Abstract
Context:
The population of Manipur is of different ethnic background from the rest of the country. Several authors have suggested population/ethnic and laboratory specific reference range of maternal thyroid profile of different trimesters.
Aims:
To find the reference range of thyroid stimulating hormone (TSH), total thyroxine (TT4) and total tri-iodothyronine (TT3) levels for normal pregnant women of native Manipur descendants.
Settings and Design:
The cross-sectional study was conducted at a teaching Institute after ethical clearance was obtained.
Subjects and Methods:
A reference populations of 375 normal pregnant women were established after screening about 600 pregnant women. The study excluded patients with hyperemesis gravid arum, past history or family history of thyroid disorders as well as the connective tissue disorders, WHO grade 1 or 2 goiter, or any medications that alter thyroid functions. The serum levels of TSH, TT4, and TT3 were measured using chemiluminescence assay.
Statistical Analysis Used:
Data for TT3 and TT4 were expressed as mean ± standard deviation, median and 5–95th percentiles.
Results:
The mean TSH in the three trimesters was 1.06 + 0.45, 1.23 + 0.30, and 1.25 + 0.36, respectively. The normal reference range thus was different from that of the kit reference range. On comparing to the Indian normative reference for the pregnant women, our results were not similar. However, the values were near similar to that of the American Thyroid Association guidelines.
Conclusions:
We conclude our study results with a new reference range for the pregnant population in Manipur and also emphasis the use of trimester-specific reference range of thyroid hormone.
Keywords: Pregnancy, reference range, thyroid hormones
INTRODUCTION
During pregnancy, there is a profound physiological change in the mother, which will complicate the interpretation of maternal thyroid function tests.[1,2,3,4,5,6] Human chorionic gonadotropin (hCG) have similar alpha-subunit to thyroid stimulating hormone (TSH) and a unique beta-subunit. As a result, hCG can stimulate the thyroid gland during the first trimester.[7] During pregnancy, the serum thyroxine-binding globulin concentrations rise almost two-fold and thyroid hormone production increase. Hence, total T4 and T3 concentrations rise during the first half of pregnancy followed by a plateauing at approximately 20 weeks of gestation. Fetal brain development in its early stage depends on the maternal thyroid hormones.[6,7,8,9,10,11,12] Reference range were studied extensively across various countries and also American Thyroid Association (ATA) had published their guidelines.[13,14,15,16,17,18,19,20,21,22] Although several studies are available from different regions of the world, it is essential to develop norms for different Indian population due to the ethnic variations.[23,24,25]
SUBJECTS AND METHODS
The study primarily aimed at establishing the trimester-specific reference ranges for TSH and thyroid hormones (total tri-iodothyronine [TT3], total thyroxine [TT4]) from healthy pregnant women of the Manipuri descendants. We also compared the results with the Indian normative reference range and the ATA guidelines reference for thyroid disease in pregnancy. This was a cross-sectional study that was conducted after approval from the Institutional Ethics Committee. The women were recruited after a prior informed consent. The sample size was calculated with an absolute error of 0.5. The serum levels of TSH, TT4, and TT3 were measured using SIEMENS ADVIA third generation chemiluminescence assay which has an intra-assay variability of <10% for all the three parameters.
Healthy women with uncomplicated single intrauterine gestations consuming iodized salt in any trimester were consecutively recruited. A detailed obstetric history such as duration of gestation, parity and number of abortions and also history related to any thyroid-related illness were obtained. Physical examination included anthropometry, goiter grading[25] and general and systemic examinations. Venous samples for the serum were drawn; serum separated and sent for analysis of thyroid hormones (TSH, TT3, and TT4). The following are the exclusion criteria to cone down the population for the reference range:
History of hyperemesis gravid arum, thyroid illness or use of thyroid medications, connective tissue disorder and abortions
Family history of thyroid illness
Presence of goiter, WHO grade 1 or 2[25]
Overt hypothyroidism (OH) or hyperthyroidism.
The data were analyzed using the SPSS 17 (Chicago, SPSS Inc.). Data for TT3 and TT4 were expressed as mean ± standard deviation (SD), median, and 5th to 95th percentiles. TSH was normalized using log transformation as it did not follow normal distribution. The 5th and 95th percentile values of serum TSH, TT3, and TT4 from this group were used to derive reference intervals for pregnant women.[26]
RESULTS
A total of 600 normal pregnant women were examined, and 375 normal pregnant women were taken as the reference population after applying the exclusion criteria. Of the 375 women, 109, 148, and 118 were in the first, second, and third trimester, respectively. Using the kit reference values, OH was found in 2 (0.5%) women (1 in the first trimester, 1 in the second trimester). The mean age groups of the women were 25.9 + 5.1, 27.3 + 5.6 and 27.1 + 5.1 in first, second, and third trimester, respectively. The basic characteristics of the women are shown in Table 1. The mean ± SD, median and 5th and 95th percentiles for TT3, TT4, and TSH in each trimester of pregnancy are given in Table 2. The reference intervals for each trimester of our study population are shown in Table 3.
Table 1.
The basic characteristics of the study population
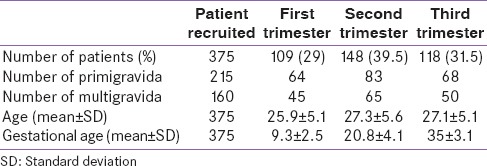
Table 2.
Thyroid hormone levels of our study population

Table 3.
Comparison of the manufacturer's reference range and the trimester specific range of our study population

DISCUSSION
The International Federation of Clinical Chemistry recommends that the determination of the normal reference values have to be described in such a way that the reference population should be appropriately described, and criteria for the selection should be clearly made.[26,27] The National Health and Nutrition Examination Survey of USA had used the exclusion criteria such as the personal or family history of thyroid illness, goiter but not ultrasound for an adult thyroid hormone reference population.[15] Marwaha et al., in their study had included the anti-thyroid antibodies and ultrasound in the Indian normative reference for the pregnant women.[13] The reference population should be as exclusive as possible as the purpose of reference intervals is to decide on thyroid status of a pregnant woman, wherein the thyroid functions cannot be compromised. This is because the adverse outcomes in fetus arising from thyroid dysfunction during pregnancy are very well-established.[5,6,7,8] In the Indian thyroid hormone reference study by Marwaha et al., a total of 331 women were studied with 107 in the first trimester, 137 in the second trimester and 87 in the third trimester. From the 5th and 95th percentile, the trimester-specific TSH values were 0.6–5.0, 0.44–5.78, and 0.74–5.7 µIU/ml, respectively.[13] Unlike the ATA guidelines for the thyroid diseases in pregnant women the TSH in Marwaha et al. study were similar to that of the normal adult population reference range Table 4. However, the reference range of the ethnic Manipuri population was near similar to that of the ATA guidelines.[14] Furthermore, in our study, we found a gradual increase in the median TSH levels as the trimester proceeds forwards. Another study from India evaluated 124 pregnant women using radioimmunoassay also showed an increase in TSH progressively with each trimester. When mean serum TT3 and TT4 were analyzed, both values increased from first to the second trimester but declined from second to the third trimester.[16] Similarly, in our study, the TT3 and TT4 increased from first to the second trimester, followed by a decline during the third trimester. A longitudinal study by Price et al., also arrived at the trimester-specific reference range. They found the serum TSH levels increase from first to the second trimester, while FT4 decreased with progress in the pregnancy. However, the serum FT3 did not change between the first and second trimesters. Furthermore, the study did not find any difference in the thyroid hormone changes between pregnant Asian and pregnant Caucasian women.[17] In a longitudinal study by Panesar et al., on pregnant Chinese women, the authors found a decrease in FT4 and FT3 by about 25% with gestational age from peak to nadir. Whereas, TSH, which was suppressed in the first trimester and increased subsequently with the advancing pregnancy.[18] A longitudinal study by Soldin et al., through the trimesters from a group of pregnant women used immunoassay and mass spectrometry to analyze the thyroid functions. The mean TSH increased with advancing gestational age from first to the second trimester, while it remained stable from second to the third trimester.[19] In an another cross-sectional study of 522 pregnant women from Japan, the authors showed a significant decrease in both free T3 (FT3) and free T4 (FT4) and increase in the TSH with advancing pregnancy.[20] Our study showed a TSH reference range, which is different from that of the proposed Indian normative range for pregnant women [Table 4]. In the study by Marwaha et al., the author suggests that the Asian pregnant women are more prone for gestational thyrotoxicosis.[13] However, in our study, we did not find women with overt hyperthyroidism. A study on thyroid function that was carried out only in the second trimester by La’ulu and Robertsshowed a higher prevalence of raised TSH in whites compared with Asians and Blacks.[21] Hence, the ethnic variations still remain a concern, and the studies that address this aspect are very scant. Hence, we can overview that there is a definite difference in the thyroid reference range across various countries and also within the Indian population due to its ethnic origin. The limitations in our study are that we did not measure the anti-thyroid antibodies as well as the free thyroid hormone levels.
Table 4.
Comparison of our TSH reference range with the proposed Indian normative range and the ATA guidelines reference range

This study concludes the following reference intervals for TT3, TT4, and TSH for the each trimester of pregnancy (TT3 [88.28–176.8, 102.24–233.27 and 88.46–205.49 ng/dl], TT4 [6.49–13.75, 7.9–16.06 and 7.77–15.3 µg/dl] and TSH [0.21–1.82, 0.72–1.71 and 0.69–1.93 IU/ml]) for the pregnant Indian women of Manipur state. We also emphasize, there is a definite difference in the trimester-specific reference range of thyroid hormones within country like India due to its ethnic variation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Man EB, Jones WS. Thyroid function in human pregnancy. V. Incidence of maternal serum low butanol-extractable iodines and of normal gestational TBG and TBPA capacities; retardation of 8-month-old infants. Am J Obstet Gynecol. 1969;104:898–908. [PubMed] [Google Scholar]
- 2.Davis LE, Leveno KJ, Cunningham FG. Hypothyroidism complicating pregnancy. Obstet Gynecol. 1988;72:108–12. [PubMed] [Google Scholar]
- 3.Leung AS, Millar LK, Koonings PP, Montoro M, Mestman JH. Perinatal outcome in hypothyroid pregnancies. Obstet Gynecol. 1993;81:349–53. [PubMed] [Google Scholar]
- 4.Allan WC, Haddow JE, Palomaki GE, Williams JR, Mitchell ML, Hermos RJ, et al. Maternal thyroid deficiency and pregnancy complications: Implications for population screening. J Med Screen. 2000;7:127–30. doi: 10.1136/jms.7.3.127. [DOI] [PubMed] [Google Scholar]
- 5.Glinoer D, Soto MF, Bourdoux P, Lejeune B, Delange F, Lemone M, et al. Pregnancy in patients with mild thyroid abnormalities: Maternal and neonatal repercussions. J Clin Endocrinol Metab. 1991;73:421–7. doi: 10.1210/jcem-73-2-421. [DOI] [PubMed] [Google Scholar]
- 6.Morreale de Escobar G, Obregón MJ, Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab. 2000;85:3975–87. doi: 10.1210/jcem.85.11.6961. [DOI] [PubMed] [Google Scholar]
- 7.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 8.Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50:149–55. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 9.Klein RZ, Haddow JE, Faix JD, Brown RS, Hermos RJ, Pulkkinen A, et al. Prevalence of thyroid deficiency in pregnant women. Clin Endocrinol (Oxf) 1991;35:41–6. doi: 10.1111/j.1365-2265.1991.tb03494.x. [DOI] [PubMed] [Google Scholar]
- 10.Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. N Engl J Med. 1994;331:1072–8. doi: 10.1056/NEJM199410203311608. [DOI] [PubMed] [Google Scholar]
- 11.Glinoer D. The regulation of thyroid function in pregnancy: Pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–33. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 12.Mandel SJ, Spencer CA, Hollowell JG. Are detection and treatment of thyroid insufficiency in pregnancy feasible? Thyroid. 2005;15:44–53. doi: 10.1089/thy.2005.15.44. [DOI] [PubMed] [Google Scholar]
- 13.Marwaha RK, Chopra S, Gopalakrishnan S, Sharma B, Kanwar RS, Sastry A, et al. Establishment of reference range for thyroid hormones in normal pregnant Indian women. BJOG. 2008;115:602–6. doi: 10.1111/j.1471-0528.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 14.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Gupta N, Nath T, Sharma JB, Sharma S. Thyroid function tests in pregnancy. Indian J Med Sci. 2003;57:252–8. [PubMed] [Google Scholar]
- 17.Price A, Obel O, Cresswell J, Catch I, Rutter S, Barik S, et al. Comparison of thyroid function in pregnant and non-pregnant Asian and western Caucasian women. Clin Chim Acta. 2001;308:91–8. doi: 10.1016/s0009-8981(01)00470-3. [DOI] [PubMed] [Google Scholar]
- 18.Panesar NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem. 2001;38(Pt 4):329–32. doi: 10.1258/0004563011900830. [DOI] [PubMed] [Google Scholar]
- 19.Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: Trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084–90. doi: 10.1089/thy.2004.14.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurioka H, Takahashi K, Miyazaki K. Maternal thyroid function during pregnancy and puerperal period. Endocr J. 2005;52:587–91. doi: 10.1507/endocrj.52.587. [DOI] [PubMed] [Google Scholar]
- 21.La’ulu SL, Roberts WL. Second-trimester reference intervals for thyroid tests: The role of ethnicity. Clin Chem. 2007;53:1658–64. doi: 10.1373/clinchem.2007.089680. [DOI] [PubMed] [Google Scholar]
- 22.Quinn FA, Gridasov GN, Vdovenko SA, Krasnova NA, Vodopianova NV, Epiphanova MA, et al. Prevalence of abnormal thyroid stimulating hormone and thyroid peroxidase antibody-positive results in a population of pregnant women in the Samara region of the Russian Federation. Clin Chem Lab Med. 2005;43:1223–6. doi: 10.1515/CCLM.2005.212. [DOI] [PubMed] [Google Scholar]
- 23.Marwaha RK, Tandon N, Gupta N, Karak AK, Verma K, Kochupillai N. Residual goitre in the postiodization phase: Iodine status, thiocyanate exposure and autoimmunity. Clin Endocrinol (Oxf) 2003;59:672–81. doi: 10.1046/j.1365-2265.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- 24.Gopalakrishnan S, Singh SP, Prasad WR, Jain SK, Ambardar VK, Sankar R. Prevalence of goitre and autoimmune thyroiditis in schoolchildren in Delhi, India, after two decades of salt iodisation. J Pediatr Endocrinol Metab. 2006;19:889–93. doi: 10.1515/jpem.2006.19.7.889. [DOI] [PubMed] [Google Scholar]
- 25.Geneva, Switzerland: World Health Organization; 1994. WHO, UNICEF, ICCIDD. Indicators for Assessing Iodine Deficiency Disorders and Their Control Through Salt Iodization. WHO/NUT/94. [Google Scholar]
- 26.Solberg HE. International federation of clinical chemistry. Scientific committee, clinical section. Expert panel on theory of reference values and international committee for standardization in haematology standing committee on reference values Approved recommendation (1986) on the theory of reference values. Part 1. The concept of reference values. Clin Chim Acta. 1987;29(165):111–8. doi: 10.1016/0009-8981(87)90224-5. [DOI] [PubMed] [Google Scholar]
- 27.Demers LM, Spencer CA. Laboratory medicine practice guidelines: Laboratory support for the diagnosis and monitoring of thyroid disease. Clin Endocrinol (Oxf) 2003;58:138–40. doi: 10.1046/j.1365-2265.2003.01681.x. [DOI] [PubMed] [Google Scholar]


