Abstract
Objective:
To isolate compounds from the roots of Garcinia cowa and to evaluated their cytotoxic activity against breast (MCF-7), prostate (DU-145), and lung (H-460) cell lines.
Materials and Methods:
The ground air-dried root was sequentially macerated with hexane, dichloromethane (DCM), ethyl acetate (EtOAc), and methanol. The DCM soluble extract was fractionated by vacuum liquid chromatography, column chromatography, and radial chromatography over silica gel with hexane, EtOAc and methanol as eluent in progressively increasing polarity manner; to yield three compounds. Their structures were elucidated based on their spectroscopic data and their comparison with those of the literature. The cytotoxicity of isolated compounds was carried out against human cell lines by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide colorimetric assay. The extract was added at various concentrations (0.1, 1, 10 and 100 mg/ml). The level of cytotoxicity was determined by calculating the level of IC50 that was based on the percentage of the cell death following the 24 h incubation with the extract.
Results:
Phytochemical study on the roots of G. cowa yielded rubraxanthone (3), cowanine (4) and 1,5-dihydroxyxanthone (5). Compound 4 with an IC50 value of 4.1 ± 1.0 μM, 5.4 ± 2.3 μM and 11.3 ± 10.0 μM against MCF-7, H-460, and DU-145, respectively while compound 3 was found to be in active.
Conclusion:
The results indicate that G. cowa roots could be important sources of natural cytotoxic compounds.
SUMMARY
Isolation of cytotoxic compounds from Garcinia cowa
Cowanine is the active constituent from the roots of Garcinia cowa
Complete nuclear magnetic resonance assignment of isolated compounds
MS fragmentation of rubraxanthone.
Keywords: Cytotoxic activity, DU-145, Garcinia cowa Roxb., H-460, MCF-7, nuclear magnetic resonance, spectroscopy, structure elucidation, xanthone
INTRODUCTION
Garcinia cowa Roxb. is a medium-sized tree, which attains a height of about 30 m, and is commonly known as asam kandis in West Sumatera or Cha muang in Thailand. It is widely distributed throughout Indonesia and the Malay peninsula, and has been used in the folk medicine for various medicinal purposes such as the use of the fruit treatment of dysentery in India,[1] and the bark as an antipyretic agent in Thailand.[2] Some pharmacological properties such as anti-tumor-promoting,[3] inhibition of human low-density lipoprotein peroxidation, and anti-platelet activities have been reported on the crude extract of leaves.[4] Previous investigation on the stem bark of this plant revealed the presence of nine prenylated poly-hydroxyxanthones, as well as pyranoxanthones.[5] The organic acid content in fresh leaves, fruits, and dried rinds of G. cowa has been investigated and found that (-)-hydroxycitric acid and its lactone constitute the major constituents.[6] Previous investigation of the latex of G. cowa revealed the presence of anti-bacterial prenylated xanthone including cowanin, cowanol, cowaxanthone, 1,3,6-trihydroxy-7-methoxy-2,5-bis (3-methyl-2-butenyl) xanthone and norcowanin.[2] Mahabusarakam et al. reported the isolation of cowagarcinones A-E, mangostin and fuscaxanthone A from the latex of G. cowa.[7] Tetraoxygenated xanthone, cowaxanthones A-E have also been obtained from the fruit of G. cowa.[8]
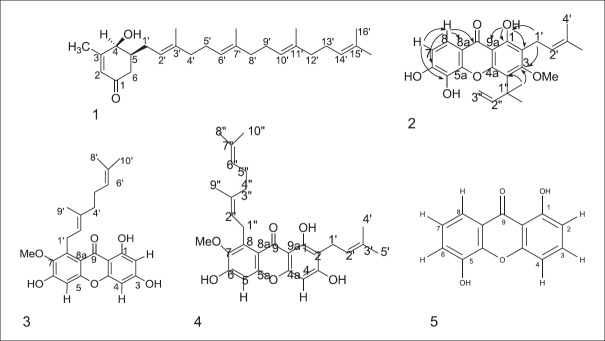
Previously, we reported the isolation of [2E,6E,10E]-(+)-4β-hydroxy-3-methyl-5β-(3,7,11,15-tetramethyl-2,6, 10, 14-hexadecatetraenyl-2- cyclohexen-1-one (1), 2-(3-methyl-2-butenyl)-1,5,6-trihydroxy-3- methoxy-4-(1,1-dimethyl-2-propenyl)-9H-xanthen-9-one (2) and rubraxanthone (3) from the stem bark of this plant [Figure 1].[9]
Figure 1.
Isolated compounds from Garcinia cowa Roxb.
In continuation of our study on G. cowa,[9] cytotoxic properties of isolated xanthones from the roots of G. cowa against cancer cell lines are reported.
MATERIALS AND METHODS
General
Vacuum liquid chromatography (VLC) was conducted on silica gel (Merck 9385) and column chromatography (CC) was conducted on either silica gel (Merck 7734) or Sephadex LH-20 (Amersham). Samples were dissolved in minimum amount of appropriate solvent or coated on silica gel, and then introduced onto the packed column. Appropriate solvents were selected and applied in increasing polarity manner. The eluates were monitored by analytical thin layer chromatography utilizing precoated silica gel (Merck 5554) adsorbent. The spots were visualized under ultraviolet lights at 254 and 366 nm, or exposed to iodine vapor. Fractions containing similar profile were combined. Radial chromatography was carried out using Harrison Research Chromatotron model 7924T on plates coated with silica gel 60 PF254 (Merck 7749) containing gypsum, at either 1 or 2 or 4 mm thickness. The samples were leaked onto the chromatotron plate and then eluted with appropriate solvents. Compound streaks were monitored under UV light and fractions were collected accordingly. The solvents were evaporated under reduced pressure. UV (in absolute ethanol) and IR (KBr) spectra were recorded on a J ASCO V-560 spectrophotometer and a Perkin-Elmer 1650 FTIR spectrophotometer, respectively. High-resolution fast atom bombardment mass spectrometry (HRFAB-MS) was obtained on a J EOL J MS HX-110A spectrometer. 1H- and 13C-NMR spectra (CDCl3) were recorded on a Varian 500 MHz nuclear magnetic resonance (NMR) spectrometer at 500 MHz (1H) and 125 MHz (13C), respectively. The spectra were interpreted with the aid of the 1H-1H COSY, HMBC, and HMQC techniques.
Cell lines
The cell lines, NCI-H-460 were purchased from the American Type Culture Collection (Manassas, VA, USA). The cancer cells were cultured in RPMI 1640 medium (Life Technologies, Paisly, UK) with 10% v/v fetal calf serum (PAA Laboratories, Linz, Austria), 100 IU/ml penicillin and 100 μg/ml streptomycin (Life Technologies, Paisly, UK) whereas the solution trypsin-EDTA were purchased from GIBCO (Auckland, New Zealand). Dimethyl sulfoxide (DMSO) was purchased from BDH Laboratory (England). Culture flask (25 cm2 and 75 cm2) and 10 ml serological pipettes were purchased from Becton Dickson (New Jersey, USA).
Plant material
The roots of G. cowa Roxb. were collected at Sarasah Bonta, Harau Valley, and West Sumatra at an altitude of 500 m. The voucher specimens (DR-183) were identified by Dr. Rusdi Tamin and were deposited in the herbarium of Andalas University, Padang, Indonesia. Plant materials were cut into small pieces (3–5 mm thickness) and air-dried under shade. The dried plant materials were ground to a powder before extraction.
The isolation of cowanin 4, rubraxanthone 3 and 1,5-dihyroxyxanthone 5 from the roots of Garcinia cowa
The ground air-dried root (2.8 kg) was sequentially macerated with hexane, dichloromethane (DCM), ethyl acetate (EtOAc) and methanol (MeOH) to yield 800 mg, 27 g, 98 g and 63 g, respectively, of dark masses after evaporation of the solvents. The DCM soluble extract (20 g) was fractionated by VLC over silica gel with hexane, EtOAc and MeOH as eluent in progressively increasing polarity manner; to yield seven combined fractions A-G. Fraction C (8 g) was further subjected to silica gel CC with hexane followed by hexane-DCM mixtures used as eluent to give five combined fractions (C1-C5). Further purification of fraction C3 (100 mg) on silica gel CC and recrystallization from EtOAc-hexane afforded cowanin (4, 18 mg), rubraxanthone (3, 15 mg) and 1,5-dihydroxyxanthone (5, 8 mg).
Cytotoxic assay
Cells were attached by incubating suspension cells (180 µl in each well except for blank) for overnight. Varying concentrations of the extracts or pure compounds in DMSO were prepared from the stock solutions by serial dilution (100 μg/ml, 10 μg/ml, 1 μg/ml, 0.1 μg/ml) in RPMI-1640 to give the volume of 200 µl in each well of the microtiter plate. The assay for each concentration was performed in quadruplicate and the culture plate was incubated at 37°C with 5% (v/v) CO2 for 96 h. After incubation, 50 μL of 2 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution was added to each well and allowed to incubate for further 4 h, after which all supernatant were discarded. Subsequently, 100 μL DMSO was added to each well and vigorously mixed to dissolve the formazan crystals. Absorbance values at 550 nm were measured with a microplate reader. Cytotoxicity was expressed as IC50 (the concentration of test sample to reduce the absorbance of treated cells by 50% with reference to the control).[10]
RESULTS AND DISCUSSION
Characterization of compound 3
From the more polar fraction of the EtOAc soluble material, the known compound rubraxanthone 3 was isolated.[9] Compound 3 was obtained as yellow needles with melting point 201–203°C (Lee and Chan 1977, m.p. 205–206°C). The EI-MS spectrum showed that the molecular ion (M+) was at m/z 410. The UV spectrum displayed the absorptions at λmax (log ε): 203 (4.54), 241 (4.50), and 312 (4.32) nm the due to xanthone nucleus.[11] The presence of hydroxyl and carbonyl groups were supported in IR spectrum by the appearance of the prominent peak at 3429 cm−1 and 1650 cm−1 respectively.[12]
The 1H-NMR spectrum showed 13 signals consisting a chelated hydroxyl proton at δ 13.52, an aromatic proton as a single peak at δ 6.72 (1H), two doublet in the aromatic region at δ 6.11 (1H, d, J = 2.0 Hz) and δ 6.21 (1H, d, J = 2.0 Hz) and a single methoxy resonance occurred at δ 3.40 (3H, s). The presence of the signals at δ 5.01 (1H, t, J = 7.0 Hz) and δ 5.20 (1H, t, J = 7.0 Hz) δ 4.07 (2H, d, J = 7.0 Hz), two triplet at δ 2.05 (2H, t, J = 7.0 Hz) and δ 1.98 (2H, t, J = 7.0 Hz), and three singlet methyls at δ 1.52, 1.55 and 1.81, were assigned as a geranyl unit.
In the 13C-NMR spectrum, there are 24 signals of carbon, which clearly showed a carbonyl carbon signal at δ 181.9 and was assigned as C-9. The assignment of the protons and carbons of 3 are summarized in Table 1.
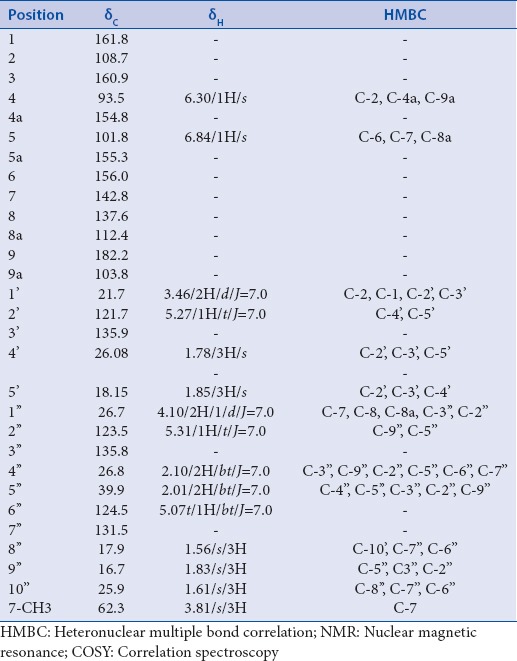
Table 1.
1H-NMR,13C-NMR, and HMBC data of rubraxanthone 3
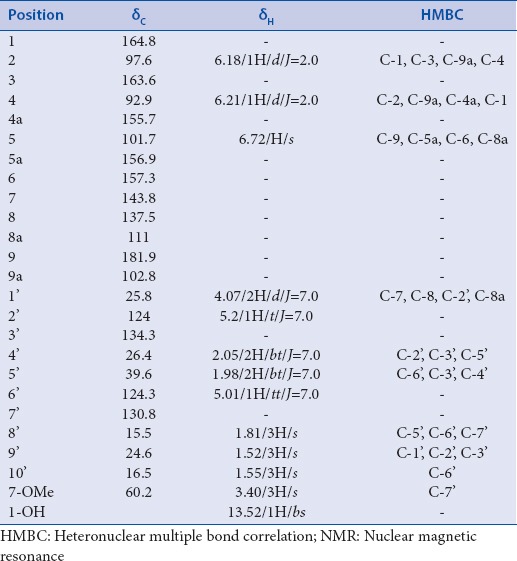
Based on the HMBC spectrum, the geranyl unit was located at C-8 according to the correlation of proton at δ 4.07 (H-1’) to carbon signals at δ 143.8 (C-7), δ 137.5 (C-8) and 111.0 (C-8a). It was particularly valuable in placing the methoxy group at C-7 due to its 2J correlation of proton signal at δ 3.40 with the carbon signal at 143.8 (C-7).
The presence of a geranyl unit was confirmed by major ions at m/z 341 (M-69)+ which is due the loss of prenyl unit. The presence of a peak at m/z 299 (M-111)+ could correspond to fragmentation involving the geranyl group and the O-methoxy group [Figure 2].
Figure 2.
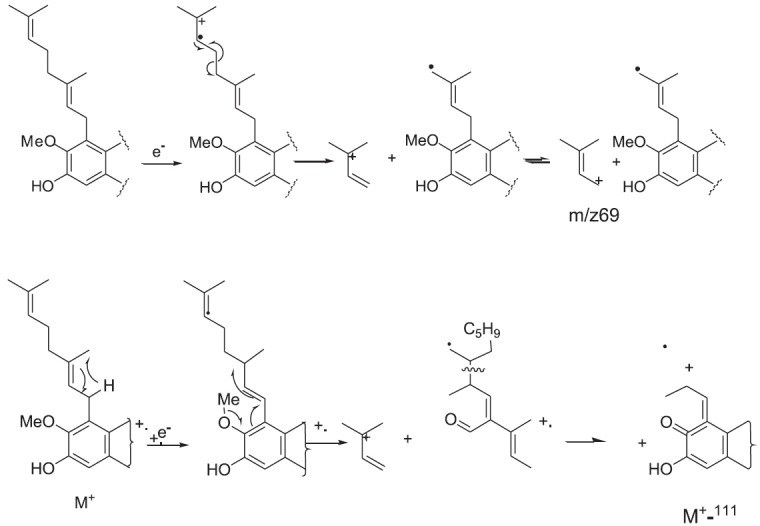
EI-MS fragmentation of rubraxanthone 3
The assignment of the methoxy group at C-7, ortho to the geranyl side-chain at C-8, was supported based on this data. Based on HMBC correlation between aromatic proton at δ 6.11 with the carbon signals at δ 164.8 (C-1), 163.6 (C-3) and δ 102.8 (C-9a), the doublet protons at δ 6.11 and δ 6.21 were assigned as meta coupled aromatic proton (J = 2.0 Hz) of H-2 and H-4, respectively. The H-4 showed correlations with carbon signals at δ 164.8 (C-1), δ 97.6 (C-2) and δ 155.7 (C-4a). Furthermore, correlations between aromatic proton at δ 6.72 with carbon signals at δ 181.9 (C-9), δ 156.9 (C-5a), δ 157.3 (C-6), and δ 111.0 (C-8a) were supported that this proton was assigned as H-5. Thus, this compound was characterized as rubraxanthone. This compound was previously isolated from stem barks of G. cowa.[9]
Characterization of compound 4
Compound 4, isolated as a yellow needless, mp 121–124°C (lit. 135–137).[2] It exhibited UV absorption bands at 243.60 nm (4.52) 315.80 nm (4.37) while hydroxyl and conjugated carbonyl absorption bands were observed at 3391 cm−1 and 1648 cm−1, respectively, in the IR spectrum. The EI mass spectra of this compound showed a molecular ion peak at m/z 478, consistant with a molecular formula C29H34O6.
The 1H-NMR spectrum displayed signals of one chelated hydroxyl group at δ 13.81 (s, 1-OH), two aromatic protons at δ 6.86 (1H, s) and δ 6.43 (1H, s) and one methoxy group at δ 3.83 (3H, s), The presence of the signals at δ 3.38 (2H, d, J = 7.0), δ 5.31 (1H, m), δ 1.68 (3H, s), and 1.81 (3H, s) were suggestive of a prenyl moiety. The remaining signals appeared as the typical signals of a geranyl unit. These signals were a doublet of methylene protons H-1’’ at δ 4.17, two broad triplets of the olefinic protons H-2’’ and H-6’’ at δ 5.27, two multiplets of the methylene protons H-4’’ and H-5’’ at δ 2.02 and δ 2.05, respectively, and three singlets of methyl groups H-8’’, H-9’’ and H-10’’ at 1.56, 1.83 and 1.61, respectively. The 13C-NMR spectrum of 2 showed 29 signals, including, one carbonyl signal at δ 182.2.
The assignments of the substituents were deduced from the HSQC and HMBC spectra [Table 2]. The geranyl unit was located at C-8 according to the correlations of benzylic methylene proton H-1’’ to C-7, C-8 and C-8a and the deshielding effect of the carbonyl group on H-1’’. The methylene protons H-1’ correlated to C-1 and C-2 whereas the methylene protons H-1’’ correlated to C-7, C-8, and C-8a, consequently, a prenyl unit was located at C-2. The aromatic proton at δ 6.84 showed two bond coupling correlation with C-6 and C-5a and three bonds coupling with C-7 and C-8a. Its confirmed the position of this proton at C-5. Another aromatic proton at δ 6.30 was attached in C-4 based on the two bond coupling correlation with C-4a and three bonds coupling to C-2 and C-9a. The position of methoxyl group a C-7 was confirmed based on the correlation between proton signals at δ 3.83 with carbon at δ 144.2 (C-7).
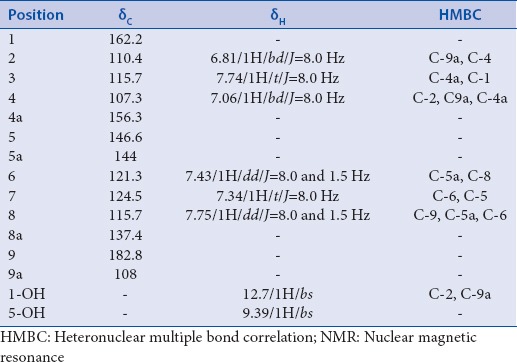
Table 2.
The 1H-NMR, 13C-NMR, COSY and HMBC data of cowanin 4
The mass spectral fragmentations supported the assigned structure. Typical fragmentation of the geranyl and prenyl substituents being observed. This compound gave strong fragment ions at m/z = 409 (M-69)+ and 353 (M-69-56)+. A weak ion at m/z = 367 (M-111)+ could correspond to fragmentation involving the geranyl group and the o-methoxy group.[13] Thus, 4 was characterized as cowanin.
Characterization of compound 5
Compound 5 was isolated as a yellow amorphous solid from the DCM fraction of roots of G. cowa. On the basis of EI-MS data, this compound showed a molecular ion peak (M+) at m/z 228 which is in agreement with molecular formula C13H8O4. The UV spectrum was showed an absorption band at λmax248.60 (4.22) and 311.60 (4.64). The IR spectrum exhibited two strong characteristic absorption bands at 3431 cm−1 and 1648 cm−1, indicative of a hydroxyl and carbonyl groups, respectively.
The 1H-NMR spectrum indicated the presence of six aromatic protons at δ 7.06 (1H, d, J = 8.0 Hz, H-4), δ 7.73 (1H, t, J = 8.0 Hz, H-3), δ 6.81 (1H, d, J = 8.0 Hz, H-2), δ 7.34 (1H, t, J = 8.0 Hz, H-6), δ 7.74 (1H, t, J = 8.0 Hz, H-7), and δ 7.43 (1H, dd, J = 7.0, 1.5 Hz, H-8). The 1H-NMR spectrum showed that all the aromatic protons were ortho coupled to each other with J value of 8.0 Hz. Furthermore, the correlation spectroscopy spectrum revealed cross peak between proton at δ 7.74 with protons at δ 6.81 and δ 7.06. Another cross peak was observed between protons at δ 7.34 with a proton at δ 7.75. Two hydroxyl protons were observed at 12.7 and 9.39. The 13C-NMR spectrum of 5 showed 11 signals, which supports the 5, has xanthone skeleton. The HMBC spectrum showed 2J correlation of the proton at δ 6.81 to the carbonyl δ 162.2 (C-1), 3J correlation to the carbon at δ 107.3 (C-4). Thus, this proton was assign as H-2. The aromatic proton at δ 7.06 was assigned as H-4 based on their 3J correlation with the carbon at C-2 (δ 110.4), C-9a (108.0) and C-4a (δ 156.3). Proton at δ 7.74 showed 3J correlation with carbon at δ 156.3 (C-4a) and δ 162.2 (C-1). Thus, this proton was assigned as H-3 [Table 3].
Table 3.
1H-NMR, 13C-NMR and HMBC data of 1,5-dihydroxyxanthone 5
The cytotoxic activity of isolated compounds, except for 5 was evaluated against three cancer cell lines due to its limited quantity. The results of this experiment are presented in Table 4.
Table 4.
Cytotoxic activity of isolated compounds toward three cancer cell lines
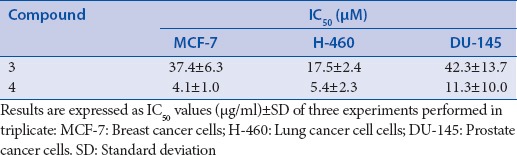
Compounds 4 exhibited potent activity with IC50 values of 4.1 ± 1.0 μM, 5.4 ± 2.3, and 11.3 ± 10.0 μM towards MCF-7, H-460, and DU-145 cells, respectively. Compound 3 displayed weak activity with IC50 ranging from 17.5 to 42.3 μM.
CONCLUSION
The potential value of the roots of G. cowa from the West Sumatra region of Indonesia has been reported for the 1st time here. Three xanthones were isolated from these roots. Structure elucidation of these compounds was carried out by spectroscopic methods. As far as our knowledge, complete NMR assignment of this compound was first reported. Cowanin was found to be a potent cytotoxic compound.
Financial support and sponsorship
The Intensified Research in Priority Areas Research Grant (IRPA, EAR No. 09-02-04-0313), International Foundation for Science (IFS no F/3967-1), National L’Oreal Fellowship Grant, and Directorate of Higher Education, Indonesian Ministry of Education (HB XIV no: 297/SP3/PP/DP2M/II/2006).
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Dachriyanus Hamidi
Prof. Dachriyanus Hamidi, is chair of Research Laboratory at Faculty of Pharmacy, Andalas University. He involve in Drug Discovery Research at Faculty of Pharmacy, Andalas University.
Acknowledgments
All authors thank the Malaysian Ministry of Science, Technology and the Environment for financial support under the Intensified Research in Priority Areas Research Grant (IRPA, EAR No. 09-02-04-0313), International Foundation for Science (IFS No. F/3967-1), National L’Oreal Fellowship Grant, and Directorate of Higher Education, Indonesian Ministry of Education (HB XIV No. 297/SP3/PP/DP2M/II/2006).
REFERENCES
- 1.Rao RR. Ethnobotany of Meghalaya: Medicinal plants used by Khasi and Garo tribes. Econ Bot. 1981;35:4–9. [Google Scholar]
- 2.na Pattalung P, Thongtheeraparp W, Wiriyachitra P, Taylor WC. Xanthones of Garcinia cowa. Planta Med. 1994;60:365–8. doi: 10.1055/s-2006-959502. [DOI] [PubMed] [Google Scholar]
- 3.Murakami A, Jiwajinda S, Koshimizu K, Ohigashi H. Screening for in vitro anti-tumor promoting activities of edible plants from Thailand. Cancer Lett. 1995;95:139–46. doi: 10.1016/0304-3835(95)03879-2. [DOI] [PubMed] [Google Scholar]
- 4.Jantan I, Jumuddin FA, Saputri FC, Rahman K. Inhibitory effects of the extracts of Garcinia species on human low-density lipoprotein peroxidation and platelet aggregation in relation to their total phenolic contents. J Med Plants Res. 2011;5:2699–709. [Google Scholar]
- 5.Shen J, Yang JS. Two new xanthones from the stems of Garcinia cowa. Chem Pharm Bull (Tokyo) 2006;54:126–8. doi: 10.1248/cpb.54.126. [DOI] [PubMed] [Google Scholar]
- 6.Jena BS, Jayaprakasha GK, Sakariah KK. Organic acids from leaves, fruits, and rinds of Garcinia cowa. J Agric Food Chem. 2002;50:3431–4. doi: 10.1021/jf011627j. [DOI] [PubMed] [Google Scholar]
- 7.Mahabusarakam W, Chairerk P, Taylor WC. Xanthones from Garcinia cowa Roxb. latex. Phytochemistry. 2005;66:1148–53. doi: 10.1016/j.phytochem.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Panthong K, Pongcharoen W, Phongpaichit S, Taylor WC. Tetraoxygenated xanthones from the fruits of Garcinia cowa. Phytochemistry. 2006;67:999–1004. doi: 10.1016/j.phytochem.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 9.Wahyuni FS, Lindsay TB, Dachriyanus, Dianita R, Jubahar J, Lajis NH, et al. A new ring-reduced tetraprenyltoluquinone and a prenylated xanthone from Garcinia cowa. Aust J Chem. 2004;57:223–6. [Google Scholar]
- 10.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 11.Ampofo SA, Waterman PG. Xanthones from three Garcinia species. Phytochemistry. 1986;25:2351–5. [Google Scholar]
- 12.Silverstein RM, Webster FX. 6th ed. Singapore: Wiley; 1997. Spectrometric Identification of Organic Compounds. [Google Scholar]
- 13.Lee HH, Chan HK. 1,3,6-Trihydroxy-7-methoxy-8-(3,7-dimethyl-2,6-octadienyl) xanthone from Garcinia cowa. Phytochemistry. 1977;16:2038–40. [Google Scholar]