Abstract
Background:
Genus Swertia is valued for its great medicinal potential, mainly Swertia chirayita (Roxb. ex Fleming) H. Karst. is used in traditional medicine for a wide range of diseases. Mangiferin one of xanthoids is referred with enormous pharmacological potentials.
Objective:
The aim of the study was to quantify and compare the anticancerous and antidiabetic drug mangiferin from 11 Swertia species from India. The study also evaluates hierarchical relationships between the species based on mangiferin content using multivariate analysis.
Materials and Methods:
The reverse phase-ultra flow liquid chromatography-diode array detector analyses was performed and chromatographic separation was achieved on a Lichrospher 100, C18e (5 μm) column (250–4.6 mm). Mobile phase consisting of 0.2% triethylamine (pH-4 with O-phosphoric acid) and acetonitrile (85:15) was used for separation with injection volume 20 μL and detection wave length at 257 nm.
Results:
Results indicated that concentration of mangiferin has been found to vary largely between Swertia species collected from different regions. Content of mangiferin was found to be highest in Swertia minor compared to other Swertia species studied herein from the Western Ghats and Himalayan region also. The same was also evident in the multivariate analysis, wherein S. chirayita, S. minor and Swertia paniculata made a separate clade.
Conclusion:
Conclusively, the work herein provides insights of mangiferin content from 11 Swertia species of India and also presents their hierarchical relationships. To best of the knowledge this is the first report of higher content of mangiferin from any Swertia species.
SUMMARY
The present study quantifies and compares mangiferin in 11 species of Swertia from India. The study also evaluates hierarchical relationships between the species based on mangiferin content using multivariate analysis. The mangiferin content was highest in S. minor compared to the studied Swertia species. To the best of our knowledge this is the first report of higher content of mangiferin from Swertia species.

Abbreviations used: LOD: Limit of detection, LOQ: Limit of quantification, RP-UFLC-DAD: Reverse phase-ultra flow liquid chromatography-diode array detector, RSD: Relative standard deviation, SAN: Swertia angustifolia, SAP: Swertia angustifolia var. pulchella, SBI: S. bimaculata, SCH: S. chirayita, SCO: S. corymbosa, SDE: S. densifolia, SDI: S. dialatata, SLA: S. lawii, SMI: S. minor; SNE: S. nervosa, and SPA: S. paniculata
Keywords: Dendrogram, mangiferin, multivariate analysis, reverse phase-ultra flow liquid chromatography, Swertia
INTRODUCTION
Genus Swertia (family Gentianaceae), comprises of ~170 species in world.[1] Nearly, 40 are endowed to India out of which 32 occur in the Himalayan regions[2] and remaining eight are confined endemic to the Western Ghats of India. The genus is valued for its medicinal potential, most importantly Swertia chirayita (Roxb. ex Fleming) H. Karst which is known for its use in traditional medicine in range of ailments including anthelmintic, hypoglycemic, and antipyretic.[3]
The pharmacological actions of any plants depend upon its chemical diversity, Swertia being no different have been reported for a wide range of such phytochemicals. The plant is reported for its marker compounds swertiamarin, swerchirin, amaroswerin, and amarogentin.[3] Studies such as antioxidant, hypoglycemic, and antiglycation activities of some Swertia species from India have been well documented.[4,5] Triterpenoids such as betulinic acid, oleanolic acid, and ursolic acid; singly or collectively are been reported from different medicinal plants including Swertia species.[6,7,8,9,10,11,12] Similarly, mangiferin a widely distributed xanthonoid found in Mangifera indica and members of Anacardeaceae is also reported from Swertia.[13]
Mangiferin exhibits diverse pharmacological activities such as antidiabetic,[14] anti-HIV,[15] anticancer,[16] anti-inflammatory,[17] antiproliferative, diuretic and antioxidant,[18,19,20] antifungal,[21] antitubercular,[22] chemopreventive,[23] hepatoprotective,[24] immunomodulatory,[25] and many other activities. The interest in this multipotential molecule is mainly due to its anticancerous and antidiabetic properties.
Keeping this in view, the present work was aimed to determine mangiferin content from 11 Swertia species from India. It also enumerates superiority among the species based upon the higher mangiferin content as determined by reversed phase-ultra flow liquid chromatography-diode array detector (RP-UFLC-DAD) analysis and evaluates similarity between the species using multivariate analysis.
MATERIALS AND METHODS
Collection of plant materials and extract preparation
Plant material of all the species were obtained from different localities of the Western Ghats and Eastern Himalayan region. The whole plant material was air-dried at the room temperature and ground to fine powder in laboratory grinder. Extracts were prepared by dissolving 500 mg plants powder in 25 ml methanol for 24 h. The filtrates were re-volumized and subjected to RP-UFLC analysis after passing through 0.2 µm nylon filters.
Chemicals and standard
All solvents and chemicals used during the study were of high-performance liquid chromatography (HPLC) grade. A HPLC grade mangiferin was procured from Sigma-Aldrich, India. An accurately weighed standard mangiferin was dissolved in known amount of methanol to obtain mg/ml concentration stock. The stock solution was then diluted to obtain desired working concentrations (1, 10, 25, 50, 100, and 250 µg/ml).
Reversephase-ultra flow liquid chromatographic analysis
The RP-UFLC analysis was performed on Shimadzu Chromatographic System consisting of a quaternary pump, manual injector, and dual λ ultraviolet absorbance DAD. The built in LC-solution software system was used for the data processing. Chromatographic separation was achieved on a lichrospher 100, C18e (5 µm) column (250–4.6 mm). Mobile phase consisting of 0.2% triethylamine (pH-4 with O-phosphoric acid) and acetonitrile (85:15) was used for separation with injection volume 20 µL. The flow rate was 1 ml/min and the detection wavelength of the dual λ absorbance detector beam was set at 257 nm. The analysis time was 8 min for both standard and samples. The system suitability test was assessed by three replicate injections of the standard solutions at a particular concentration.
Statistical and multivariate analysis
Statistical analysis was performed using the statistical software Graph Pad Prism Evaluation version (GraphPad Software, USA). The data were reported as means and ± standard deviation (SD). The chromatographic profiles of all extracts were analyzed using built in Shimadzu LC-solution software version 1.25 (Shimadzu corporation, Japan). Multivariate analysis for correlations was analyzed using Biodiversity Pro, version 2 (N Mc Aleece, PJD Lambshead, GLJ Paterson and JD Gage, The Natural History Museum & The Scottish Association for Marine Science.)eco-statistical software to understand the possible natural groupings and correlation in and among the samples collected. The hierarchical clustering analysis performed was based on the relative peak area of the mangiferin from all the samples.
RESULTS AND DISCUSSION
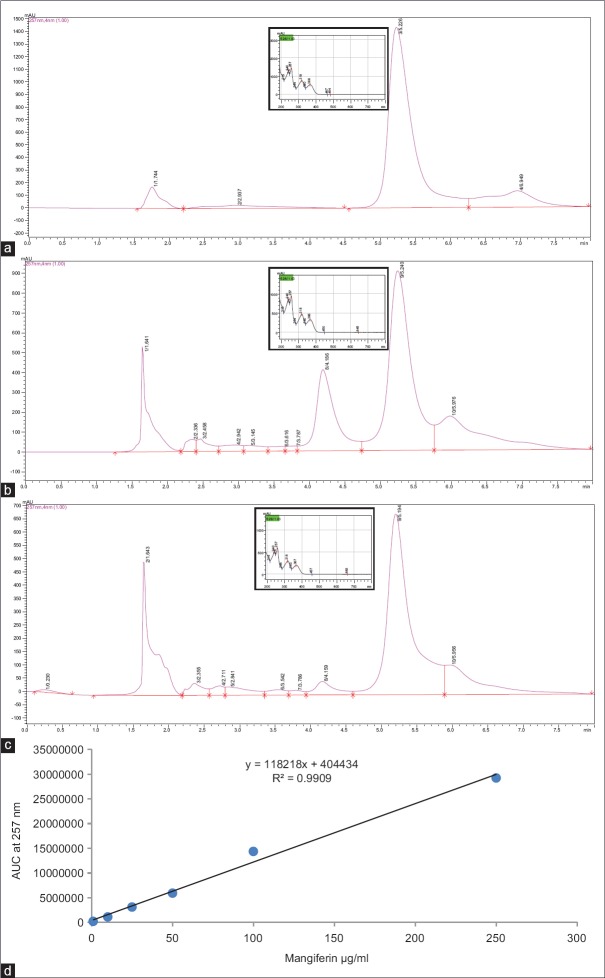
In this study, 11 Swertia species, five from the Western Ghats, and six from Himalayan region of India were considered for the study. Quantitative determination of mangiferin in the various species was achieved using RP-UFLC-DAD method and results were expressed as mg/g on dry weight basis. The analysis yielded clear, sharp peaks for standard and sample runs [Figure 1a–c]. Calibration curve was constructed from six different concentrations of standard mangiferin against their respective area under curve with coefficient of determination (R2) not < 0.990 [Figure 1d]. A lowest calibrator concentration of 1 µg/ml was used during the study with 0.158 µg/ml limit of detection and 0.479 µg/ml limit of quantification values. The relative SD values were <2% indicating precision and reproducibility of the used method. Validation test was performed by injecting equal volume spiking of standard with S. chirayita sample extract to attain recovery at 95–100% range.
Figure 1.
Reverse phase-ultra flow liquid chromatography profiles of (a) standard mangiferin (250 μg/ml); (b) Swertia minor extract; (c) Swertia chirayta extract; (d) six point calibration curve (1, 10, 25, 50, 100, and 250 μg/ml); figures in inset shows spectrum maximum wavelength for peak at 5.220 ± 0.053 min
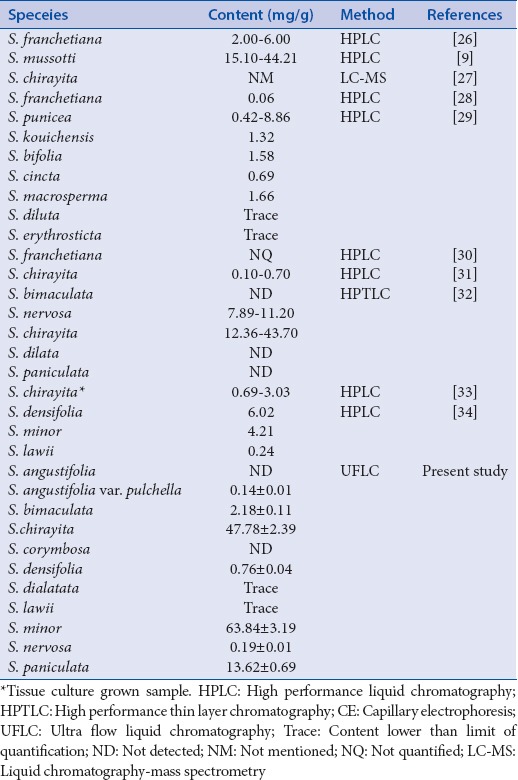
Mangiferin was retained and detected at 5.220 ± 0.053 min in standards and samples. Out of the 11 Swertia species collected, S. minor (63.84 ± 3.19 mg/g) and S. chirayita (47.78 ± 2.39 mg/g) had a higher content of mangiferin, followed by S. paniculata with 13.62 ± 0.69 mg/g and Swertia bimaculata (2.18 ± 0.11 mg/g) [Table 1]. Swertia angustifolia var. pulchella, Swertia densifolia and Swertia nervosa were among the other species with mangiferin content below 1 mg/g [Table 1]. Among the rest, Swertia dialatata and Swertia lawii showed mangiferin content less than LOQ hence termed as trace. Whereas, it was not detected in S. angustifolia and Swertia corymbosa. Thus, the paper provides a data on mangiferin content of 11 Swertia species from India. Mangiferin content (mg/g) has earlier been determined in Swertia species by using various chromatographic techniques [Table 1][8,31] and it was observed that the mangiferin content determined in the present study for S. chirayita and S. minor was the highest among all.
Table 1.
Content (mg/g) of mangiferin reported in different Swertia species
The multivariate analysis was performed using area under curve for mangiferin from all the samples. A percent similarity dendrogram was obtained based on area of mangiferin run of RP-UFLC-DAD analysis. The Swertia species were arranged in ascending order of mangiferin content from top to bottom [Figure 2]. S. chirayita, S. minor and S. paniculata made a separate clade at bottom with a higher content and a similarity of 44.45%. Among this S. minor showed higher similarity with S. chirayita (85.67%). This followed by S. densifolia, S. bimaculata, S. aungustifolia var. pulchella and S. nervosa with medium content and a percent similarity of 55.55%. S. lawii and S. dialatata with trace amount of mangiferin showed 46.20% similarity. S. corymbosa and S. aungustifolia with no content of mangiferin remained separated from all others with a lower percent similarity (4.40%). Similar analysis has been also performed by Deshmukh et al.[35] in different banana varieties and Wohlmuth et al.,[36] in developing method to improve detection of adulteration in Ginkgo biloba, hence justifying use of such tools in understanding hierarchical relations.
Figure 2.
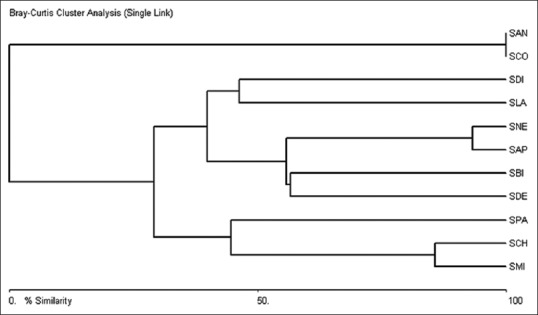
Dendrogram generated using area under curve obtained from reverse phase-ultra flow liquid chromatography-diode array detector analysis of mangiferin from various Swertia species
CONCLUSION
In conclusion, the work herein provides insights of mangiferin content from 11 Swertia species of India and also presents their hierarchical relationships. Besides, we also stress upon S. minor from the Western Ghats of India, to have a higher content of mangiferin than any other species reported hereto. To best of the knowledge this is the first report of a higher content of mangiferin from any Swertia species.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Sandeep R. Pai
Dr. Sandeep R. Pai, Scientist (DST-YS), research interests in plant based compounds, extraction and analytical methods, presently working on elicitation of secondary metabolites using biotechnological tools and prospecting of medicinally important compounds from plants with over 10 years research experience.
Acknowledgment
Authors are indebted to officer-in-charge, RMRC, ICMR, Belagavi and Head, Department of Botany, Shivaji University, Kolhapur for their kind support. Authors also thank Dr. Manoj Lekhak, Department of Botany, Shivaji University, Kolhapur for his help in collection of Swertia species. SRP is also indebted to SERB, DST, and New Delhi for providing financial support during the work (SB/YS/LS-71/2013).
REFERENCES
- 1.Brahmachari G, Mondal S, Gangopadhyay A, Gorai D, Mukhopadhyay B, Saha S, etal Swertia (Gentianaceae): Chemical and pharmacological aspects. Chem Biodivers. 2004;1:1627–51. doi: 10.1002/cbdv.200490123. [DOI] [PubMed] [Google Scholar]
- 2.Kumar N, Singh B, Kaul VK, Ahuja PS. Chemical and biological aspects of iridoid bearing plants of temperate region. Vol. 32, Part L, Atta-ur-Rahman, editor. Studies in Natural Products Chemistry Pages Bioactive Natural Products. 2005:247–302. [Google Scholar]
- 3.Joshi P, Dhawan V. Swertia chirayita: Overview. Curr Sci. 2005;89:635–40. [Google Scholar]
- 4.Kshirsagar P, Chavan J, Nimbalkar M, Yadav S, Dixit G, Gaikwad N. Phytochemical composition, antioxidant activity and HPLC profiles of Swertia species from Western Ghats. Nat Prod Res. 2015;29:780–4. doi: 10.1080/14786419.2014.986124. [DOI] [PubMed] [Google Scholar]
- 5.Kshirsagar P, More T, Arvindekar A, Gaikwad N. Antioxidant, antihyperglycemic and antiglycation properties of some Swertia species from Western Ghats. Int J Pharm Pharm Sci. 2014;6:303–6. [Google Scholar]
- 6.Kshirsagar PR, Pai SR, Nimbalkar MS, Gaikwad NB. Quantitative determination of three pentacyclic triterpenes from five Swertia L. species endemic to Western Ghats, India, using RP-HPLC analysis. Nat Prod Res. 2015;29:1783–8. doi: 10.1080/14786419.2015.1004174. [DOI] [PubMed] [Google Scholar]
- 7.Li G, Zhang X, You J, Song C, Sun Z, Xia L, et al. Highly sensitive and selective pre-column derivatization high-performance liquid chromatography approach for rapid determination of triterpenes oleanolic and ursolic acids and application to Swertia species: Optimization of triterpenic acids extraction and pre-column derivatization using response surface methodology. Anal Chim Acta. 2011;688:208–18. doi: 10.1016/j.aca.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Gupta M, Bisht D, Khatoon S, Srivastava S, Rawat AK. Determination of ursolic acid a biomarker in different Swertia species through high performance thin layer chromatography. Chin Med. 2011;2:121–4. [Google Scholar]
- 9.Yang H, Ding C, Duan Y, Liu J. Variation of active constituents of an important Tibet folk medicine Swertia mussotii Franch. (Gentianaceae) between artificially cultivated and naturally distributed. J Ethnopharmacol. 2005;98:31–5. doi: 10.1016/j.jep.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Pai SR, Nimbalkar MS, Pawar NV, Dixit GB. Optimization of extraction techniques and quantification of betulinic acid (BA) by RP-HPLC method from Ancistrocladus heyneanus Wall. Ex Grah. Ind Crops Prod. 2011;34:1458–64. [Google Scholar]
- 11.Upadhya V, Ankad GM, Pai SR, Hegde HV, Kholkute SD. Accumulation and trends in distribution of three triterpenoids in various parts of Achyranthes coynei determined using RP-UFLC analysis. Pharmacogn Mag. 2014;10:398–401. doi: 10.4103/0973-1296.141761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pai SR, Upadhya V, Hegde HV, Joshi RK, Kholkute SD. New report of triterpenoid betulinic acid (BA) along with oleanolic acid (OA) from Achyranthes aspera by RP-UFLC analysis and confirmation using HPTLC and FTIR techniques. J Planar Chromatogr Modif TLC. 2014;27:38–41. [Google Scholar]
- 13.Chavan JJ, Ghadage DM, Kshirsagar PR, Kudale SS. Optimization of extraction techniques and RP-HPLC analysis of antidiabetic and anticancer drug mangiferin from roots of ‘Saptarangi’ (Salacia chinensis L.) J Liq Chromatogr Relat Technol. 2015;38:963–9. [Google Scholar]
- 14.Miura T, Ichiki H, Iwamoto N, Kato M, Kubo M, Sasaki H, et al. Antidiabetic activity of the rhizoma of Anemarrhena asphodeloides and active components, mangiferin and its glucoside. Biol Pharm Bull. 2001;24:1009–11. doi: 10.1248/bpb.24.1009. [DOI] [PubMed] [Google Scholar]
- 15.Guha S, Ghosal S, Chattopadhyay U. Antitumor, immunomodulatory and anti-HIV effect of mangiferin, a naturally occurring glucosylxanthone. Chemotherapy. 1996;42:443–51. doi: 10.1159/000239478. [DOI] [PubMed] [Google Scholar]
- 16.Naoki Y, Kengo M, Masaki K, Yasuhiro Y, Toshiya K, Zheng Q, et al. The inhibitory effects of mangiferin, a naturally occurring glucosyl xanthone, in bowel carcinogenesis of male F344 rats. Cancer Lett. 2001;163:163–70. doi: 10.1016/s0304-3835(00)00678-9. [DOI] [PubMed] [Google Scholar]
- 17.Garrido G, González D, Lemus Y, García D, Lodeiro L, Quintero G, et al. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VIMANG) Pharmacol Res. 2004;50:143–9. doi: 10.1016/j.phrs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Hsu MF, Raung SL, Tsao LT, Lin CN, Wang JP. Examination of the inhibitory effect of norathyriol in formylmethionyl-leucyl-phenylalanine-induced respiratory burst in rat neutrophils. Free Radic Biol Med. 1997;23:1035–45. doi: 10.1016/s0891-5849(97)00132-9. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez GM, Re L, Giuliani A, Núñez-Sellés AJ, Davison GP, León-Fernández OS. Protective effects of Mangifera indica L. extract, mangiferin and selected antioxidants against TPA-induced biomolecules oxidation and peritoneal macrophage activation in mice. Pharmacol Res. 2000;42:565–73. doi: 10.1006/phrs.2000.0727. [DOI] [PubMed] [Google Scholar]
- 20.Martínez G, Delgado R, Pérez G, Garrido G, Núñez Sellés AJ, León OS. Evaluation of the in vitro antioxidant activity of Mangifera indica L. extract (Vimang) Phytother Res. 2000;14:424–7. doi: 10.1002/1099-1573(200009)14:6<424::aid-ptr643>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez S, Wolfender JL, Hakizamungu E, Hostettmann K. An antifungal naphthoquinone, xanthones and secoiridoids from Swertia calycina. Planta Med. 1995;61:362–4. doi: 10.1055/s-2006-958102. [DOI] [PubMed] [Google Scholar]
- 22.Bian QY, Luo NC, Xiao PG. Four xanthone glycosides from Swertia calycina Franch. Pharm Pharmacol Commun. 1998;41:597–8. [Google Scholar]
- 23.Yoshimi N, Matsunaga K, Katayama M, Yamada Y, Kuno T, Qiao Z, et al. The inhibitory effects of mangiferin, a naturally occurring glucosylxanthone, in bowel carcinogenesis of male F344 rats. Cancer Lett. 2001;163:163–70. doi: 10.1016/s0304-3835(00)00678-9. [DOI] [PubMed] [Google Scholar]
- 24.Komatsu K, Purusotam B, Yamaji S, Kadota S, Namba T. A comparative study on swertiae herbs from Japan, Nepal and China and their hypoglycemic activities in streptozotocin (STZ)-induced diabetic rats. Nat Med. 1997;51:265–8. [Google Scholar]
- 25.Leiro J, Arranz JA, Yáñez M, Ubeira FM, Sanmartín ML, Orallo F. Expression profiles of genes involved in the mouse nuclear factor-kappa B signal transduction pathway are modulated by mangiferin. Int Immunopharmacol. 2004;4:763–78. doi: 10.1016/j.intimp.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Duan Y, Hu F, Liu J. Lack of altitudinal trends in phytochemical constituents of Swertia franchetiana (Gentianaceae) Biochem Syst Ecol. 2004;32:861–6. [Google Scholar]
- 27.Suryawanshi S, Mehrotra N, Asthana RK, Gupta RC. Liquid chromatography/tandem mass spectrometric study and analysis of xanthone and secoiridoid glycoside composition of Swertia chirata, a potent antidiabetic. Rapid Commun Mass Spectrom. 2006;20:3761–8. doi: 10.1002/rcm.2795. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Wang L, Wang X. Separation and determination of the major active components in Tibetan folk medicinal species Swertia franchetiana by HPLC with DAD. J Liq Chromatogr Relat Technol. 2007;30:1687–96. [Google Scholar]
- 29.Tian L, Chen J, Huang F, Fang J. Simultaneous determination of four active components in Swertia by RP-HPLC. Chin J Nat Med. 2008;6:444–9. [Google Scholar]
- 30.Sun Y, Zhang X, Xue X, Zhang Y, Xiao H, Liang X. Rapid identification of polyphenol C-glycosides from Swertia franchetiana by HPLC-ESI-MS-MS. J Chromatogr Sci. 2009;47:190–6. doi: 10.1093/chromsci/47.3.190. [DOI] [PubMed] [Google Scholar]
- 31.Phoboo S, Pinto MD, Bhowmik PC, Jha PK, Shetty K. Quantification of major phytochemicals of Swertia chirayita, a medicinal plant from Nepal. Ecoprint. 2010;17:59–68. [Google Scholar]
- 32.Pandey DK, Basu S, Jha TB. Screening of different East Himalayan species and populations of Swertia L. based on exomorphology and mangiferin content. Asian Pac J Trop Biomed. 2012;2:S1450–6. [Google Scholar]
- 33.Kumar V, Chauhan RS, Sood H. In vitro production and efficient quantification of major phytopharmaceuticals in an endangered medicinal herb, Swertia chirata. Int J Biotechnol Bioeng Res. 2013;4:495–506. [Google Scholar]
- 34.Nangare A, Mendhulkar V. Mangiferin quantification in some Swertia species collected from south-west zone of Maharashtra. Int J Bioassays. 2014;3:3488–91. [Google Scholar]
- 35.Deshmukh MH, Pai SR, Nimbalkar MS, Patil RP. Biochemical characterization of banana cultivars from Southern India. Int J Fruit Sci. 2009;9:305–22. [Google Scholar]
- 36.Wohlmuth H, Savage K, Dowell A, Mouatt P. Adulteration of Ginkgo biloba products and a simple method to improve its detection. Phytomedicine. 2014;21:912–8. doi: 10.1016/j.phymed.2014.01.010. [DOI] [PubMed] [Google Scholar]