Abstract
Background:
Protium heptaphyllum (Aubl.) March is popularly used as an analgesic and anti-inflammatory agent.
Objective:
This study aimed to evaluate the chemical composition of P. heptaphyllum essential oil, its cytotoxicity in a breast cancer cell line (MCF-7), antimicrobial activity, and its antimutagenicity in vivo.
Materials and Methods:
The chemical composition of the essential oil collected in three 3 years was determined by gas chromatography-mass spectrometry. The cytotoxicity was evaluated using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Annexin V conjugated with fluorescein isothiocyanate, caspase-3, and tumor necrosis factor-alpha (TNF-α) assays were performed to evaluate apoptosis and inflammatory events. The antimutagenic activity at doses of 25, 50, and 100 mg/kg was determined using a micronucleus test in murine bone marrow.
Results:
The essential oil showed a predominance of monoterpene compounds, being the terpinolene, p-cymene-8-ol, and p-cymene, present in the essential oil extracted in the 3 years. The essential oil showed a protection against cyclophosphamide-induced genotoxicity, and the cytotoxicity index polychromatic erythrocytes/normochromatic erythrocytes ratio in animals treated with oil at all doses (1.34 ± 0.33; 1.15 ± 0.1; 1.11 ± 0.13) did not differ from the negative control animal (1.31 ± 0.33), but from the cyclophosphamide group (0.61 ± 0.12). Cytotoxicity, at a concentration of 40.0 μg/mL, and antimicrobial activity were not observed for the essential oil (minimum inhibitory concentration ≥0.5 mg/mL). The essential oil did not change the levels of caspase-3 in the TNF-α level.
Conclusion:
The essential oil showed antimutagenic activity due to its chemical composition.
SUMMARY
Terpinolene, p-cymene-8-ol, and p-cymene are the main constituents of the essential oil of P. heptaphyllum collected within 3-years
The essential oil of P. heptaphyllum did not show antimicrobial activity (MIC >0.5 mg/mL) against E. coli, S. aureus, E. faecalis, and C. albicans
The essential oil of P. heptaphyllum has activity against S. mutans (MIC = 0.5 mg/mL)
The essential oil showed a protection against cyclophosphamide-induced genotoxicity in the micronuclei assay.
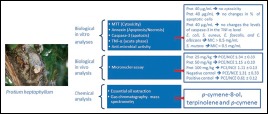
Abbreviations used: GC–MS: Gas Chromatography–Mass Spectrometry, MTT: 3-(4,5dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Annexin V-FITC: Annexin V conjugated with fluorescein isothiocyanate, TNF-α: Tumor necrosis factor alpha, MIC: Minimum Inhibitory Concentration
Keywords: Antimutagenicity, cytotoxicity, essential oil, micronuclei assay, Protium heptaphyllum
INTRODUCTION
Protium heptaphyllum (Aubl.) March, Burseraceae, known as almécega, breu, and almíscar, originated in South America.[1] This species, as well as other members of the family Burseraceae, exudes an oily resin, called breu-branco, which is obtained from the trunk. The resin is chemically composed of a mixture of triterpenes from the α-amyrin (ursane) and β-amyrin (oleane) series and an essential oil rich in mono- and sesquiterpenes.[2,3,4,5]
The essential oil from the P. heptaphyllum resin has received special attention because of its chemical composition and possible pharmacological activities.[6,7,8,9,10,11,12,13,14,15,16,17,18] The anti-inflammatory effect of the mixture of α- and β-amyrin and the essential oil was described in several studies.[6,13,15]
Considering the anti-inflammatory activity of P. heptaphyllum essential oil and resin, the present study aimed to evaluate the chemical composition, cytotoxicity, and pro-apoptotic capability of the essential oil, on a mammary adenocarcinoma cell line (MCF-7), and its in vivo antimutagenic activity.
MATERIALS AND METHODS
Plant material
The resin of the stem of the species of P. heptaphyllum (Aubl.) March was collected in March 2009, May 2011, and August 2013 on Ilha de Guriri, Espírito Santo. A stem was deposited in the herbarium of the University of Vila Velha (UVV/ES 1802) and identified by botanist Solange Zanotti Schneider. In this study, we used the sample collected in 2011 for the biological assays.
Essential oil extraction
The essential oil extraction was performed as described previously[19,20] with modifications. Deionized water (1.5 L) was added to 250 g of the resin, and the extraction was performed over 2 h. The hydrolate obtained was allowed to stand in the dark at 4°C, and the oil was separated by cryogenic separation. The oily fraction was filtered over anhydrous magnesium sulfate and then stored in an amber vial at 4°C. The yield of the extraction was determined by the proportional relationship between the weight of the resin and the weight of the oil that was obtained.
Gas chromatography coupled to mass spectrometry
The analysis of the chemical constituents of the essential oil of the resin of P. heptaphyllum was performed using a gas chromatograph (Trace Ultra, Thermo Scientific®) coupled to a mass spectrometer (DSQII, Thermo Scientific®) as previously described.[21]
The identification of the substances contained in the oil and resin was performed by comparing the similarity of the obtained mass spectra obtained with those in the literature.[22,23] The relative percentages of these compounds were calculated from the mean areas of the chromatograms.
Cell line
A mammary adenocarcinoma (MCF-7, ATCC-HTB22™) cell line was used and maintained in Dulbecco's modified Eagle's medium (DMEM) culture medium (Sigma-Aldrich, St. Louis, MO) supplemented with a 10 mL solution of penicillin G, streptomycin, and l-glutamine (Sigma-Aldrich, St. Louis, MO) and 20% fetal bovine serum (Gibco, Invitrogen Corporation, Grand Island, NY).
Preparation of samples for assays
The test sample used in the biological assays was the essential oil of the resin (O) dissolved in phosphate-buffered saline (PBS), dimethyl sulfoxide (DMSO) (0.09%), and propylene glycol (1%). The final concentration of dichloromethane in the assay was <0.003%.
Cellular cytotoxicity assay with colorimetric method of 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
Cytotoxicity was determined using the colorimetric 3- (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method (Sigma-Aldrich, St. Louis, MO), in which the tetrazolium salt is converted into the formazan salt by living cells, forming a blue color.[24]
MCF-7 tumor cells were plated in sterile 96-well plates at a concentration of 5 × 104 cells/mL. Then, 10 µL of essential oil, final concentration of 1.71–40.0 µg/mL, were added. The microplate was incubated at 37°C in 5% CO2 for 72 h. Doxorubicin (DOX) (Sigma-Aldrich, St. Louis, MO) was used as a positive control at a final concentration of 0.9 μM.[25] Then, 10 µL of MTT solution (5 mg/mL) diluted in DMEM was added, and the plate was incubated for 4 h. Finally, we added 150 µL of isopropanol acidified with 0.04 M HCl and evaluated the samples with an ELISA reader (Thermoplate TP-Reader at 570 nm). The analyses were performed in triplicate, and the percentage of inhibition was calculated using the formula below:
% inhibition = (absorbance of negative-control well) × 100 negative control.
Determination of apoptosis by flow cytometry analysis
Apoptosis was carried out using an annexin V-FITC Kit (Sigma Aldrich, St. Louis, MO).[21] Briefly, MCF-7 cells were plated in a sterile 24-well plate at a concentration of 5 × 106 cells/mL. Then, 10 µL of the oil essential sample (final concentration of 40 µg/mL) was added. A solution of hydrogen peroxide (Sigma-Aldrich, St. Louis, MO) (final concentration of 10 µM) was used as the positive control. The plate was incubated at 37°C in 5% CO2 for 72 h. After this period, the cells were colored with fluorescein isothiocyanate-conjugated annexin V and propidium iodide for 15 min at room temperature, protected from light, and then analyzed by flow cytometry. The percentage of positive cells determined over 10,000 acquired events was analyzed by a FACSCalibur system equipped with a 488 nm argon laser and FCS Express 4 Flow Cytometry software (De Novo Software, Los Angeles, CA).[21]
Determination of caspase-3 for colorimetric assay
The caspase-3 activity was determined using a colorimetric method in which the presence of caspase-3 lysate produces p-nitroaniline, which generates a yellow color.[26] MCF-7 cells were plated in a sterile 24-well plate at a concentration of 1 × 105 cells/mL. Then, 10 µL of essential oil (final concentration of 40 µg/mL) was added. As a positive control, we used DOX (final concentration of 0.9 µM). The plate was incubated at 37°C in 5% CO2 for 72 h. After this period, the determination of caspase-3 was performed according to the manufacturer's specification (Sigma-Aldrich, St. Louis, MO) using an ELISA reader (TP-Thermoplate Reader at 405 nm). The analyses were the average of 8 replicates, and the results were expressed in ΔmOD405 nm/min.[26]
Determination of tumor necrosis factor-alpha
To quantify the levels of tumor necrosis factor-alpha (TNF-α), MCF-7 tumor cells were plated in a sterile 24-well plate at a concentration of 1 × 106 cells/mL.[21] Then, 10 µL of sample of essential oil (final concentration 40 µg/mL) was added, and the plate was incubated at 37°C in 5% CO2 for 72 h. As a positive control, we used DOX (final concentration of 0.9 µM). After this period, we proceeded to read the plate in an ELISA reader (TP-Thermoplate Reader at 450 nm) using a commercial kit (Invitrogen, San Jose, USA) according to the manufacturer's instructions. Analyses were performed in replicates of eight.[21]
Determination of minimum inhibitory concentration assay
The determination of minimum inhibitory concentration (MIC) was carried out in 96-well culture plates, according to the rules of CLSI.[27] The microorganisms used were Candida albicans (ATCC 10231™), Staphylococcus aureus (ATCC™ 25923), Enterococcus faecalis (ATCC 29212™), Escherichia coli (ATCC 25922™), and Streptococcus mutans (ATCC 25175™). The final concentration of the cells was adjusted in the range of 104 CFU/mL by the McFarland scale. To each well of the plate, except for the blank used, Hilton Muller culture medium was added, before adding the samples or positive or negative controls. The intermediate concentrations were prepared by diluting a stock solution of 10 mg/mL in DMSO and further diluted in PBS solution, resulting in final concentrations from 0.5 to 0.03125 mg/mL. In all plates, positive controls and negative controls were included, with hydrogen peroxide and hypochlorite as negative controls. At the end of the preparation, the plates were incubated at 36°C for 24 h and subsequently added to the indicator 50 µL of triphenyltetrazolium chloride (TTC) (0.5% aqueous solution) chloride. After 4 h of incubation, the MIC was determined as the lowest concentration capable of inhibiting visible growth of cells conferred by TTC (nonstained cells are dead). All tests were performed in triplicate.
Antimutagenic assay
The micronucleus assay was conducted in male and female Swiss mice 3 months of age weighing between 20 and 40 g. The animals were conditioned in cages with controlled temperature and humidity, a 12 h light–dark cycle, and ad libitum access to water and food. The mice were provided by the Vivarium of Complexo Biopráticas of University Vila Velha. The animals were divided into five groups, with equal numbers of males and females in each group. The negative control group (CN, n = 6) received the vehicle (peanut oil) orally and the positive control group (CP, n = 6) received cyclophosphamide (45 mg/kg) intraperitoneally 24 h before euthanasia. The groups treated with the P. heptaphyllum essential oil were Protium 25 (PROT25, n = 6), Protium 50 (PROT50, n = 6), and Protium 100 (PROT100, n = 6). These groups orally received the P. heptaphyllum essential oil diluted in a vehicle at doses of 25, 50, and 100 mg/kg, respectively, twice daily for 3 days. The penultimate dose was applied simultaneously with cyclophosphamide 24 h before euthanasia. The micronucleus test developed initially by Schmid,[28] and all assays, including collection of the bone marrow specimens to preparing the slides, were run as described by Ribeiro et al.,[29] following the guidelines recommended by the Guideline for the Testing of Chemicals (OECD).[30] Also, this evaluation was performed by a biologist blind to the experimental groups. The results were presented as the frequency of polychromatic erythrocytes-micro nucleated (PCE-MN) and cytotoxicity index PCE/normochromatic erythrocytes (NCE) ratio. The protocol was approved by the Ethics Committee and Bioethics and Animal Welfare UVV (Protocol No. 116/2010).
Statistical analysis
The results were expressed as the mean plus or minus the standard error of the mean and antimutagenic for the test, ±the standard deviation. They were analyzed by one-way ANOVA. The significance of the difference between the means was determined using the post-hoc Tukey method, adjusted for multiple comparisons with significance above 5% (P < 0.05).
RESULTS
The essential oil obtained from the resin of P. heptaphyllum showed a yellowish color and a refreshing woody odor. The yield (g/g) of the extraction process of essential oils was 14.4%, 10.2%, and 10.7% for the years 2009, 2011, and 2013, respectively, being higher than the 10.0% (w/w) reported.[6]
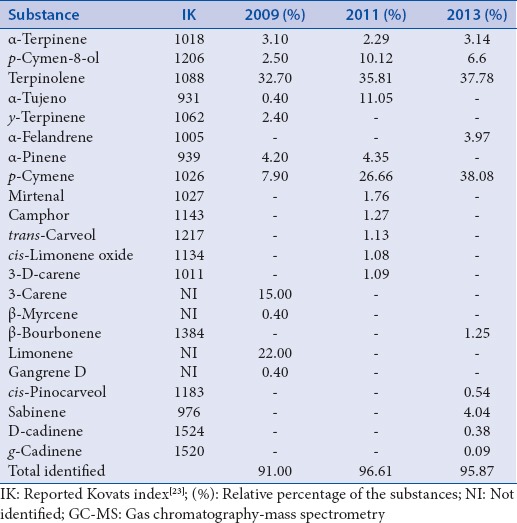
The chemical constitution of the oil of P. heptaphyllum collected within 3 years was characterized by a large number of monoterpenes [Table 1]. For essential oil extracted in 2009, the major constituents were 3-carene, limonene, and terpinolene. In 2011, the main constituents identified were p-cymene-8-ol, the α-tujeno, p-cymene, and terpinolene [Table 1]. To the oil extracted in 2013, the main constituents were p-cymene-8-ol, terpinolene, and p-cymene, the ratio of 1:6:6.
Table 1.
Chemical composition of the oil of Protium heptaphyllum obtained in the years 2009, 2011, and 2013 analyzed by GC–MS
The essential oil of P. heptaphyllum did not show antimicrobial activity (MIC > 0.5 mg/mL) against E. coli, S. aureus, E. faecalis, and C. albicans, but showed a weak activity against S. mutans (MIC = 0.5 mg/mL).
In the assay for cell viability using MTT, the P. heptaphyllum essential oil did not exhibit significant cytotoxicity against MCF-7 cancer cells at a concentration of 40.0 µg/mL.
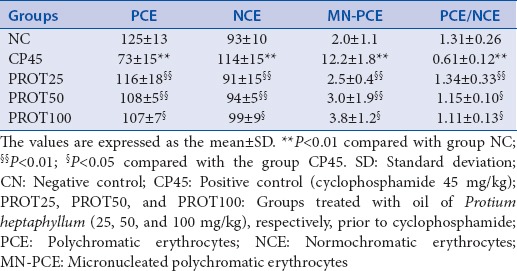
The antimutagenic activity of the essential oil of the P. heptaphyllum resin was evaluated using a micronucleus test. The micronucleus test revealed a reduction in MN-PCE and the normal cytotoxicity index PCE/NCE ratio in animals treated with oil at all doses [Table 2]. The essential oil of the P. heptaphyllum resin did not induce cytotoxicity or caspase-3 activity or TNF-α and it did not induce apoptosis in MCF-7 cells. However, it showed antimutagenic activity [Table 2]. These data suggest that this essential oil could be used as a chemo-preventive agent for cancer.[31]
Table 2.
Numbers of PCE and NCE in 200 cells and MN-PCE in 2000 PCEs and the cytotoxicity index (PCE/NCE) ratio from the bone marrow of mice
DISCUSSION
The phytochemical analysis results agree with other studies that have evaluated the chemical composition of the essential oil from the resin of P. heptaphyllum in different localities, such as Manaus, Maranhão, and Acre; the prevalence of monoterpenes was also observed in these studies.[6,7,32]
In this study, the essential oil of P. heptaphyllum did not show a pronounced antimicrobial activity against the microorganisms selected for this study. In another study, the essential oil of P. heptaphyllum showed moderate antimicrobial activity against several microbial strains.[9] However, the use of resin extract produced by different solvents showed activity against C. albicans (MIC values: 1000 μg/ml), E. coli (MIC values: 1000 μg/ml), and S. aureus 125 (MIC values: µg/mL for E. coli).[33] The results obtained in the cellular cytotoxicity assay with colorimetric method of the MTT showed that the essential oil was not effective against the cancer cells at a concentration of 40.0 µg/mL, in agreement with the results of a study carried out with the crude resin of P. heptaphyllum[21] with the same concentration against MCF-7 tumor cells.
About the antimutagenic activity of the essential oil of the P. heptaphyllum resin, other studies have also used the micronucleus test to confirm the potential antimutagenic effect of natural products,[34,35,36] because this assay can detect damage to chromosomes and the mitotic machinery.[33] The results obtained in this assay indicate that the oil induced protection against cyclophosphamide-induced genotoxicity, suggesting that P. heptaphyllum has some potential antimutagenic activity.
The anti-oxidant activity of the P. heptaphyllum essential oil using a 2,2-diphenyl-1-picrylhydrazyl in vitro assay was previously verified.[9] It was also observed a prominent antioxidant activity using an in vivo enzymatic activity assay in mice with ethanol-induced gastric ulcers treated with the oil at doses of 12.5–100.0 mg/kg.[8] This result was verified by the increase in the activity of antioxidant enzymes, such as glutathione, glutathione reductase, glutathione peroxidase, and superoxide dismutase and a decrease in lipid peroxidation after exposure,[6] both of which reduced oxidative stress. The antioxidant activity could potentially explain the antimutagenic activity of the essential oil in the present study because the generation of reactive oxygen species and oxidative stress plays a critical role in DNA and chromosome damage.[37,38,39]
The antimutagenic activity observed in this study is possibly a result of the presence of monoterpenes contained in the oil which were observed in the chemical analysis.
CONCLUSION
The possible chemopreventive activity of the essential oil of the P. heptaphyllum resin, most likely through the action of monoterpenes, was indicated by the antimutagenic effect and the absence of cytotoxic and pro-apoptotic effects. Further studies, however, are required for a detailed evaluation of the mechanism.
Financial support and sponsorship
The work was supported by program Project Grant 472171/2013-5 awarded by CNPq/Brazil (Conselho Nacional de Desenvolvimento Científico e Tecnológico. The Fundação de Amparo à Pesquisa do Espírito Santo – FAPES is acknowledged for the master fellowship program.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Denise Coutinho Endringer
Dr. Denise Coutinho Endringer, is a Professor at the University Vila Velha (UVV) and the Federal Institute of Espirito Santo. She is the coordinator of the Graduation Program of Pharmaceutical Sciences at UVV. Her area of expertise focuses on pharmacognosy, performing phytochemicals biomonitoring studies, in vitro evaluation of biological activities of natural products, mainly in vitro inhibition of ECA, COX 1, COX 2, and aromatase (CYP19). She graduated from the Federal University of Espirito Santo and defended both, her Master and her PhD, at the Federal University of Minas Gerais. Her PhD was a cooperation between UFMG and the Purdue University, West Lafayette, IN, USA.
Acknowledgment
The work was supported by program Project Grant 472171/2013-5 awarded by CNPq/Brazil (Conselho Nacional de Desenvolvimento Científico e Tecnológico). The Fundação de Amparo à Pesquisa do Espírito Santo – FAPES is acknowledged for the master fellowship program. We also acknowledge the University of Vila Velha – UVV for providing the knowledge and structure necessary to accomplish this work.
REFERENCES
- 1.Corrêa MP, Pena MA. Rio de Janeiro: Ministeério da Agricultura, Instituto Brasileiro de Desenvolvimento Florestal; 1984. Dicionário das Plantas Úteis do Brasil e das Exóticas Cultivadas; p. 82. [Google Scholar]
- 2.Pernet R. Phytochimiedes Burseraceae. Lloydia. 1972;35:280–7. [Google Scholar]
- 3.Bandeira PN, Machado MI, Cavalcante FS, Lemos TL. Essential oil composition of leaves, fruits and resin of Protium heptaphyllum (Aubl.) March. J Essent Oil Res. 2001;13:33–4. [Google Scholar]
- 4.Siani AC, Ramos MF, Guimarães AC, Susunaga GS, Zoghbi MG. Volatile constituents from oleoresin of Protium heptaphyllum (Aubl.) March. J Essent Oil Res. 1999;11:72–4. [Google Scholar]
- 5.Maia RM, Barbosa PR, Cruz FG, Roque NF, Fascio M. Triterpenes from the resin of Protium heptaphyllum March (Burseraceae): characterization in binary mixtures. Quím Nova. 2000;23:623–6. [Google Scholar]
- 6.Siani AC, Ramos MF, Menezes-de-Lima O, Jr, Ribeiro-dos-Santos R, Fernadez-Ferreira E, Soares RO, et al. Evaluation of anti-inflammatory-related activity of essential oils from the leaves and resin of species of Protium. J Ethnopharmacol. 1999;66:57–69. doi: 10.1016/s0378-8741(98)00148-2. [DOI] [PubMed] [Google Scholar]
- 7.Rao VS, Maia JL, Oliveira FA, Lemos TL, Chaves MH, Santos FA. Composition and anti-nociceptive activity of the essential oil from Protium heptaphyllum Resin. Nat Prod Commun. 2007;2:1199–202. [Google Scholar]
- 8.Araujo DA, Takayama C, Faria-de FM, Socca EA, Dunder RJ, Manzo LP, et al. Gastroprotective effects of essential oil from Protium heptaphyllum on experimental gastric ulcer models in rats. Braz J Pharmacogn. 2011;21:721–9. [Google Scholar]
- 9.Bandeira PN, Fonseca AM, Costa SM, Lins MU, Pessoa OD, Monte FJ, et al. Anti-bacterial and anti-oxidant activities of the essential oil of resin of Protium heptaphyllum. Nat Prod Commun. 2006;1:117–20. [Google Scholar]
- 10.Holanda Pinto SA, Pinto LM, Cunha GM, Chaves MH, Santos FA, Rao VS. Anti-inflammatory effect of alpha, beta-Amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacology. 2008;16:48–52. doi: 10.1007/s10787-007-1609-x. [DOI] [PubMed] [Google Scholar]
- 11.Holanda Pinto SA, Pinto LM, Guedes MA, Cunha GM, Chaves MH, Santos FA, et al. Antinoceptive effect of triterpenoid alpha, beta-amyrin in rats on orofacial pain induced by formalin and capsaicin. Phytomedicine. 2008;15:630–4. doi: 10.1016/j.phymed.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Aragão GF, Carneiro LM, Junior AP, Vieira LC, Bandeira PN, Lemos TL, et al. A possible mechanism for anxiolytic and antidepressant effects of alpha- and beta-amyrin from Protium heptaphyllum (Aubl.) March. Pharmacol Biochem Behav. 2006;85:827–34. doi: 10.1016/j.pbb.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Aragão GF, Cunha Pinheiro MC, Nogueira Bandeira P, Gomes Lemos TL, de Barros Viana GS. Analgesic and anti-inflammatory activities of the isomeric mixture of alpha- and beta-amyrin from Protium heptaphyllum (Aubl.) March. J Herb Pharmacother. 2007;7:31–47. doi: 10.1300/j157v07n02_03. [DOI] [PubMed] [Google Scholar]
- 14.Lima-Júnior RC, Oliveira FA, Gurgel LA, Cavalcante IJ, Santos KA, Campos DA, et al. Attenuation of visceral nociception by alpha- and beta-amyrin, a triterpenoid mixture isolated from the resin of Protium heptaphyllum, in mice. Planta Med. 2006;72:34–9. doi: 10.1055/s-2005-873150. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira FA, Vieira-Júnior GM, Chaves MH, Almeida FR, Florêncio MG, Lima RC, Jr, et al. Gastroprotective and anti-inflammatory effects of resin from Protium heptaphyllum in mice and rats. Pharmacol Res. 2004;49:105–11. doi: 10.1016/j.phrs.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira FA, Lima-Junior RC, Cordeiro WM, Vieira-Júnior GM, Chaves MH, Almeida FR, et al. Pentacyclic triterpenoids, alpha, beta-amyrins, suppress the scratching behavior in a mouse model of pruritus. Pharmacol Biochem Behav. 2004;78:719–25. doi: 10.1016/j.pbb.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira FA, Chaves MH, Almeida FR, Lima RC, Jr, Silva RM, Maia JL, et al. Protective effect of alpha- and beta-amyrin, a triterpene mixture from Protium heptaphyllum (Aubl.) March. trunk wood resin, against acetaminophen-induced liver injury in mice. J Ethnopharmacol. 2005;98:103–8. doi: 10.1016/j.jep.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira FA, Costa CL, Chaves MH, Almeida FR, Cavalcante IJ, Lima AF, et al. Attenuation of capsaicin-induced acute and visceral nociceptive pain by alpha- and beta-amyrin, a triterpene mixture isolated from Protium heptaphyllum resin in mice. Life Sci. 2005;77:2942–52. doi: 10.1016/j.lfs.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 19.Skrubis BG. The drying of laurel leaves. Perfumer Flavorist. 1982;7:37–40. [Google Scholar]
- 20.Ming L, Figueiredo R, Machado S, Andrade R. Yield of essential oil of and citral content in different parts of lemongrass leaves (Cymbopogon citratus (DC.) Stapf.) Poaceae. Proceedings of the International Symposium on Medicinal and Aromatic Plants. Acta Hortic. 1996;1:555–9. [Google Scholar]
- 21.Lima EM, Nascimento AM, Lenz D, Scherer R, Meyrelles SS, Boëchat GA, et al. Triterpenes from the Protium heptaphyllum resin – Chemical composition and cytotoxicity. Rev Bras Farmacognosia. 2004;24:399–407. [Google Scholar]
- 22.NIST Mass Spectral Program, NIST/EPA/NIH 2005. National Institute of Standard Tests/United Stated Environment Protection Agency/National Institute of Health. Mass Spectral Library with Search Program. Data Version: NIST 05, Software Version 2.0d [Google Scholar]
- 23.Adams RP. Carol Stream: Allured Publishing Corporation; 2001. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; p. 469. [Google Scholar]
- 24.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 25.Zheng J, Liu Q, Yang J, Ren Q, Cao W, Yang J, et al. Co-culture of apoptotic breast cancer cells with immature dendritic cells: A novel approach for DC-based vaccination in breast cancer. Braz J Med Biol Res. 2012;45:510–5. doi: 10.1590/S0100-879X2012007500061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Posmantur R, Wang KK, Gilbertsen RB. Caspase-3-like activity is necessary for IL-2 release in activated Jurkat T-cells. Exp Cell Res. 1998;244:302–9. doi: 10.1006/excr.1998.4214. [DOI] [PubMed] [Google Scholar]
- 27.Wayne (USA): Clinical and Laboratory Standards Institute; 2007. Clinical and Laboratory Standards Institute. Performance standards for anti-microbial susceptibility testing; Seventeenth informational supplement. [Google Scholar]
- 28.Schmid W. The micronucleus test. Mutat Res. 1975;31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro LR, Salvatori DM, Marques EK. Mutagênese ambiental. Teste do Micronúcleoem Medula Óssea de roedores in vivo. In: Ribeiro LR, Salvatori DM, editors. Mutagênese Ambiental. Canoas: Ulbra; 2003. pp. 173–200. [Google Scholar]
- 30.OECD. Guideline for the Testing of Chemicals. Mammalian Erythocyte Micronucleous Test. Organization for Ecomomic Cooperation and Development, Paris, July, 1997 Guideline 474 p 1-10 [Google Scholar]
- 31.Sporn MB, Suh N. Chemoprevention of cancer. Carcinogenesis. 2000;21:525–30. doi: 10.1093/carcin/21.3.525. [DOI] [PubMed] [Google Scholar]
- 32.Marques DD, Sartori RA, Lemos TL, Machado LL, Souza JS, Monte FJ. Chemical composition of the essential oils from two subspecies of Protium heptaphyllum. Acta Amazon. 2010;40:227–30. [Google Scholar]
- 33.Violante IM, Hamerski L, Garcez WS, Batista AL, Chang MR, Pott VJ, et al. Antimicrobial activity of some medicinal plants from the cerrado of the centralwestern region of Brazil. Braz J Microbiol. 2012;43:1302–8. doi: 10.1590/S1517-83822012000400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aparecida Resende F, de Andrade Barcala CA, da Silva Faria MC, Kato FH, Cunha WR, Tavares DC. Antimutagenicity of ursolic acid and oleanolic acid against doxorubicin-induced clastogenesis in Balb/c mice. Life Sci. 2006;79:1268–73. doi: 10.1016/j.lfs.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 35.Kalil IC, Gibson BA, Ribeiro CA, Benincá LS, Brasil GA, Andrade TU, et al. Anti-mutagenic activity of Carica papaya L. Assayed in vivo by micronucleus test. Rev Ciênc Farm Básica Apl. 2011;32:419–23. [Google Scholar]
- 36.Vinod V, Tiwari PK, Meshram GP. Evaluation of mutagenic and antimutagenic activities of neem (Azadirachta indica) seed oil in the in vitro Ames Salmonella/microsome assay and in vivo mouse bone marrow micronucleus test. J Ethnopharmacol. 2011;134:931–7. doi: 10.1016/j.jep.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Soto-Reyes E, Del Razo LM, Valverde M, Rojas E. Role of the alkali labile sites, reactive oxygen species and antioxidants in DNA damage induced by methylated trivalent metabolites of inorganic arsenic. Biometals. 2005;18:493–506. doi: 10.1007/s10534-005-0858-7. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed MK, Habibullah-Al-Mamun M, Hossain MA, Arif M, Parvin E, Akter MS, et al. Assessing the genotoxic potentials of arsenic in tilapia (Oreochromis mossambicus) using alkaline comet assay and micronucleus test. Chemosphere. 2011;84:143–9. doi: 10.1016/j.chemosphere.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 39.Shao B, Zhu L, Dong M, Wang J, Wang J, Xie H, et al. DNA damage and oxidative stress induced by endosulfan exposure in zebrafish (Danio rerio) Ecotoxicology. 2012;21:1533–40. doi: 10.1007/s10646-012-0907-2. [DOI] [PubMed] [Google Scholar]


