Abstract
Background:
Salsola imbricata Forssk. is a shrub widely growing in Egypt, used as a camel food, traditionally, used as anti-inflammatory agent. Literature survey showed no report about the anti-inflammatory activity of S. imbricata.
Aim of the Study:
This work was designed to study the phenolic constituents and to provide evidence for the traditional use of S. imbricata as an anti-inflammatory agent.
Materials and Methods:
The in vitro anti-inflammatory activity of the total aqueous methanol extract and some isolated compounds were investigated in RAW 264.7 macrophage cells using nitric oxide assay. All chemical structures were identified on the basis of electrospray ionization-mass spectrometry, one- and two-dimension nuclear magnetic resonance.
Results:
Nine phenolic compounds, among them two new natural products; isorhamnetin-3-O-β-D-glucuronyl (1’’’→4’’) glucuronide (1) and its dimethyl ester; isorhamnetin-3-O-β-D-di glucuronate dimethyl ester (2), two isorhamnetin glycosides: Isorhamnetin-3-O-β-D-galactopyranoside (3), isorhamnetin-3-O-β-D-glucopyranoside (4), and isorhamnetin (5). In addition, an alkaloidal phenolic; trans N-feruloyl tyramine (6), three phenolic acids: Isovanillic acid (7), ferulic acid (8), and p-hydroxy benzoic acid (9) were isolated from salsola imbricata leaves. All compounds were isolated and identified for the first time from this plant except compound (6). The extract and the tested compounds showed distintict anti-inflammatory activities with no toxicity on RAW 264.7 macrophage cells.
Conclusion:
The extract and the tested compounds showed distintict anti-inflammatory activities with no toxicity on RAW 264.7 macrophage cells.
SUMMARY
Investigation of the chemical constituents of the leaves of Salsola imbricata led to isolation of two new isorhamnetin derivatives: isorhamnetin.3-O-β-D.glucuronyl (1’“→”) glucuronide (1) and its dimethyl ester (2), together with seven known phenolic compounds. The extract and the tested compounds showed distintict anti-inflammatory activities with no toxicity on RAW 264.7 macrophage cells.
Abbreviations used: PC: Paper chromatography, MPLC : Medium Pressure Liquid Chromatography, HMBC: Heteronuclear multiple bond correlation, HMQC: Heteronuclear single quantum correlation, NMR: Nuclear magnetic resonance
Keywords: Amaranthaceae, anti-inflammatory, isorhamnetin-3-O-β-D-glucuronyl (1′′′→4′′) glucuronide, plant phenolics, Salsola imbricata
INTRODUCTION
The Amaranthaceae (formerly Chenopodiaceae) is a large family that contains approximately 175 genera and 2000 species, including the genus Salsola (from Latin salsus, meaning salty).[1] Salsola imbricata Forssk. (syn. Chenopodium baryosmum, Salsola foetida, Caroxylon imbricata, and Salsola baryosma) is a shrub wildly growing in Egypt, used as a camel food.[2,3] Traditionally, S. imbricata is used as a diuretic and anti-inflammatory agent.[4] It has also been reported to possess antioxidant[5] and antidiabetic activity;[6] in addition, it inhibits tyrosinase[7,8] and leads to central nervous system depression.[9] S. imbricata was reported to contain triterpene glycoside derivatives,[10] triterpenes,[5] isoflavonoids, flavonoids, coumarins,[8,11] alkaloidal phenolics,[7,12] and sterols.[13]
Inflammation is a normal protective response induced by tissue injury or infection to combat invaders in the body (microorganisms and nonself-cells) and to remove dead or damaged host cells. The level of nitric oxide (NO) induced may reflect the degree of inflammation. Recently, some plant secondary metabolites have been reported to inhibit NO production such as 6-gingerol, tanshinone IIA, and arctigenin.[14] Most of the traditionally known biological activities are not supported by experimental or clinical data. In this context, this work was designed to study the phenolic constituents and to provide evidence for the traditional use of S. imbricata as an anti-inflammatory agent.
MATERIALS AND METHODS
General methods
1H-nuclear magnetic resonance (NMR) spectra were measured with a Bruker avance 400 MHz NMR spectrometer. 1H chemical shifts (δ) were measured in ppm, relative to TMS and 13C-NMR chemical shifts to dimethyl sulfoxide-d6 and converted to the TMS scale by adding 39.5. Electrospray ionization-mass spectrometry (ESI-MS) were measured on an AB SCIEX API 3200QTRAP liquid chromatography (LC)/MS/MS System, ultraviolet (UV) recordings were made on a Shimadzu UV-Visible-1601 spectrophotometer. Paper chromatographic (PC) analysis and preparative PC separation were carried out on Whatman No. 1 and 3 MM papers, using solvent systems 15% HOAc and BAW (n-BuOH-HOAc-H2O, 4:1:5, upper layer). Medium pressure LC (MPLC) was performed on Sepaore ×50 (Buchi Labortechnik, swissland).
Plant materials
S. imbricata leaves were collected in the Western desert near Baharia Oasis (Egypt) in April 2012. Authentication was performed by Dr. M. El-Gebali, former researcher of Botany at the National Research Centre (NRC) of Cairo, Egypt. In addition, a voucher specimen was deposited at the Herbarium of the NRC.
Extraction and isolation
One thousand grams air-dried powdered leaves of S. imbricata were extracted successively for three times under reflux in aq. methanol MeOH-H2O (3:1). The filtrates were collected and evaporated to dryness under vacuum to yield a dark brown amorphous powder of the aq. methanol extract (112 g). The residue was suspended in water and partitioned between methylene chloride (for defatting), ethyl acetate, and n-butanol (BuOH), successively. The n-BuOH extract (21 g) was fractionated by column chromatography on Diaion HP-20 column (5 cm × 50 cm, 200 g) using H2O and MeOH in H2O (25:75 v/v, 2 L) as an eluent to give mainly two fractions. The second fraction (1 g) was purified with MPLC using RP-18 column (40 g) eluting with water-MeOH in order of decreasing polarity, yielding two amorphous yellow powder compounds (1,2). The ethyl acetate extract (33 g) was chromatographed on Sephadex LH-20 column (11 cm × 50 cm, 300 g). Elution started with water then water/MeOH mixtures while gradually decreasing the polarity. Collected fractions were monitored using PC. Three major fractions were collected and then applied to further fractionation on Sephadex LH-20 columns. The first fraction (2 g) gave three compounds (3-5), the second fraction (0.5 g) gave one compound 6, and the third one (1.5 g) yielded three compounds (7-9). All the isolated compounds were furtherly purified on Sephadex LH-20 columns using methanol (high-performance LC grade) to give pure compounds. All isolated compounds were identified by ESI-MS, UV,1 H-, and 13C-NMR, heteronuclear multiple-bond correlation (HMBC), and heteronuclear single-quantum correlation (HMQC).
Isorhamnetin-3-O-β-D-glucuronyl-(1’’’→4’’)- β-glucuronic acid (1)
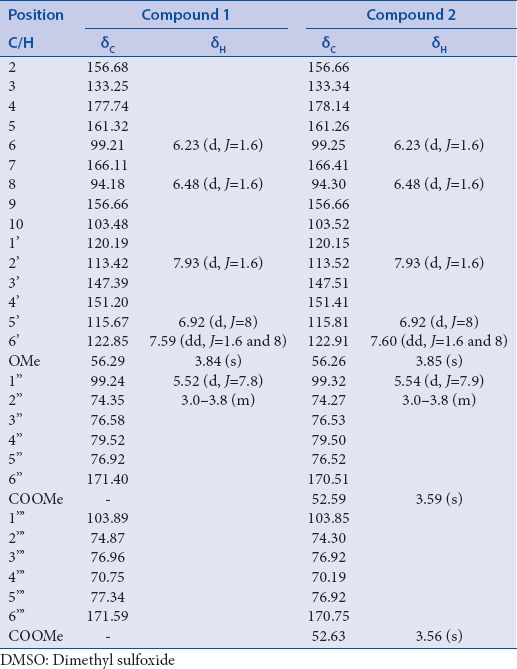
Amorphous yellow powder, Rf - values: 0.92 (HOAc), 0.08 (BAW). UV λmax nm in MeOH: 252, 352; +NaOMe: 268, 326, 405; +NaOAc 269, 322, 360; +NaOAc-H3 BO3 252, 353; AlCl3 264, 298 (sh), 355; and AlCl3+ HCl 273, 363. Normal acid hydrolysis (2N aqueous HCl, 100°C, 2 h) gave glucuronic acid and isorhamnetin. Positive ESI-MS: m/z [M + H]+669 and 317 [M-diglucuronic acid]+. 1H- and 13C-NMR spectra [Table 1].
Table 1.
1H, 13C spectral data of 1 and 2 (in DMSO, in ppm, J in Hz)
Isorhamnetin-3-O-β-D-glucuronate methyl ester (1’’’→4’’)-β-glucuronate methyl ester (2)
Amorphous yellow powder, Rf - values: 0.87 (HOAc), 0.23 (BAW). UV λmax nm in MeOH: 254, 352; +NaOMe: 267, 326, 404; +NaOAc 272, 362; +NaOAc-H3 BO3 253, 354; AlCl3 265, 356; and AlCl3 + HCl 272, 360. Normal acid hydrolysis (2N aqueous HCl, 100°C, 2 h) gave glucuronic acid and isorhamnetin. Positive ESI-MS: m/z [M + H]+697 and 317 [M-diglucuronic dimethyl ester]+. 1H- and 13C-NMR spectra [Table 1].
Biological assays
Cytotoxicity study on macrophages.
Cell culture
Raw murine macrophages (RAW 264.7) were purchased from the American Type Culture collection. Cells were routinely cultured in RPMI-1640. Media were supplemented with 10% fetal bovine serum, 2 mM L-glutamine, containing 100 U/ml penicillin G, 100 U/ml streptomycin, and 250 ng/ml amphotericin B. Cells were maintained in humidified air containing 5% CO2 at 37°C. RAW 264.7 cells were collected by scraping them from the solid support. All experiments were repeated four times, unless mentioned, and the data is represented as a mean ± standard deviation. Cell culture material was obtained from Cambrex, Bio-Science (Copenhagen, Denmark).
Cell viability assay
The viability of RAW 264.7 cells, treated with isolated extracts and compounds, was determined with the 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide cell viability assay.[15]
Estimation of nitric oxide
Raw murine macrophages (RAW 264.7) were seeded in 96-well plates at 0.5 × 105 cells/well for 2 h in RPMI without phenol red. The cells were stimulated with lipopoly saccharide (LPS) at final concentrations of 100 µg/ml. After two extra hours stimulated cells were either treated with 100 µg/ml (safe dose) of samples. Dexamethasone (50 ng/ml) was employed as a potent anti-inflammatory drug. Negative controls included cells left with the LPS alone or left completely untreated. After incubation for 24 h, the supernatants were removed and assessed for NO. Nitrite accumulation was used as an indicator of NO production using a microplate assay based on the Griess reaction. The Griess reaction is a two-step diazotization reaction in which acidified nitrites generate a nitrosating agent that reacts with sulfanilic acid to form diazonium ion. This ion is then coupled to N-(1-naphthyl) ethylenediamine to produce the chromophoric pink azo-derivative that can be determined spectro photometrically at 540 nm.[16]
Procedures
In each well of a flat bottom 96 well-microplate, 40 µl freshly prepared Griess reagent (40 mg/ml deionized water) was mixed with 40 µl cell supernatant or different concentrations of sodium nitrite ranging from 0 to 100 µmol/L. The plate was incubated for 10 min in the dark, and the absorbance of the mixture was determined at 540 nm using the micro plate enzyme-linked immunosorbent assay reader. A standard curve relating NO in µmol/L to the absorbance was constructed from which the NO level in the cell supernatant was computed by interpolation.
Calculation
The NO level of each of the tested cell supernatants was expressed as NO level of the tested cell supernatant ×100/NO level of the control.
RESULTS AND DISCUSSION
Structure elucidation of the new compounds
Compounds 1 and 2 were isolated from the n-butanol extract as an amorphous yellow powder, which appeared to be a flavonol derivatives with a substituted 3-hydroxyl group and free hydroxyl groups at C-5, C-7, and C-4’.[17] This was identified from the UV spectra of both compounds 1 and 2 in the presence of shift reagents. Normal acid hydrolysis of 1 and 2 (2 N aqueous HCl, 100°C, 3 h) gave glucuronic acid (CoPC) and isorhamnetin (CoPC and UV). Positive ESI-MS spectrum showed a molecular ion [M + 1]+ and loss of a dihexanoic acid moiety (isorhamnetin aglycone) was observed at m/z 669 and 317, respectively, for compound 1. Compound 2 showed a molecular ion [M + 1]+ and loss of dihexanoic acid dimethyl ester moieties (isorhamnetin aglycone) was observed at m/z 697 and 317, respectively, thus identifying compounds 1 and 2 to be isorhamnetin-3-O-diglucuronic acid and isorhamnetin-3-O-diglucuronic dimethyl ester, respectively. To determine and confirm the structure of 1 and 2 and the site of attachments,1 H-NMR, 13C-NMR, HMQC, and HMBC spectroscopic analysis were performed. 1H- and 13C-NMR spectra of 1 and 2 [Table 1] were similar to those of isorhamnetin aglycone with substituted 3-OH.[18,19] Substitution at the 3-O position of the isorhamnetin moiety of 1 and 2 was confirmed by 13C-NMR from the up field shit of C-3 up to 2 ppm compared to that of isorhamnetin aglycone together with HMBC correlation between the anomeric proton H-1” at about δ 5.54 (d, J = 7.9) of glucuronic acid and the carbon at about δ ppm 133.25 of C-3[20] [Figure 1]. In 13C-NMR spectrum a large downfield shift of the glucuronic acid C-4” carbon [Table 1] was observed, compared to the corresponding signal of flavanol attached to glucuronic acid at position 3 suggesting an interglycosidic linkage at 4” position of the first glucuronic acid.[21,22] This was also corroborated by the HMBC correlation between the anomeric proton H-1’“ δ ppm 4.70 (d, J = 7. 6) of the terminal glucuronic acid and a carbon δ ppm 79.50 assigned to C-4’’of the initial glucuronic acid.[20] Furthermore, the β-configuration of each glucuronyl moiety was deduced from the large coupling constants (7.6–7.9 Hz) at the anomeric position[23] in the1 H-NMR spectra Table 1 in the1 H-NMR spectra [Table 1]. Therefore, compound 1 was identified as isorhamnetin-3-O-β-D-glucuronyl-(1’“→4”)-β-D-glucuronic acid. The presence of dimethyl group attached to glucuronic acid moiety was confirmed by1 H-NMR; signals at δ ppm 3.56 and 3.52 and 13C-NMR signals at δ 52.63 and 52.59, characteristic for glucuronic acid methyl ester attached to flavonoid moiety,[24,25,26,27] Therefore, compound 2 was identified as isorhamnetin-3-O-β-D-glucuronyl methyl ester-(1’“→4”)-β-D-glucuronic acid methyl ester [Figure 1].
Figure 1.
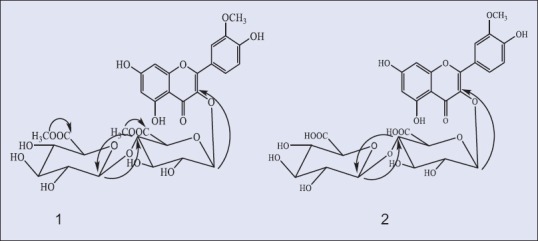
Heteronuclear multiple bond correlation correlations of compounds 1 and 2
Identification of the known compounds
Seven known compounds (3-9) were isolated from ethyl acetate fraction. All compounds were identified by comparing observed data with published one for these compounds. These compounds were identified as isorhamnetin-3-O-β-D-galactopyranoside,[28] isorhamnetin-3-O-β-D-glucopyranoside,[29] isorhamnetin,[30] trans N-feruloyl tyramine,[31] isovanillic acid,[31] (ferulic acid,[32] and p-hydroxy benzoic acid,[33] respectivley.
Cytotoxicity and nitric oxide index
All examined samples did not show cytotoxicity at a concentration of 100 µg/ml [Figure 2]. NO, overproduced by activated macrophages via inducible NO synthase (iNOS), is suggested to be a significant pathogenic factor in various inflammatory tissue injuries.[34] In order to elucidate the anti-inflammatory action of S. imbricata, this study was designed to isolate its active constituents and examine their effects on NO production, detected as nitrite in the culture medium of macrophages induced by LPS through iNOS expression, to reflect the degree of anti-inflammatory activity.
Figure 2.
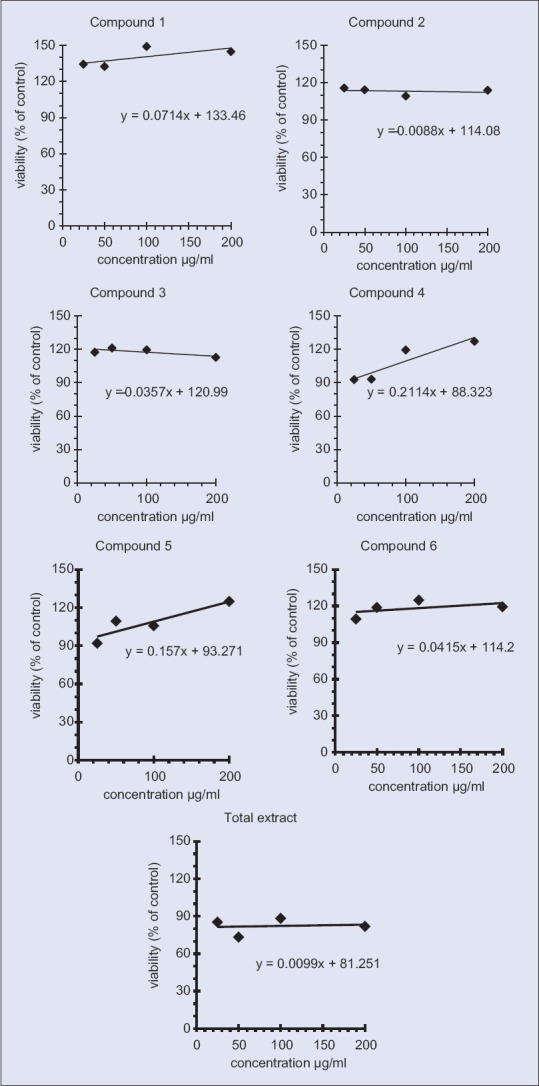
Cytotoxicity of used samples on RAW 264.7 showing dose response curve
The results indicated that the inflammatory (LPS 100 μg/ml) induced NO production up to 1.2 fold of the control, while that the potent anti-inflammatory dexamethasone (50 μg/ml) significantly reduced the levels of NO production compared to that of the LPS [Figure 3].
Figure 3.
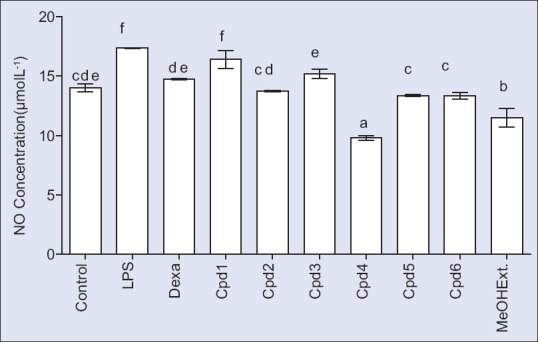
Anti-inflammatory activity of the total methanol extract and some isolated compounds in RAW 264.7 macrophage cells
Figure 3 showed different anti-inflammatory effects at the used concentration level 100 µg/ml of all samples. Compound 2 showed a significant reduction of NO level production compared to LPS. Compounds 5 and 6 showed a significant reduction compared to both dexamethasone and LPS. Furthermore, the methanol extract and compound 4 exhibited a significant reduction of NO as compared to dexamethasone, LPS and even that of the control. Literature data confirm that isorhamnetin and its derivatives have potent anti-inflammatory activity.[34,35] In our study, we found that compound 4, isorhamnetin-3-O-glucopyranoside was more potent than galactopyranoside and the two new compounds 1 and 2 which contain a diglucuronic moiety. It was also observed that the methoxylated derivative (compound 2) decreased NO concentration compared to that of compound 1. Moreover, compound 4, isorhamnetin-3-O-glucopyranoside showed higher potency than the isorhamnetin aglycone. Fortunately, displaying higher anti-inflammatory activity, compounds 2, 4, 5, and 6 in addition to the methanol extract do not exhibit cytotoxicity to RAW 264.7.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Mohamed A. El Raey
Dr. Mohamed A. El Raey, Ph.D, Researcher of Natural Products Chemistry, department of Phytochemistry and Plant Systematic, National Research Center, Dokki, Cairo, Egypt.
Acknowledgments
This study was supported by the cooperation between the National Research Center, Dokki, Cairo, Egypt, and Faculty of Pharmacy, October 6 University Giza, Egypt.
REFERENCES
- 1.Mabberley DJ. UK: Cambridge University Press; 2008. Mabberley's Plant-Book: A Portable Dictionary of Plants, their Classifications, and Uses. [Google Scholar]
- 2.Tackholm V. Egypt: Cairo University; 1974. Students’ Flora of Egypt; p. 2. [Google Scholar]
- 3.Batanouny KH. Cairo, Egypt: The Palm Press C; 1999. Wild Medicinal Plants in Egypt (An Inventory to Support Onservation and Sustainable Use) [Google Scholar]
- 4.Al-Saleh FH, Ali HH, Mirza M. Chemical constituents of some medicinal plants growing in Bahrain. Fitoterapia. 1993;64:251–6. [Google Scholar]
- 5.Ahmad Z, Mehmood S, Fatima I, Malik A, Ifzal R, Afza N, et al. Structural determination of salsolins A and B, new antioxidant polyoxygenated triterpenes from Salsola baryosma, by 1D and 2D NMR spectroscopy. Magn Reson Chem. 2008;46:94–8. doi: 10.1002/mrc.2119. [DOI] [PubMed] [Google Scholar]
- 6.Khacheba I, Djeridane A, Kameli AK, Yousfi M. The inhibitory effect of some algerian plants phenolics extracts on the α-glucosidase and α-amylase activities and their antioxidant activities. Curr Enzym Inhib. 2014;10:59–68. [Google Scholar]
- 7.Khan KM, Maharvi GM, Abbaskhan A, Hayat S, Khan MT, Makhmoor T, et al. Threetyrosinase inhibitors and antioxidant compounds from Salsola foetida. Helv Chim Acta. 2003;86:457–64. [Google Scholar]
- 8.Ahmad S, Maharvi GM, Ashraf M, Riaz N, Afza N, Khan KM, et al. Phytochemical studies on Salsola baryosma. J Chem Soc Pak. 2006;28:176–8. [Google Scholar]
- 9.Taha A, Alsayed H. Brine shrimp bioassay of ethanol extracts of Sesuvium verrucosum, Salsola baryosma and Zygophyllum quatarense medicinal plants from Bahrain. Phytother Res. 2000;14:48–50. doi: 10.1002/(sici)1099-1573(200002)14:1<48::aid-ptr536>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Hamed AI, Masullo M, Sheded MG, Mahalel UA, Tawfik MM, Perrone A, et al. Triterpene saponins from Salsola imbricata. Phytochem Lett. 2011;4:353–6. [Google Scholar]
- 11.Saleem M, Akhter N, Shaiq Ali M, Nazir M, Riaz N, Moazzam M, et al. Structure determination of salisomide and salisoflavan, two new secondary metabolites from Salsola imbricata, by 1D and 2D NMR spectroscopy. Magn Reson Chem. 2009;47:263–5. doi: 10.1002/mrc.2361. [DOI] [PubMed] [Google Scholar]
- 12.Hussein NS, El-Bassuony AA. Hydroxycinnamoylamides from Salsola baryosoma. Rev Latinoam Quimica. 2004;32:15–20. [Google Scholar]
- 13.Andhiwal C, Kishore K. Sterols of Salsola-foetida. J. Indian Chem. Soc. 1984;61:729–730. [Google Scholar]
- 14.Yang EJ, Yim EY, Song G, Kim GO, Hyun CG. Inhibition of nitric oxide production in lipopolysaccharide-activated RAW 264.7 macrophages by Jeju plant extracts. Interdiscip Toxicol. 2009;2:245–9. doi: 10.2478/v10102-009-0022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–10. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 16.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 17.Mabry T, Markham K, Thomas MB. New York: Springer-Verlag; 1970. The Systematic Identification of Flavonoids; pp. 41–164. [Google Scholar]
- 18.Wang DM, Pu WJ, Wang YH, Zhang YJ, Wang SS. A new isorhamnetin glycoside and other phenolic compounds from Callianthemum taipaicum. Molecules. 2012;17:4595–603. doi: 10.3390/molecules17044595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olszewska MA, Roj JM. Phenolic constituents of the inflorescences of Sorbus torminalis (L.) Crantz. Phytochem Lett. 2011;4:151–7. [Google Scholar]
- 20.Hirayama C, Ono H, Meng Y, Shimada T, Daimon T. Flavonoids from the cocoon of Rondotia menciana. Phytochemistry. 2013;94:108–12. doi: 10.1016/j.phytochem.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi K, Mitsunaga T, Batubara I. Synthesis of quercetin glycosides and their melanogenesis stimulatory activity in B16 melanoma cells. Bioorg Med Chem. 2014;22:937–44. doi: 10.1016/j.bmc.2013.12.062. [DOI] [PubMed] [Google Scholar]
- 22.Hasan A, Ahmed I, Jay M, Voirin B. Flavonoid glycosides and an anthraquinone from Rumex chalepensis. Phytochemistry. 1995;39:1211–3. doi: 10.1016/0031-9422(95)00071-e. [DOI] [PubMed] [Google Scholar]
- 23.Nawwar M, Ayoub N, Hussein S, Hashim A, El-Sharawy R, Wende K, et al. A flavonol triglycoside and investigation of the antioxidant and cell stimulating activities of Annona muricata Linn. Arch Pharm Res. 2012;35:761–7. doi: 10.1007/s12272-012-0501-4. [DOI] [PubMed] [Google Scholar]
- 24.Nawwar MA, Souleman AM, Buddrus J, Linscheid M. Flavonoids of the flowers of Tamarix nilotica. Phytochemistry. 1984;23:2347–9. [Google Scholar]
- 25.Ma YM, Wang P, Chen L, Feng CL. Chemical composition of the leaves of Periploca sepium. Chem Nat Compd. 2010;46:464–5. [Google Scholar]
- 26.Satake T, Kamiya K, An Y, Oishi Nee Taka T, Yamamoto J. The anti-thrombotic active constituents from Centella asiatica. Biol Pharm Bull. 2007;30:935–40. doi: 10.1248/bpb.30.935. [DOI] [PubMed] [Google Scholar]
- 27.Moehle B, Heller W, Wellmann E. UV-Induced biosynthesis of quercetin 3-O-β-D-glucuronide in dill cell cultures. Phytochemistry. 1985;24:465–7. [Google Scholar]
- 28.Yan X, Murphy BT, Hammond GB, Vinson JA, Neto CC. Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon) J Agric Food Chem. 2002;50:5844–9. doi: 10.1021/jf0202234. [DOI] [PubMed] [Google Scholar]
- 29.Su XC, Chen L, Aisa HA. Flavonoids and sterols from Alhagi sparsifolia. Chem Nat Compd. 2008;44:365. [Google Scholar]
- 30.Cao X, Wei Y, Ito Y. Preparative isolation of isorhamnetin from stigma maydis using high-speed countercurrent chromatography. J Liq Chromatogr Relat Technol. 2009;32:273–280. doi: 10.1080/10826070802603369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CY, Wang YD, Wang HM. Chemical constituents from the roots of Synsepalum dulcificum. Chem Nat Compd. 2010;46:448–9. [Google Scholar]
- 32.Balde AM, Claeys M, Pieters LA, Wray V, Vlietinck AJ. Ferulic acid esters from stem bark of Pavetta owariensis. Phytochemistry. 1991;30:1024–6. [Google Scholar]
- 33.Ren Q, Chen W, Zhao H, Wu Z, Zhang H. Organic acids from Capparis spinosa fruit. Chem Nat Compd. 2012;48:868–9. [Google Scholar]
- 34.Chen YC, Lee HZ, Chen HC, Wen CL, Kuo YH, Wang GJ. Anti-inflammatory components from the root of Solanum erianthum. Int J Mol Sci. 2013;14:12581–92. doi: 10.3390/ijms140612581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chirumbolo S. Anti-inflammatory action of isorhamnetin. Inflammation. 2014;37:1200–1. doi: 10.1007/s10753-014-9846-9. [DOI] [PubMed] [Google Scholar]


