10]
Structure characterization of the flavonoids by tandem mass spectrometry
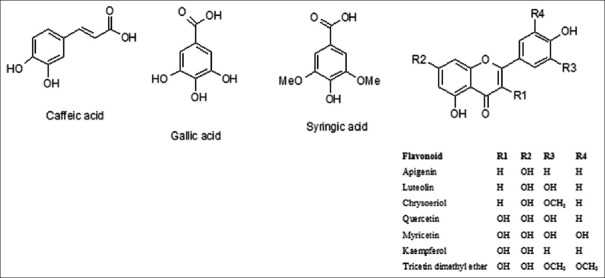
Mass spectrometric methods can be used to obtain information on the carbohydrate sequence and the aglycone. Flavonoid aglycones are structurally diverse group of natural products. The most important variations in their structure are in the level of oxygenation (hydroxyl or methoxyl groups) and the point of attachment of ring B (flavonoids and isoflavonoids). When MSn experiments are performed on instruments with ion trap analyzers, it is possible to perform tandem experiments many times (MSn) on sequential product ions. From the mass spectra of flavonoid glycosides using MSn, we can obtain molecular mass, structure of the aglycone (pattern of hydroxylation on aglycone, point of attachment of ring B on ring C), information about acylation of sugar hydroxyl groups, possible methylation or sulphation of aglycone hydroxyl(s), number of sugar rings, their configuration, and in some cases placement of glycosidic bonds.
In structure characterization, we first judged if the flavonoid glycoside is a C-glycosylated or O-glycosylated. The carbon-carbon bond of C-glycosyl flavonoids is resistant to rupture and in C-glycosides mainly the fragmentation of the sugar unit is observed. Losses of 120 and 90 u were observed, corresponding to cross-ring cleavages in the sugar unit. Fragmentation pathway of O-glycosylated flavonoids start with the cleavage of the glycosidic bonds and elimination of the sugar moieties with charge retention on aglycone. In compounds containing two or more sugars to the same aglycone carbon, ions arising from the cleavage of the glycosidic bonds between sugar units are weak. Although the aglycone and the glycane were all identified, the accurate structure of the flavonoids glycoside could not be always determined because identity and the site of connection of monosaccharide cannot be determined by LC-MS. The structures of compounds were finally identified by comparison with the literature.[7,20,21,22,23]
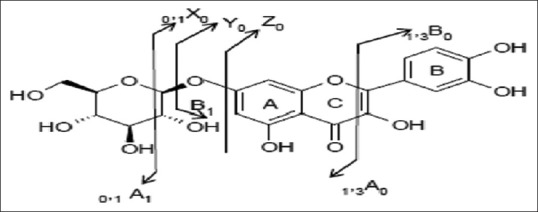
Major diagnostic fragments of flavonoid aglycone identification are those involving the cleavage of two C-C bonds of the C-ring giving two fragment ions which provide information about the number and type of substituents in A- and B-rings. These fragment ions are designated according to the nomenclature proposed by Ma et al., 1997. For free aglycone i, jA and i, jB labels refer to the fragments containing intact A- and B-rings, respectively, in which the superscripts i and j indicate the C-ring bonds that have been broken. The possible fragmentation patterns and ion nomenclature of flavonoid glycosides are illustrated on quercetin-O-hexoside in Figure 2. For flavonoid glycosides, the classical nomenclature is proposed by Domon and Castello for glycoconjugates to denote major fragments:k, l Xj, Yj, Zj represent the ions still containing the aglycone, where j is the number of inter glycosidic bond broken (counted from the aglycon), and k and ldenote the cleavage within the carbohydrate rings.[7,24,25,26,27] Fragmentations of quercetin-O-hexoside and quercetin-O-deoxyhexoside identified in plant extract are shown in Figures 3 and 4, respectively.
Figure 2.
Fragmentation of quercetin-O-hexoside
Figure 3.
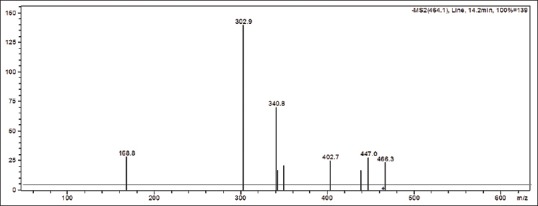
Fragmentation of quercetin-O-hexoside (peak 14) in negative mode
For flavonoid aglycone and its glycosides in the negative mode, the spectra showed both the deprotonated molecule [M − H]− of the glycoside and the ion corresponding to the deprotonated aglycone [A − H]−. The latter ion was formed by loss of the pentose (132 units), hexose (164 units), or deoxyhexose (146 units) moieties from the glycosides. The presence of deprotonated aglycone [A − H]− at m/z 301 demonstrated the presence of the quercetin, while m/z 269 represented apigenin, m/z 317 indicated myricetin, and m/z 285 demonstrated luteolin and kaempferol. For flavonoid aglycone and its glycosides in the positive mode, the spectra showed both the protonated molecule [M + H]+ of the glycoside and the ion corresponding to the protonated aglycone [A + H]+. The presence of protonated aglycone [A + H]− at m/z 273 demonstrated the presence of the naringenin, m/z 301 represented chrysoeriol, and m/z 331 indicated tricetin dimethyl ether.[3,4,5,13,14,15,16,17,18,19]
Structure characterization of phenolic acids by tandem mass spectrometry
In hydrolysable tannins, hydroxyphenolic acids such as gallic acid can be esterified by D-glucose, yielding gallotannins. Thus, anion of gallic acid (m/z 169) was often found in MS analyses of tannins. Loss of (m/z 152) indicated the presence of galloyl moiety. Moreover, the presence of syringic acid derivatives was indicated by the presence of (m/z 198).[3,5,15,11,12]
Standard-free relative quantification by peak area of liquid chromatography and mass spectrometry
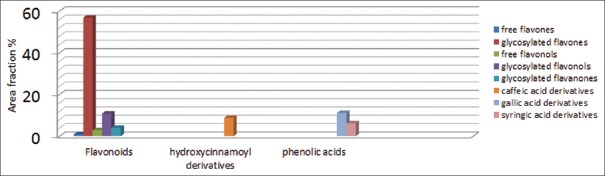
The use of LC/MS allowed the identification and quantification of 36 compounds in the Egyptian carob pods methanolic extract. Twenty-six compounds were identified in the negative mode corresponding to 85.4% of plant dry weight [Table 1], while ten compounds were identified in the positive mode representing 16.1% of plant dry weight [Table 2], with the prevalence of flavonoids (75.4% of plant dry weight) predominantly represented by two methylapigenin-O-pentoside isomers (20.9 and 13.7% of plant dry weight) and luteolin-O-hexoside (10.6% of plant dry weight). On the other hand, phenolic acids (17.3% of plant dry weight) were present in various forms mainly syringic acid derivative and trigalloyl hexose (representing 6.2 and 3.3% of plant dry weight, respectively). Two hydroxycinnamoyl derivatives were detected in relatively appreciable amount (8.8% of plant dry weight) and identified as hexosylcaffeic acid derivatives (7.9 and 0.9% of plant dry weight). Figure 5 illustrates a graphical representation of major constituents in carob pods methanolic extract.
Figure 4.
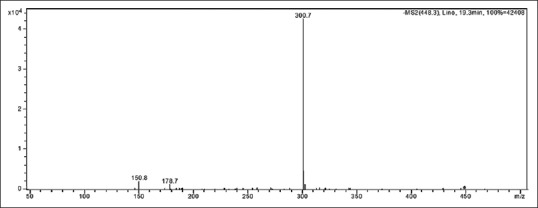
Fragmentation of quercetin-O-deoxyhexoside (peak 23) in negative mode
Figure 5.
Graphical representation of major constituents in carob pods methanolic extract
CONCLUSION
The present data shows that LC/ESI-MS/MSn is a useful tool for characterization and quantification of different phenolic compounds present in Egyptian carob pods, which is performed for the first time. The results of this study clarifies that carob pods not only has a high content of phenolic antioxidants, comparable to other Mediterranean foods such as olives[3] but also contains a rich variety of individual components from several classes: flavonoids including; free flavones (0.9% of plant dry weight), glycosylated flavones (56.8% of plant dry weight), free flavonols (2.9% of plant dry weight), glycosylated flavonols (10.8% of plant dry weight), and glycosylated flavanones (4.0% of plant dry weight); in addition to the presence of hydroxycinnamoyl derivatives (caffeic acid derivatives; 8.8% of plant dry weight) and phenolic acid derivatives (gallic acid derivatives; 11.1% of plant dry weight and syringic acid derivatives; 6.2% of plant dry weight).
To date, carob consumption has been based on the pleasant taste of the drink, especially in the Mediterranean region. The identification of various compounds present in carob pods opens a new door to an increased understanding of the different health benefits brought about by the consumption of popular carob and its products.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Asmaa Ibrahim Owis
Asmaa Ibrahim Owis, is Lecturer at the Department of Pharmacognosy, Faculty of Pharmacy, Beni-Suef University, Beni-Suef. The research of interest is in the area of pharmacognosy, phytochemistry and natural products research. Additionally, she is the Deputy Director for Quality Assurance Unit, Faculty of Pharmacy, Beni-Suef University, Beni-Suef. Moreover, she is the Principle investigator and Co-PI for two research project, (worth 40 000 Egyptian pounds, each) from Development Unit of Scientific Research, Beni-Suef University, Beni-Suef, Egypt.
REFERENCES
- 1.Steinmann D, Ganzera M. Recent advances on HPLC/MS in medicinal plant analysis. J Pharm Biomed Anal. 2011;55:744–57. doi: 10.1016/j.jpba.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Battle I, Tous J. Rome, Italy: Bioversity International; 1997. Carob tree: Ceratonia siliqua L. – Promoting the Conservation and Use of Underutilized and Neglected Crops. 17. [Google Scholar]
- 3.Owen RW, Haubner R, Hull WE, Erben G, Spiegelhalder B, Bartsch H, et al. Isolation and structure elucidation of the major individual polyphenols in carob fibre. Food Chem Toxicol. 2003;41:1727–38. doi: 10.1016/s0278-6915(03)00200-x. [DOI] [PubMed] [Google Scholar]
- 4.Ortega N, Macià A, Romero MP, Trullols E, Morello JR, Anglès N, et al. Rapid determination of phenolic compounds and alkaloids of carob flour by improved liquid chromatography tandem mass spectrometry. J Agric Food Chem. 2009;57:7239–44. doi: 10.1021/jf901635s. [DOI] [PubMed] [Google Scholar]
- 5.Papagiannopoulos M, Wollseifen HR, Mellenthin A, Haber B, Galensa R. Identification and quantification of polyphenols in carob fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MSn. J Agric Food Chem. 2004;52:3784–91. doi: 10.1021/jf030660y. [DOI] [PubMed] [Google Scholar]
- 6.Corsi L, Avallone R, Cosenza F, Farina F, Baraldi C, Baraldi M. Antiproliferative effects of Ceratonia siliqua L.on mouse hepatocellular carcinoma cell line. Fitoterapia. 2002;73:674–84. doi: 10.1016/s0367-326x(02)00227-7. [DOI] [PubMed] [Google Scholar]
- 7.Plazonic A, Bucar F, Males Z, Mornar A, Nigovic B, Kujundzic N. Identification and quantification of flavonoids and phenolic acids in burr parsley (Caucalis platycarpos L.), using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Molecules. 2009;14:2466–90. doi: 10.3390/molecules14072466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matzke MM, Brown JN, Gritsenko MA, Metz TO, Pounds JG, Rodland KD, et al. A comparative analysis of computational approaches to relative protein quantification using peptide peak intensities in label-free LC-MS proteomics experiments. Proteomics. 2013;13:493–503. doi: 10.1002/pmic.201200269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maggar EM. Artemisia herba alba and Artemisia monosperma: The discovery of the first potential Egyptian plant sources for the pharmaceutical commercial production of artemisinin and some of its related analogues. JAPS. 2012;2:77–91. [Google Scholar]
- 10.Barreira JC, Dias MI, Zivkovic J, Stojkovic D, Sokovic M, Santos-Buelga C, et al. Phenolic profiling of Veronica spp. grown in mountain, urban and sandy soil environments. Food Chem. 2014;163:275–83. doi: 10.1016/j.foodchem.2014.04.117. [DOI] [PubMed] [Google Scholar]
- 11.Nuengchamnong N, Ingkaninan K. On-line characterization of phenolic antioxidants in fruit wines from family Myrtaceae by liquid chromatography combined with electrospray ionization tandem mass spectrometry and radical scavenging detection. LWT Food Sci Technol. 2009;42:297–302. [Google Scholar]
- 12.Nuengchamnong N, Boonpathanasak S, Tepwitukkij P. Rapid screening of antioxidant compounds in homemade fruit fermented juice using an on line LC-ESI-MS/MS and DPPH assay. Chiang Mai J Sci. 2011;38:430–8. [Google Scholar]
- 13.Colombo R, Yariwake JH, Queiroz EF, Ndjoko K, Hostettmann K. On-line identification of minor flavones from sugarcane juice by LC/UV/MS and post-column derivatization. J Braz Chem Soc. 2009;20:1574–9. [Google Scholar]
- 14.Sánchez-Rabaneda F, Jáuregui O, Casals I, Andrés-Lacueva C, Izquierdo-Pulido M, Lamuela-Raventós RM. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao) J Mass Spectrom. 2003;38:35–42. doi: 10.1002/jms.395. [DOI] [PubMed] [Google Scholar]
- 15.Karimi H, Farmani A, Nourizadeh H. Quantitative structure-retention relationship analysis of polyphenols in carob fibre. World Appl Sci J. 2011;15:1465–8. [Google Scholar]
- 16.Pereira OR, Silva AM, Domingues MR, Cardoso SM. Identification of phenolic constituents of Cytisus multiflorus. Food Chem. 2012;131:652–9. [Google Scholar]
- 17.Custódio L, Escapa AL, Patarra J, Aligué R, Alberício F, Neng NR, et al. Sapwood of carob tree (Ceratonia siliqua L.) as a potential source of bioactive compounds. Rec Nat Prod. 2013;7:225–9. [Google Scholar]
- 18.Qu J, Wang Y, Luo G, Wu Z. Identification and determination of glucuronides and their aglycones in Erigeron breviscapus by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2001;928:155–62. doi: 10.1016/s0021-9673(01)01111-6. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez-Rabaneda F, Jáuregui O, Lamuela-Raventós RM, Viladomat F, Bastida J, Codina C. Qualitative analysis of phenolic compounds in apple pomace using liquid chromatography coupled to mass spectrometry in tandem mode. Rapid Commun Mass Spectrom. 2004;18:553–63. doi: 10.1002/rcm.1370. [DOI] [PubMed] [Google Scholar]
- 20.de Rijke E, Zappey H, Ariese F, Gooijer C, Brinkman UA. Liquid chromatography with atmospheric pressure chemical ionization and electrospray ionization mass spectrometry of flavonoids with triple-quadrupole and ion-trap instruments. J Chromatogr A. 2003;984:45–58. doi: 10.1016/s0021-9673(02)01868-x. [DOI] [PubMed] [Google Scholar]
- 21.March RE, Lewars EG, Stadey CJ, Miao XS, Zhao X, Metcalfe CD. A comparison of flavonoid glycosides by electrospray tandem mass spectrometry. IJMS. 2006;248:61–85. [Google Scholar]
- 22.Stobiecki M. Application of mass spectrometry for identification and structural studies of flavonoid glycosides. Phytochemistry. 2000;54:237–56. doi: 10.1016/s0031-9422(00)00091-1. [DOI] [PubMed] [Google Scholar]
- 23.Tolonen A, Uusitalo J. Fast screening method for the analysis of total flavonoid content in plants and foodstuffs by high-performance liquid chromatography/electrospray ionization time-of-flight mass spectrometry with polarity switching. Rapid Commun Mass Spectrom. 2004;18:3113–22. doi: 10.1002/rcm.1736. [DOI] [PubMed] [Google Scholar]
- 24.Cuyckens F, Claeys M. Mass spectrometry in the structural analysis of flavonoids. J Mass Spectrom. 2004;39:1–15. doi: 10.1002/jms.585. [DOI] [PubMed] [Google Scholar]
- 25.Domon B, Costello CE. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj J. 1988;5:397–409. [Google Scholar]
- 26.Hughes RJ, Croley TR, Metcalfe CD, March RE. A tandem mass spectrometric study of selected characteristic flavonoids. Int J Mass Spectrom. 2001;210:371–85. [Google Scholar]
- 27.Ma Y, Li Q, van den Heuvel H, Claeys M. Characterization of flavone and flavonol aglycones by collision-induced dissociation tandem mass spectrometry. Rapid Commun Mass Spectrom. 1997;11:1357–64. doi: 10.1002/(SICI)1097-0231(19991015)13:19<1932::AID-RCM735>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]