Abstract
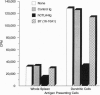
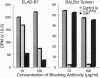
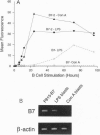
Effective T-cell activation requires antigen/major histocompatibility complex engagement by the T-cell receptor complex in concert with one or more costimulatory molecules. Recent studies have suggested that the B7 molecule, expressed on most antigen presenting cells, functions as a costimulatory molecule through its interaction with CD28 on T cells. Blocking the CD28/B7 interaction with CTLA4Ig inhibits T-cell activation in vitro and induces unresponsiveness. We demonstrate that another molecule(s), termed B7-2, is expressed constitutively on dendritic cells, is differentially regulated on B cells, and costimulates naive T cells responding to alloantigen. B7-2 is up-regulated by lipopolysaccharide in < 6 hr and is maximally expressed on the majority of B cells by 24 hr. In contrast, B7 is detected only on a subset of activated B cells late (48 hr) after stimulation. In addition, Con A directly induces B7-2 but not B7 expression on B cells. Finally, although both anti-B7 monoclonal antibodies and CTLA4Ig blocked T-cell proliferation to antigen-expressing B7 transfectants, only CTLA4Ig had any significant inhibitory effect on T-cell proliferation to antigens expressed on natural antigen presenting cells, such as dendritic cells. Thus, B7 is not the only costimulatory molecule capable of initiating T-cell responses since a second ligand, B7-2, can provide a necessary second signal for T-cell activation.
Full text
PDF
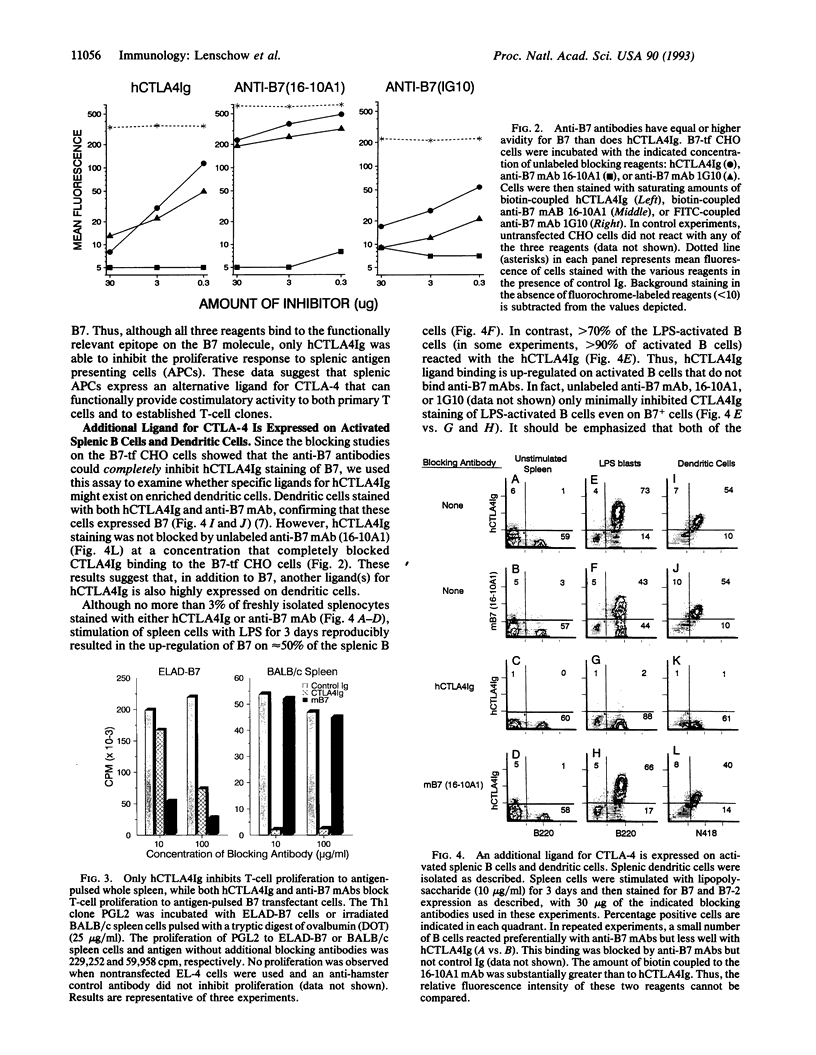
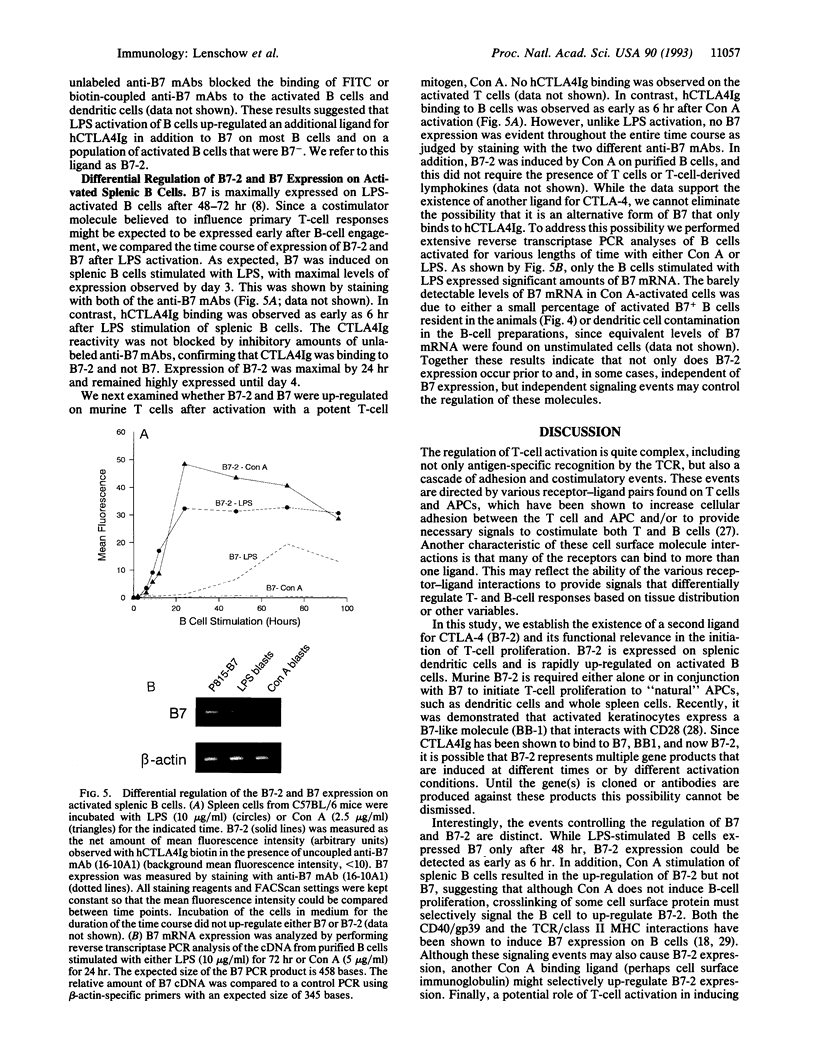

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Damle N. K., Klussman K., Linsley P. S., Aruffo A. Differential costimulatory effects of adhesion molecules B7, ICAM-1, LFA-3, and VCAM-1 on resting and antigen-primed CD4+ T lymphocytes. J Immunol. 1992 Apr 1;148(7):1985–1992. [PubMed] [Google Scholar]
- Freeman G. J., Gray G. S., Gimmi C. D., Lombard D. B., Zhou L. J., White M., Fingeroth J. D., Gribben J. G., Nadler L. M. Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J Exp Med. 1991 Sep 1;174(3):625–631. doi: 10.1084/jem.174.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Pinnas M., Wong T., Fitch F. W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991 Mar 15;146(6):1750–1758. [PubMed] [Google Scholar]
- Go C., Miller J. Differential induction of transcription factors that regulate the interleukin 2 gene during anergy induction and restimulation. J Exp Med. 1992 May 1;175(5):1327–1336. doi: 10.1084/jem.175.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. A., Callas E., Allison J. P. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992 Jul 15;149(2):380–388. [PubMed] [Google Scholar]
- Harding F. A., McArthur J. G., Gross J. A., Raulet D. H., Allison J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992 Apr 16;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Hibbs M. L., Xu H., Stacker S. A., Springer T. A. Regulation of adhesion of ICAM-1 by the cytoplasmic domain of LFA-1 integrin beta subunit. Science. 1991 Mar 29;251(5001):1611–1613. doi: 10.1126/science.1672776. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Taylor P. S., Norton S. D., Urdahl K. B. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991 Oct 15;147(8):2461–2466. [PubMed] [Google Scholar]
- Larsen C. P., Ritchie S. C., Pearson T. C., Linsley P. S., Lowry R. P. Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J Exp Med. 1992 Oct 1;176(4):1215–1220. doi: 10.1084/jem.176.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow D. J., Zeng Y., Thistlethwaite J. R., Montag A., Brady W., Gibson M. G., Linsley P. S., Bluestone J. A. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992 Aug 7;257(5071):789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- Lindsten T., Lee K. P., Harris E. S., Petryniak B., Craighead N., Reynolds P. J., Lombard D. B., Freeman G. J., Nadler L. M., Gray G. S. Characterization of CTLA-4 structure and expression on human T cells. J Immunol. 1993 Oct 1;151(7):3489–3499. [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Urnes M., Grosmaire L. S., Damle N. K., Ledbetter J. A. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991 Sep 1;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Clark E. A., Ledbetter J. A. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Greene J. L., Tan P., Bradshaw J., Ledbetter J. A., Anasetti C., Damle N. K. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992 Dec 1;176(6):1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Wallace P. M., Johnson J., Gibson M. G., Greene J. L., Ledbetter J. A., Singh C., Tepper M. A. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992 Aug 7;257(5071):792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- Miller J., Germain R. N. Efficient cell surface expression of class II MHC molecules in the absence of associated invariant chain. J Exp Med. 1986 Nov 1;164(5):1478–1489. doi: 10.1084/jem.164.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi N., Freeman G. J., Gault A., Godfrey D., Nadler L. M., Glimcher L. H. Signalling through the MHC class II cytoplasmic domain is required for antigen presentation and induces B7 expression. Nature. 1992 Nov 19;360(6401):266–268. doi: 10.1038/360266a0. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Mitra R. S., Lee K., Turka L. A., Green J., Thompson C., Shimizu Y. Discordant expression of CD28 ligands, BB-1, and B7 on keratinocytes in vitro and psoriatic cells in vivo. Am J Pathol. 1993 Apr;142(4):1029–1040. [PMC free article] [PubMed] [Google Scholar]
- Ranheim E. A., Kipps T. J. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med. 1993 Apr 1;177(4):925–935. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razi-Wolf Z., Freeman G. J., Galvin F., Benacerraf B., Nadler L., Reiser H. Expression and function of the murine B7 antigen, the major costimulatory molecule expressed by peritoneal exudate cells. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4210–4214. doi: 10.1073/pnas.89.9.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser H., Freeman G. J., Razi-Wolf Z., Gimmi C. D., Benacerraf B., Nadler L. M. Murine B7 antigen provides an efficient costimulatory signal for activation of murine T lymphocytes via the T-cell receptor/CD3 complex. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):271–275. doi: 10.1073/pnas.89.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N., Koide S., Crowley M., Witmer-Pack M., Livingstone A. M., Fathman C. G., Inaba K., Steinman R. M. Presentation of exogenous protein antigens by dendritic cells to T cell clones. Intact protein is presented best by immature, epidermal Langerhans cells. J Exp Med. 1989 Mar 1;169(3):1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H., Mueller D. L., Jenkins M. K., Quill H. T-cell clonal anergy. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 2):605–610. doi: 10.1101/sqb.1989.054.01.072. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Kaplan G., Witmer M. D., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979 Jan 1;149(1):1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turka L. A., Linsley P. S., Lin H., Brady W., Leiden J. M., Wei R. Q., Gibson M. L., Zheng X. G., Myrdal S., Gordon D. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11102–11105. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- van Seventer G. A., Shimizu Y., Shaw S. Roles of multiple accessory molecules in T-cell activation. Curr Opin Immunol. 1991 Jun;3(3):294–303. doi: 10.1016/0952-7915(91)90027-x. [DOI] [PubMed] [Google Scholar]