Abstract
Objective:
Morning blood pressure surge (MBPS) is an independent predictor of cardiovascular events. However, little is known about the association between glycemic control and MBPS, and its effect on vascular injury in patients with type 2 diabetes mellitus (T2DM). The current study examined the association between glycemic control and MBPS and the involvement of MBPS in the development of vascular dysfunction in T2DM patients.
Materials and Methods:
One hundred and twenty-two consecutive T2DM outpatients from the Department of Cardiology and Endocrinology were enrolled in this study. We did MBPS in T2DM patients, 85 (male) (69.7%) patients and 37 (female) patients (30.3%); mean age 60.1 ± 9.39; (n = 122) using 24 h ambulatory blood pressure monitoring and assessed vascular function by brachial artery flow-mediated dilation (FMD) and nitroglycerin-mediated dilation (NMD).
Results:
The correlation between MBPS and various clinical variables were examined by single regression analysis in all subjects. MBPS showed significant and positive correlation with pulse rate (P = 0.01), fasting blood sugar (P = 0.002), and postprandial blood sugar (P = 0.05). To further confirm the association of insulin resistance (IR) with MBPS in T2DM patients, we examined the correlation between homeostasis model assessment-IR (HOMA-IR), an established marker of IR and MBPS in diabetic (DM) patients who were not taking insulin no significant association with MBPS in T2DM patients (P = 0.41), angiotensin-converting enzyme/angiotensin receptor blocker (P = 0.07). We examined the relationship between MBPS and vascular injury by measuring endothelium-dependent FMD and endothelium-independent NMD in T2DM patients. Among the various traditional risk factors for atherosclerosis such as DM duration (P = 0.04), platelet reactivity (P = 0.04) and morning surge (P = 0.002) emerged as significant factors. HOMA-IR was a negative correlation with FMD.
Conclusions:
The current study demonstrated that poor glycemic control and IR have predictive value for the occurrence of MBPS in T2DM patients, which might be significantly associated with endothelial dysfunction.
Keywords: Flow-mediated dilation, morning blood pressure surge, type 2 diabetes mellitus
INTRODUCTION
Many studies in the past decade have demonstrated diurnal variation in the onset of acute cardiovascular disorders in hypertensive patients, such as acute coronary syndrome, and ischemic and hemorrhagic stroke occurring in the morning (6:00 am to noon) after a nadir in these events during the night. Blood pressure (BP) falls during the night[1] because of the reduction of sympathetic activity, that is, brought about by sleep and then increases steeply when in the morning the subject awakes and resumes his/her daily activities.[2] This increase occurs together with a peak incidence of cerebral and cardiac events in the morning hours.[3] Moreover, a recent prospective study suggests that higher morning BP surge (MBPS) might be an independent risk factor of atherosclerotic events beyond ambulatory BP and nocturnal BP falls.[4] The molecular mechanisms associating MBPS peak and vulnerable atherosclerotic plaque are not clear, although inflammation, which plays a central role in the cascade of events that result in plaque erosion and fissuring also were related to MBPS.[5]
Patients with diabetes mellitus (DM) tend to exhibit accelerated arteriosclerosis and are consequently at higher risk of cardiovascular disease (CVD) including stroke and coronary heart disease.[6] DM is often complicated with other comorbidities that contribute to increased risk of CVD (i.e., hypertension, chronic kidney disease, and dyslipidemia).
It has been increasingly recognized that the early MBPS (i.e., the increase in BP that occurs during the period from night to early morning), which can be detected by ambulatory BP monitoring (ABPM), provides a clinically relevant measure to predict CVD risk independent of age and 24 h systolic BP (SBP).[7] This concept is supported by data indicating that cerebral and cardiac events occur most often in the morning.[8] It is possible that inadequate glycemic control[9] or the occurrence of insulin resistance[10] (IR) activate sympathetic activity, which leads to MBPS in DM patients. Furthermore, hypertensive patients with exaggerated MBPS exhibit elevated levels of macrophages, T-lymphocytes, and tumor necrosis factor alpha in atherosclerotic plaques obtained from the carotid artery compared with those without exaggerated MBPS, suggesting an association between MBPS and vascular injury in hypertensive patients.[11] Taken together, these results suggest that poor glycemic control could accelerate vascular injury in DM patients by causing MBPS.
In recent years, flow-mediated dilation (FMD) has become a popular technique in cardiovascular medicine and clinical physiology, as evidence has occurred that depressed FMD is an independent prognostic index of the incident and recurrent cardiovascular events which add predictive value to the established risk factors.[12,13,14,15,16]
The FMD measurement has gained growing interest as several studies indicated that a decreased FMD response predicts arterial disease progression with intimal thickening and increased cardiovascular mortality.[17,18] Several cardiovascular risk factors have been shown to lead to acute and chronic FMD impairment.[19]
IR is defined as a disorder of insulin-mediated glucose release. Although hyperinsulinemia itself is a marker for IR, it can also be determined using methods such as Hyperinsulinemic - euglycemic clamp technique[20], intravenous glucose tolerance test, and insulin compression test. Since these techniques are complicated and difficult to utilize, and there are some difficulties in the daily routine usage and application to wide populations, easier indexes which were depended on clinical measurements were improved. Homeostasis model assessment-IR (HOMA-IR) is well-correlated with hyperglycemic-euglycemic clamp technique[20,21,22,23] which was accepted to determine the insulin sensitivity as a valuable standard.[24] It has been shown that insulin has various vascular benefits, such as increasing the nitric oxide (NO) activity, releasing NO synthesis gene expression[25,26] and causing vasodilatation.[27] As it is known that insulin increases the endothelium-dependent dilation, this effect is impaired with the presence of IR.
It is known that endothelial dysfunction has a close relation with hyperglycemia, hypertension, dyslipidemia, fibrinolysis, obesity, and IR, has a major role in the progression of microvascular complications.[28,29,30,31]
In this study, we evaluated (1) the association of IR and glycemic control with MBPS and (2) the association between MBPS and vascular endothelial dysfunction as assessed by endothelium-dependent flow-mediated dilation (FMD) and nitroglycerin-mediated dilation (NMD) in type 2 DM (T2DM) patients.
MATERIALS AND METHODS
Study patients
One hundred twenty-two consecutive outpatients from the Department of Cardiology and Endocrinology were enrolled in this study during the period of March 2014–November 2014. Ethical Committee approval and informed consent from each patient were obtained. Exclusion criteria were the presence of clinically evident micro/macrovascular complications of diabetes, i.e., the clinical or electrocardiogram (ECG) evidence of CAD, past or present episodes of stroke, and/or transient ischemic attack, clinical evidence of peripheral vascular disease, features suggestive of diabetic retinopathy on fundus examination, the presence of overt proteinuria or serum creatinine >2 mg/dl, clinical evidence of diabetic neuropathy, associated comorbid illness which is likely to influence the endothelial function such as congestive cardiac failure, liver diseases, chronic infections, and renal diseases.
Procedural protocol
Clinical examination included BP measurement, cardiovascular examination, and body mass index (BMI) measurement. Biochemical assessment included fasting blood sugar (FBS), postprandial blood sugar levels, and comprehensive lipid profile. Plasma glucose, insulin, and lipid estimation were done after an overnight Fasting for 12 hours. HOMA-IR ratio, which was calculated as fasting insulin (µU/ml) multiplied by fasting plasma glucose (mg/dl) and divided by 405, was used to assess the insulin sensitivity in the subjects without insulin therapy. Diabetes mellitus was defined as per the American Diabetes Association criteria.
Determination of vascular function with ultrasound
The ultrasound method for measuring endothelium-dependent and endothelium-independent arterial dilation has been used as described previously. The brachial artery diameter was measured on B-mode ultrasound images, with the use of a 7.5 MHz linear array transducer with image point HX Ultra sound Equipment (iE33 2D Echo, Philips Ultrasound Bothell, Washington, USA). The right brachial artery was studied in all the subjects. Brachial artery endothelial function was studied after the subject had abstained from alcohol, caffeine, and smoking for 8 h. Scans were obtained with the subject at rest, during reactive hyperemia and again at rest. The subjects were asked to lie quietly for at least 10 min before the first scan. The brachial artery was scanned in longitudinal section 2 cm above the elbow, the center of the artery was identified when the clearest picture of the anterior and posterior intimal layers was obtained. The transmit (focus) zone was set to the depth of the near wall because of the greater difficulty in evaluating the ‘M’ line (the interface between the media and adventitia) of the near wall as compared with that of the far wall. Depth and gain settings were set to optimize images of the interface between the lumen and the arterial wall, and the images were magnified. Settings for operating the machine were not changed during the study.
When a satisfactory transducer position was found, the skin was marked, and the arm was kept in the same position throughout the study. A resting scan was obtained. The arterial diameter was measured. Increased flow was then induced by the inflation of a sphygmomanometer cuff placed around the forearm (distal to the scanned part of the artery) to a pressure of 200 mmHg for 5 min followed by release. A second scan was performed continuously of 30, 60, and 90 s after deflation of the cuff. The diameter of the artery was measured at the peak of R wave (corresponding to end-diastole).
Flow-mediated dilation was calculated, and the average result of the three observations was recorded. Flow-mediated dilation results are presented as the percent change diameter postischemia (d2) – diameter baseline (d1) divided by diameter baseline (d1) multiplied by 100. Severe endothelial dysfunction was defined as FMD <5.5% as has been described. Fifteen minutes was allowed for vessel to recovery, and then a further resting scan was taken then sublingual glyceryl trinitrate (200 mcg puff) was administered and 4 min after, the last scan was done. ECG was monitored throughout the scans, and artery diameter measured at the peak of R wave (corresponding to end-diastole). An average of three values was taken for each measurement. None of these patients were treated with dietary therapy alone. Of 122 patients, 46 (37.70%) patients were on insulin therapy, 61 (87.14%) patients were on metformin therapy, 27 (38.6%) patients were on glimepiride, 3 (4.3%) patients were on vildagliptin, 14 (20%) patient were on voglibose, and 11 (15.7%) patients were on saxagliptin. Fifty-five (78.6%) were undergoing treatment with β-blockers, angiotensin-converting enzyme/angiotensin receptor blocker (ACE/ARB) were 54 (77.1%), 17 (24.3%) were on calcium channel blockers (CCBs), and 20 (28.6%) were additional using diuretics along with other drugs. BMI was calculated as body weight (in kilograms)/height (in square meters).
Blood pressure measurements and analysis of ambulatory blood pressure monitoring data
Noninvasive ABPM was performed in a hospital setting with an automated system (BR-102 Plus Schiller, Switzerland) that records BP using the auscultatoric and oscillometric method and pulse rate every 1 h for 24 h, as described previously.[32] Awake and sleep time were defined on the basis of written diaries recorded by the patients during the ABPM. The morning BP was defined as the average of the four BP values obtained during the first 2 h after waking up. The lowest BP was defined as the average of the three BP readings centered around the lowest night time reading (i.e., the lowest night time reading plus the readings immediately before and after). The MBPS was calculated as the morning SBP minus the lowest SBP as reported previously. Smoothness index is defined as the homogeneity of the BP reduction induced by antihypertensive treatment over the 24 h.
Statistical analysis
Statistical software
The Statistical software namely SAS 9.2 (SAS institute USA), SPSS 15.0 (IBM Version), Stata 10.1 (stata Corp), Med Calculator 9.0.1 (software bvba of tend Belgium), Systat version 12.0 (Systat software Inc), and R environment version 2.11.1 (the R foundation) were used.
Statistical methods
Descriptive and inferential statistical analysis has been carried out in this study. Results on continuous measurements are presented as mean ± standard deviation (minimum-maximum), median (limits of observed values) was used for the DM duration, HOMA-IR, urine microalbumin, and urine albumin to creatinine ratio. Unpaired samples were analyzed nonparametrically using Mann–Whitney U-test. Correlation coefficients were calculated by simple and multiple regression analyses. Receiver-operating characteristic (ROC) curves were constructed to access the best MBPS for the identification of the presence of vascular dysfunction. The value P < 0.05 was considered statistically significant.
RESULTS
The clinical characteristics of 122 patients were summarized in Table 1. Of 122 patients, 85 (69.7%) were male and 37 (30.3%) were female patients. The duration of diabetes was 0.22–36 years, smokers were 20 (16.39%), alcoholic patients were 14 (11.47%), 13 patients were hypothyroid (10.8%), postmenopausal women were 34 (30.6%). Of 122 patients, 51 (41.8%) patients were on insulin therapy, 111 (91%) patients were on metformin therapy, 47 (38.5%) patients were on glimepiride, 6 (4.9%) patients were on vildagliptin, 26 (21.3%) patient were on voglibose, and 17 (13.9%) patients were on saxagliptin. Eighty-one (66.4%) were undergoing treatment with β-blockers, ACE/ARB were 89 (73%), 44 (36.1%) were on CCBs, and 40 (32.8%) were additional using diuretics along with other drugs. No significant difference existed in MBPS between patients receiving treatment and not receiving treatment with β-blockers (P = 0.23), ACE/ARBs (P = 0.63) or CCBs (P = 0.38), and diuretics (P = 0.51). MBPS did not significant between those receiving and not receiving treatment with statins.
Table 1.
Demographic data
In this study, out of 122 patients, morning surge was present in 108 (88.52%) and absent in 14 (11.46%) patients, smoothness index was 0.73 ± 0.10 (P = 0.039), impaired HOMA-IR (3 and above) was observed in 37 (48.68%) patients. Severely impaired FMD, i.e., <5.5 was observed in 69 (57.02%) patients. MBPS was significantly lower in patients with normal FMD than in those with impaired FMD (P = 0.002). FMD was not significantly lower in patients receiving β-blockers (P = 0.07), ACE/ARB (P = 0.75), CCB (P = 0.34), diuretics (P = 0.62), and statins (P = 0.68) also did not show significant difference between normal and severely impaired FMD.
Correlations of the clinical variables with morning blood pressure surge in type 2 diabetes mellitus patients
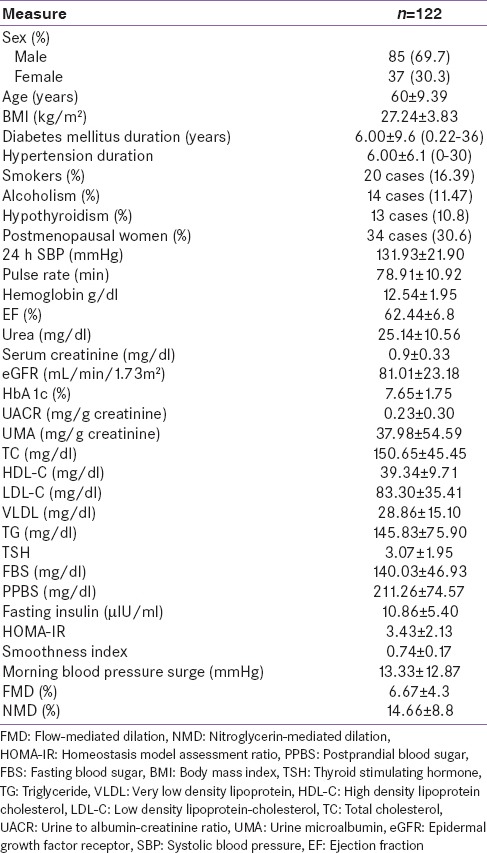
On univariate analysis MBPS shows significant and positive correlation with pulse rate (P = 0.001), FBS (P = 0.002), PBBS (P = 0.002), nondippers (P = 0.000) and FMD (P = 0.035). On further analyzed by multivariate regression analysis of MBPS [Table 2] showing negative correlation with BMI (r = −0.12, B = 0.88), HBA1c (r = −0.61, B = 0.54), FMD (r = −0.12, B = 0.88), and ACE/ARB (r = −3.12, B = 0.04). We next examined the relationship between MBPS and vascular injury by measuring endothelium-dependent FMD and endothelium-independent NMD in type 2 DM (T2DM) patients. Only DM duration was (P = 0.04), pulse rate (P = 0.04), and extreme dipper (P = 0.000) were statistically significant. By regression analysis, there was a negative correlation with FMD and MBPS (P = 0.03) [Figure 1].
Table 2.
Multiple regression analysis and morning blood pressure surge
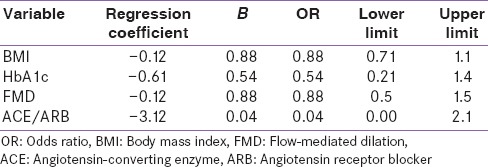
Figure 1.
Co-relation between morning blood pressure surge and vascular dysfunction flow-mediated dilation
To further confirm the association of IR with MBPS in T2DM patients, we examined the correlation between HOMA-IR, an established marker of IR and MBPS in DM patients who were not undergoing insulin which were negatively correlated on logistic regression analysis.
ROC curve was constructed for the best cutoff value of MBPS at 19.67 mmHg sensitivity was 0.435, specificity was 0.90, positive predictive value was 85.71, and negative predictive value was 0.62 [Figure 2].
Figure 2.
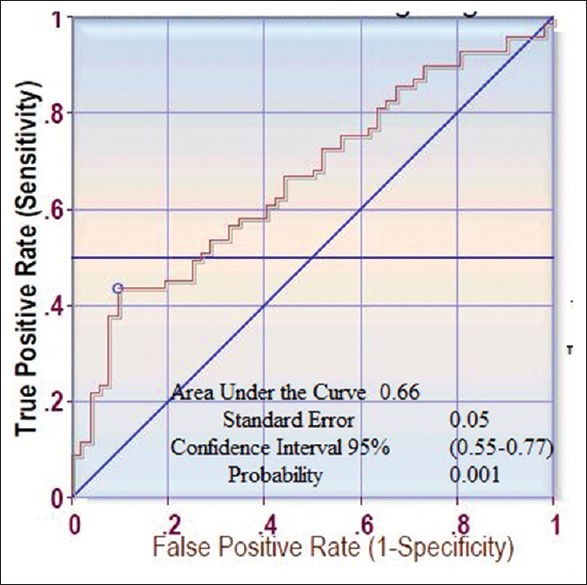
The receiver-operating characteristic curve for the morning blood pressure surge and vascular dysfunction flow-mediated dilation
DISCUSSION
In the current study, we demonstrated that MBPS was significantly and independently associated in a negative fashion with FMD but not NMD. These data suggested that poor glycemic control or IR is associated with the occurrence of MBPS in T2DM patients, which might be associated with the development of endothelial dysfunction in these patients. It was suggested that triglyceride (TG) level might provide an index for IR, we did the correlations of the established markers of IR, HOMA-IR with MBPS, after the subjects were restricted to DM patients not receiving insulin therapy. However, a significant correlation between HOMA-IR and MBPS was not observed in this study.
MBPS is considered to result from increased activities of the sympathetic nervous system, the renin-angiotensin system, and the hypothalmic-pituitary-adrenal (HPA) axis during the latter half of the sleep cycle. Because IR is known to stimulate sympathetic activity by affecting the metabolism of adipocytokines, such as leptin, the association of TG level and HOMA - IR with MBPS might be explained through increased sympathetic activity by IR. Furthermore, it remains possible that insulin-stimulated reabsorption of sodium at the renal tubule might contribute to the development of MBPS. The activity of the HPA axis shows a clear circadian rhythm exhibiting a rapid rise during the latter half of the sleep cycle, with the highest levels occurring in the early morning in parallel with the time course change of BP, suggesting the involvement of a diurnal change in HPA axis in the generation of MBPS. It was reported that T2DM patients exhibit higher baseline levels of serum cortisol. Therefore, it is possible that IR might make the association with MBPS significant by its stimulatory effect on HPA axis early in the morning. Chronic hyperglycemia is reported to cause endothelial dysfunction by accumulating advanced glycosylation end products in the vascular wall, resulting in the development of vascular injury. In fact, hypertensive DM patients exhibit higher levels of plasma advanced glycosylation end products than their nonhypertensive counterparts.
Impaired FMD of the brachial artery, which is mainly caused by the loss of endothelium-derived NO, has been established as a relevant marker for endothelial dysfunction. MBPS appears to play the most important role in the development of endothelial dysfunction, as reflected by the strongest association of MBPS with CVD risk. Because MBPS might acutely increase the mechanical stretch on endothelial cells, it augmented the production of endothelium-derived superoxide, resulting in the inactivation of NO. Indeed, it was reported that marked fluctuations in BP in sinoaortic denervated rats significantly impaired endothelial function by reducing acetylcholine-induced NO release from aortic rings. In the current study, HbA1c level correlated significantly in a negative manner with FMD in univariate regression analyses, but not in multiple regression analyses including MBPS as an independent variable, suggesting the intimate involvement of MBPS in the association of HbA1c level and FMD. The previous study, demonstrating the association of a blunted dip in BP during sleep with endothelial dysfunction in T2DM patients, might support our data suggesting MBPS as an important factor to accelerate vascular damage in T2DM patients.
Our study showed that MBPS was associated with impaired FMD, a marker for endothelium injury, but not NMD, a marker of vascular smooth muscle function. Because it was reported that DM patients showed vascular injury more predominantly in the endothelium than on the arterial wall, the preferential association of MBPS with FMD, but not NMD, might validate the importance of MBPS in the development of vascular injury in T2DM patients. Alternatively, T2DM patients with preexisting CVD were negated from this study, and thus their atherosclerotic changes were in the early but not advanced stage. In the early stage of DM, endothelial dysfunction, as reflected by impaired FMD, might predominately occur over arterial smooth muscle dysfunction as represented by impaired NMD.
The limitations of our study are as follows. First, ABMP is ideally conducted at home in a routine daily environment. Second, because DM patients enrolled in the study were treated with various drugs, including antihypertensive drugs and statins, the effect of those treatments on MBPS could not be totally neglected.
CONCLUSION
The current study demonstrated that poor glycemic control and IR have predictive value for the occurrence of MBPS in T2DM patients, which might be significantly associated with endothelial dysfunction.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–22. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, et al. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res. 1983;53:96–104. doi: 10.1161/01.res.53.1.96. [DOI] [PubMed] [Google Scholar]
- 3.Muller JE. Circadian variation in cardiovascular events. Am J Hypertens. 1999;12(2 Pt 2):35S–42S. doi: 10.1016/s0895-7061(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 4.Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: A prospective study. Circulation. 2003;107:1401–6. doi: 10.1161/01.cir.0000056521.67546.aa. [DOI] [PubMed] [Google Scholar]
- 5.Marfella R, Siniscalchi M, Nappo F, Gualdiero P, Esposito K, Sasso FC, et al. Regression of carotid atherosclerosis by control of morning blood pressure peak in newly diagnosed hypertensive patients. Am J Hypertens. 2005;18:308–18. doi: 10.1016/j.amjhyper.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Kannel WB, Schwartz MJ, McNamara PM. Blood pressure and risk of coronary heart disease: The Framingham Study 1969. Chest. 2009;136(5 Suppl):e23. doi: 10.1378/chest.56.1.43. [DOI] [PubMed] [Google Scholar]
- 7.White WB. The risk of waking-up: Impact of the morning surge in blood pressure. Hypertension. 2010;55:835–7. doi: 10.1161/HYPERTENSIONAHA.109.148908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79:733–43. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- 9.Del Rio G, Carani C, Baldini A, Marrama P, Della Casa L. Chronobiology of catecholamine excretion in normal and diabetic men. J Endocrinol Invest. 1990;13:575–80. doi: 10.1007/BF03348628. [DOI] [PubMed] [Google Scholar]
- 10.Smith MM, Minson CT. Obesity and adipokines: Effects on sympathetic overactivity. J Physiol. 2012;590(Pt 8):1787–801. doi: 10.1113/jphysiol.2011.221036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marfella R, Siniscalchi M, Portoghese M, Di Filippo C, Ferraraccio F, Schiattarella C, et al. Morning blood pressure surge as a destabilizing factor of atherosclerotic plaque: Role of ubiquitin-proteasome activity. Hypertension. 2007;49:784–91. doi: 10.1161/01.HYP.0000259739.64834.d4. [DOI] [PubMed] [Google Scholar]
- 12.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–75. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 13.Karatzis EN, Ikonomidis I, Vamvakou GD, Papaioannou TG, Protogerou AD, Andreadou I, et al. Long-term prognostic role of flow-mediated dilatation of the brachial artery after acute coronary syndromes without ST elevation. Am J Cardiol. 2006;98:1424–8. doi: 10.1016/j.amjcard.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 14.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol. 2008;51:997–1002. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 15.Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, et al. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol. 2009;134:52–8. doi: 10.1016/j.ijcard.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 18.Halcox JP, Donald AE, Ellins E, Witte DR, Shipley MJ, Brunner EJ, et al. Endothelial function predicts progression of carotid intima-media thickness. Circulation. 2009;119:1005–12. doi: 10.1161/CIRCULATIONAHA.108.765701. [DOI] [PubMed] [Google Scholar]
- 19.Kelm M. Flow-mediated dilatation in human circulation: Diagnostic and therapeutic aspects. Am J Physiol Heart Circ Physiol. 2002;282:H1–5. doi: 10.1152/ajpheart.2002.282.1.H1. [DOI] [PubMed] [Google Scholar]
- 20.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 21.Pacini G, Bergman RN. MINMOD: A computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed. 1986;23:113–22. doi: 10.1016/0169-2607(86)90106-9. [DOI] [PubMed] [Google Scholar]
- 22.Shen SW, Reaven GM, Farquhar JW. Comparison of impedance to insulin-mediated glucose uptake in normal subjects and in subjects with latent diabetes. J Clin Invest. 1970;49:2151–60. doi: 10.1172/JCI106433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korytkowski MT, Berga SL, Horwitz MJ. Comparison of the minimal model and the hyperglycemic clamp for measuring insulin sensitivity and acute insulin response to glucose. Metabolism. 1995;44:1121–5. doi: 10.1016/0026-0495(95)90003-9. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, et al. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101:1539–45. doi: 10.1161/01.cir.101.13.1539. [DOI] [PubMed] [Google Scholar]
- 26.Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, et al. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: A specific vascular action of insulin. Circulation. 2000;101:676–81. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- 27.Cardillo C, Kilcoyne CM, Nambi SS, Cannon RO, 3rd, Quon MJ, Panza JA. Vasodilator response to systemic but not to local hyperinsulinemia in the human forearm. Hypertension. 1998;32:740–5. doi: 10.1161/01.hyp.32.4.740. [DOI] [PubMed] [Google Scholar]
- 28.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138(5 Pt 2):S419–20. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 29.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–10. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tooke JE. Microvascular function in human diabetes. A physiological perspective. 1995;44:721–6. doi: 10.2337/diab.44.7.721. [DOI] [PubMed] [Google Scholar]
- 31.King GL, Wakasaki H. Theoretical mechanisms by which hyperglycemia and insulin resistance could cause cardiovascular diseases in diabetes. Diabetes Care. 1999;22(Suppl 3):C31–7. [PubMed] [Google Scholar]
- 32.Imai Y, Sasaki S, Minami N, Munakata M, Hashimoto J, Sakuma H, et al. The accuracy and performance of the A&D TM 2421, a new ambulatory blood pressure monitoring device based on the cuff-oscillometric method and the Korotkoff sound technique. Am J Hypertens. 1992;5:719–26. doi: 10.1093/ajh/5.10.719. [DOI] [PubMed] [Google Scholar]


