Abstract
Background:
Diabetic ketoacidosis (DKA) is one of the commonly encountered diabetes mellitus emergencies.
Aim:
This study aimed at describing the clinical profiles and hospitalization outcomes of DKA patients at the University Teaching Hospital (UTH) in Lusaka, Zambia and to investigate the role of coma on mortality outcome.
Materials and Methods:
This was a cross-sectional analytical study of hospitalized DKA patients at UTH. The data collected included clinical presentation, precipitating factors, laboratory profiles, complications, and hospitalization outcomes. Primary outcome measured was all-cause in-hospital mortality.
Results:
The median age was 40 years. Treatment noncompliance was the single highest identified risk factor for development of DKA, followed by new detection of diabetes, then infections. Comatose patients were significantly younger, had lower baseline blood pressure readings, and higher baseline respiratory rates compared to noncomatose patients. In addition, comatose patients had higher baseline admission random blood glucose readings. Their baseline sodium and chloride levels were also higher. The prevalences of hypokalemia, hypernatremia, and hyperchloremia were also higher among comatose patients compared to noncomatose patients. Development of aspiration during admission with DKA, pneumonia at baseline, development of renal failure, and altered mental status were associated with an increased risk of mortality. Development of renal failure was independently predictive of mortality.
Conclusion:
The mortality rate from DKA hospitalizations is high at UTH. Treatment noncompliance is the single highest identifiable precipitant of DKA. Aspiration, development of renal failure, altered sensorium, and pneumonia at baseline are associated with an increased risk of mortality. Development of renal failure during admission is predictive of mortality.
Keywords: Coma, diabetic ketoacidosis, hospitalization outcomes, precipitating factors
INTRODUCTION
Diabetes mellitus (DM) is a chronic disease associated with abnormal blood glucose regulation. It is characterized by a myriad of metabolic abnormalities and long-term complications. Its most serious acute metabolic complications include the two hyperglycemic emergencies; diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state.[1] These hyperglycemic emergencies are associated with very high mortality rates in the developing world.[2]
DKA is a frequently encountered hospital emergency and is associated with significant morbidity and mortality. According to the American Diabetes Association, DKA mortality outcome has been reported to be <5% in treatment-experienced centers of America, Europe, and Asia.[3] In Africa, mortality from hyperglycemic emergencies is not well documented but comparatively high in published studies.[4,5,6]
There are a number of factors that have been found useful in predicting the outcome of DKA in different clinical setups worldwide. For instance, mortality as a major adverse outcome has been associated with a number of factors such as diabetic foot, advanced age, sepsis, hypertension, and hypokalemia.[2] Describing the clinical characteristics and outcome predictors in DKA patients is useful in guiding clinicians to manage some poor prognostic indicators more aggressively to improve outcome.
There are no previously published reports on treatment outcomes of DKA patients in Zambia. The aims of this study were to establish the clinical characteristics of DM patients that were admitted with DKA, to describe the predictors of clinical outcomes, and to ascertain the prognostic value of coma.
MATERIALS AND METHODS
This study was conducted at the Medicine Department of the University Teaching Hospital (UTH) in Lusaka, Zambia. UTH is Zambia's national referral hospital, which also acts as a teaching hospital for the University of Zambia School of Medicine and several nursing colleges in Zambia.
This was an analytical cross-sectional study that was conducted from October 2013 to July 2014. Consenting DKA patients who were 16-year-old and above were recruited and followed up throughout their hospital stay. Comatose patients were only enrolled if their next of kin consented to participation in the study.
DKA was diagnosed when the patient had an admission random blood glucose of >13.9 mmol/L, ketonuria of 2+ or more on urine dipstick, with evidence of acidosis demonstrated by 1 or more of the following; pH <7.3, HCO3 −<18 mEq/L, and a high anion gap (>12).
The operational definition of coma was an admission Glasgow Coma Scale (GCS) of between 3 and 8.[7]
Exclusion criteria were recent alcohol binge in the last 36 h (males >10 units in 2 h, females >8 units in 2 h), history of poisoning with salicylate, ethylene glycol, paraldehyde, methanol, isopropanol or propylene glycol, and pregnancy. Convenience sampling was utilized to maximize on patient recruitment rate into the study.
A full history was taken by either the attending physician or the researcher in patients who met the diagnostic criteria. A physical examination was then done on the recruited participants and included blood pressure, heart rate, respiratory rate and type, urinalysis and determination of ketonuria, and temperature. In addition, a vigorous effort to identify possible infection sources and sites was undertaken. The researchers had to verify the accuracy of the history and physical examination findings before recruitment into the study if they were done and recorded by a non-member of the study team.
About 4 mL of blood was drawn from each patient to measure glucose, pH, sodium, potassium, chloride, and bicarbonate levels in the laboratory. Participants were also subjected to diagnostic counseling and testing for HIV infection.
Each participant was followed up until the end of hospitalization (defined either as death of a patient or discharged alive).
Data were collected on a hard copy using a structured data collection sheet and later stored and analyzed in statistical package for social sciences version 20 (IBM corp., New York, USA). Frequency tables were used to describe the sociodemographic characteristics of the patients. Comparisons of medians were done using Kruskal–Wallis one-way ANOVA for continuous data to test for statistical significance. Fisher's exact test was used to examine associations between categorical data and outcome.
All baseline biochemical and clinical variables were analyzed to identify variables that potentially had a significant association with primary outcome (mortality). All variables with a P < 0.20 were further subjected to binary logistic regression using backward Wald algorithm to determine adjusted odds ratios and independent predictors of mortality. A two-sided P < 0.05 was considered as statistically significant.
RESULTS
Patient characteristics
Eighty enrolled patients were included in the final analysis. Their ages ranged from 16 to 85-year-old, and the mean age of the patients was 43.73 (standard deviation 15.61) years. Sixty percent of the patients were 35 years or older. The male to female ratio was 1. The most common identifiable risk factor for development of DKA was nonadherence to prescribed anti-diabetic treatments (42.5%). Of the 58 patients who were previously diagnosed with diabetes, only 32.8% reported strict adherence to their treatments. The second leading precipitant was new diabetes diagnosis upon admission with DKA at 27.5%. Infection was the third identifiable precipitant at 22.5%. The infections associated with the development of DKA in decreasing order of magnitude were urinary tract infection, pneumonia, diabetic foot and other infected wound and upper respiratory tract infections. Of the 10 study subjects with infected wounds, diabetic foot was the most common (70%) while the remainder had decubitus ulcers (30%). There were no obvious triggers identified in the remaining 7.5% of the study participants. Comorbidities included hypertension (36.25%), tuberculosis (2.5%), prostate cancer (1.25%), and HIV infection (15%).
Comparison of biodemographic characteristics between comatose and noncomatose diabetic ketoacidosis patients
The median GCS for comatose patients was 7 while that of their noncomatose counterparts was 15. Tables 1 and 2 show their baseline bio-demographic differences. Comatose patients were younger (30.0 vs. 42.5 years, P = 0.005) and had lower baseline blood pressure readings than their counterparts. They also had a higher respiratory rate and blood glucose levels at baseline. As expected, the comatose patients also had higher median blood sodium levels. There was no statistically significant difference in hospital length of stay between the two groups [Table 1].
Table 1.
Bio-demographic baseline data of the participants
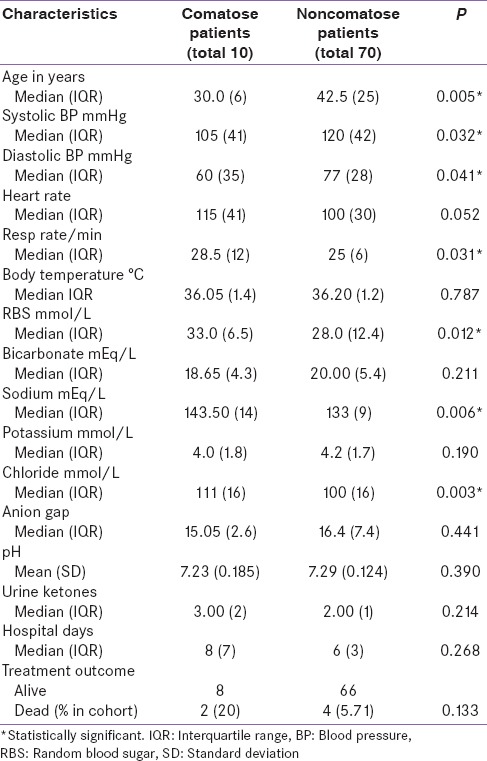
Table 2.
Clinico-laboratory derangements of DKA
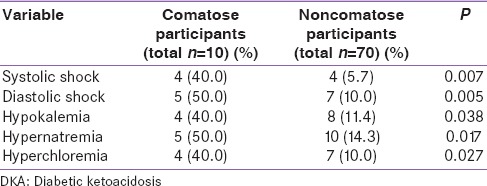
There were statistically significant differences in the presence of both systolic and diastolic shock between comatose and noncomatose patients, with a larger proportion of comatose patients having a shock on admission. Serum potassium, serum sodium, and serum chloride were also significantly higher in comatose patients compared to noncomatose patients [Table 2].
Complications
As shown in Figure 1, a number of complications were recorded in a quarter of the study participants.
Figure 1.
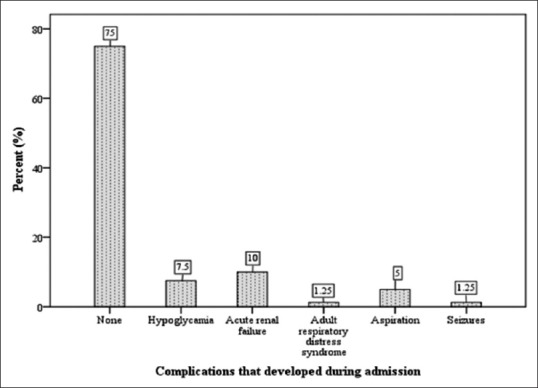
Complications during hospitalization for diabetic ketoacidosis
There were no obvious complications detected in 75% of the patients. Hypoglycemia accounted for 7.5% of the complications reported during treatment and this was corrected with intravenous dextrose infusion and all affected were eventually discharged. Ten percent developed acute renal failure while 1.25% developed adult respiratory distress syndrome, 5% had aspiration associated with comatose state, and a single participant (1.25%) had a seizure.
Risk factors associated with mortality
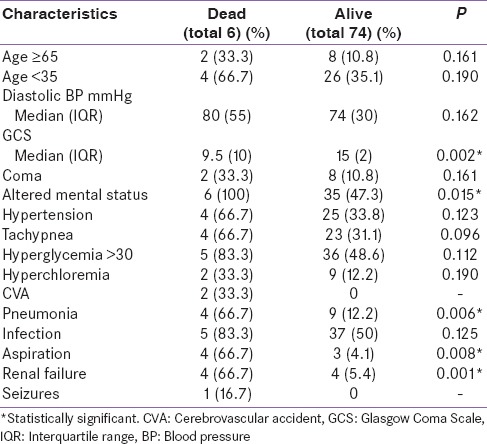
Table 3 shows that there were statistically significant associations between mortality outcome and each of the following variables; altered mental status, pneumonia diagnosis at baseline, renal failure, cerebrovascular accident (CVA), and development of aspiration [Table 3].
Table 3.
Risk factors of mortality
All variables in Table 3 with a P < 0.20 were submitted to binary logistic regression using backward Wald algorithm, and renal failure was found to be an independent predictor of mortality is presented in Table 4.
Table 4.
Mortality prediction

There was a very wide confidence interval for the only independent predictor of mortality which suggests that this was very likely a pure chance finding. The positive outcomes of this analysis were too low to have a conclusive result.
Six participants (7.5%) died. Although, the study found an association between altered mental status and mortality outcome, coma as a feature at admission was neither predictive of nor associated with an increased risk of mortality.
DISCUSSION
The study showed that mortality outcome was high compared to statistics from advanced treatment centers in other parts of the world. Although, altered sensorium was found to have a statistically significant association with mortality outcome, the study findings suggest that comatose state on admission did not necessarily increase the risk of mortality. Development of renal failure during hospitalization was found to be an independent predictor of mortality outcome in this study.
Comatose patients were significantly younger than noncomatose patients. Although, there was no statistically significant difference, the median age of those who died during hospitalization for DKA was equally younger compared to those who were eventually discharged. This contrasts findings in other studies in both advanced and resource challenged settings including Sweden, Nigeria, and Libya where mortality outcomes were higher in patients older than 65 years.[8,9,10] Several other studies have shown that advanced age is associated with poorer outcomes of DKA. A possible explanation is that younger patients in the study may have had severe insulin deficiency and hence suffered more adverse outcome compared to their older counterparts who may have had relatively higher insulin reserves with preserved beta cell function associated with Type 2 diabetes. In addition, older patients may have chronically been on some form of diabetes treatment compared to younger patients who may have been more recently diagnosed and presenting with increased morbidity and mortality risk. In the face of the severe insulin deficiency that afflicts the generally younger patients with Type 1 diabetes, the younger population may have also succumbed more to adverse outcome as there were insulin administration interruptions that affected most of the patients due to frequent stock-outs of essential monitoring tools and manpower shortages. Older patients who were more likely to have had Type 2 DM may have been able to tolerate insulin dose omissions better as long as they were getting some rehydration. In addition, according to Welch and Zib in 2004, development of DKA is rare in Type 2 diabetes compared to Type 1 which afflicts younger patients mostly.[11] The higher prevalence of DKA in younger patients statistically increases their risk of mortality from DKA.
The factors which were associated with development of DKA in this study were treatment noncompliance, infection, and new onset diabetes. These were the commonly identified factors in similar studies.[12] The leading precipitant of DKA was identified as nonadherence to prescribed anti-diabetic drugs. Majority of the 58 participants who were already diagnosed (93.1%) had been on anti-diabetic medications. Two of these participants reported having been exclusively on lifestyle modification strategies as advised at their primary health care clinics. Another two were using self-prescribed undisclosed herbal treatments only. They, however, recovered without complications and were later discharged from in-hospital treatment. Compliance was poor in more than half (58.6%) of the previously known diabetics but there was no significant difference between the comatose and noncomatose groups. This situation may be as a result of a weak primary health care establishment and lack of basic health information. Nearly a third of the participants (27.5%) were previously unaware of their diabetes, and this was the second leading risk factor identified in the study. The third highest identified risk factor for development of DKA was infection at 22.5% while 7.5% of the participants did not have an obviously identifiable precipitant. The predominant identifiable infections in decreasing order of magnitude were urinary tract infections, pneumonia, infected wounds, and upper respiratory tract infections.
Forty percent of the study participants had preexisting hypertension, and the prevalence was similar in the comatose and noncomatose groups. This was a rather high prevalence when the median ages are considered (30.0 vs. 42.5 years for comatose and noncomatose groups respectively) but the study did not investigate for possible secondary causes especially in patients younger than 40 as that was beyond the scope of the study. In addition, two subjects (of 10) who were older than 65 years had ischemic CVAs over 3 weeks prior to admission and this was confirmed on the brain computed axial tomography (CAT) scan. There may have been overlap of the symptoms, signs, and laboratory profiles with DKA, but this could not be certainly established. As described by Jovanovic et al.,[13] CVA may have actually led to the development of DKA, although, the patients had this complication for a relatively long duration.
Two patients were on consolidation phase of anti-tuberculosis therapy, and both reported marked recovery from their pulmonary tuberculosis symptoms at the time of admission with DKA. One participant had prostate cancer. The total prevalence of HIV infection was 15%, and this is lower than the reported district prevalence of 20.8% as published by Central Statistics Office.[14] Furthermore, there was no statistically significant difference between the coma groups (comatose 20% vs. noncomatose 14.3% [P = 0.851]). The study did not demonstrate any association between HIV infection and mortality outcome. The test was not done in eight subjects. Three patients declined the test while the remaining five were affected with either test reagents stock outs or nonresponse from trained HIV counselors to test the patients before hospital discharge.
As expected, comatose patients were noted to have significantly worse clinical and biochemical profiles than noncomatose patients at baseline. Comatose patients had lower baseline blood pressures, higher prevalence of systolic shock, and higher respiratory rates at baseline. Comatose participants also had a higher prevalence of hypokalemia, hypernatremia, and hyperchloremia than their noncomatose counterparts. There was no statistically significant difference in anion gap between the two groups. In a similar study by Otieno et al., altered consciousness was associated with systolic hypotension and severe metabolic acidosis.[15] Total prevalence of hypokalemia from this study was 15%. Studies from other parts of the world have reported widely varied prevalences. For instance, a low prevalence was described in a study done in a multicenter retrospective cross-sectional study at emergency departments in the United States with a sample size of 155,000 adults diagnosed with DKA.[16] Only 7 (0.0045%) of the subjects had hypokalemia. A Nigerian study by Ogbera et al. reported a much higher prevalence (35%) than our findings.[10]
The common complication encountered was acute renal failure followed by hypoglycemia, aspiration, seizures, and adult respiratory distress syndrome. Renal failure may have been due to prolonged prerenal volume contraction from late hospital presentation and inadequate intravenous fluid infusion. It was also noted that there were delays in infusing prescribed intravenous fluids upon admission that affected 78.7% of all the participants. Aspiration while in hospital was found in 66.7% of those who eventually died during hospitalization. All affected patients had altered mental status. Some measures such as head elevation and nasogastric tube insertion which may have helped to prevent aspiration were not readily available for deserving patients at all times. One patient who developed generalized tonic-clonic seizures eventually died during admission. The patient had not been stable enough to be transported for a brain CAT scan as the machine was located far from the admission area. The imaging would have helped in assessing for cerebral edema, a rare complication of DKA in adults which is associated with a mortality of 20–40%.[1] Although this patient had severe metabolic acidosis with a pH of 6.90 and severe pneumonia, etiology of the seizures was not fully investigated, and consent for postmortem was declined by the family.
The mortality rate was high at 7.5% compared to that reported in advanced treatment centers (<5%) by American Diabetes Association.[3] It was however noted to be lower than the observations made in earlier African studies in Kenya (29.8%),[5,15] Democratic Republic of Congo (27.5%),[17] South Africa (11.9%)[18] and Libya (14.4% and 11.9%).[9,19] There were gaps in monitoring and management that may have contributed to this high mortality rate. Most of our patients had limited access to optimum care prescribed on admission and beyond. There was erratic administration of prescribed insulin and fluid replacements in about 78.7% of subjects enrolled into the study. This was particularly observed at the beginning of the study when the nursing staffing had declined as a result of an industrial strike action. Regular monitoring was hampered by frequent stockouts of basic equipment such as point-of-care blood glucose monitoring kits, urinalysis strips, laboratory biochemistry reagents, and appropriate intravenous fluids at times. Currently, there is no point-of-care blood gas analyzer readily available to the department for regular monitoring to allow timely interventions.
Risk factors associated with mortality outcomes included admission with pneumonia, development of aspiration, development of renal failure, and altered mental status. Coma was not necessarily associated with increased risk of mortality outcome. All patients who died had some alteration in mental status. Although, there was no statistically significant difference, these patients who died had a higher prevalence of hypernatremia, hypokalemia, high baseline blood glucose levels >30 mmol/L, and hyperchloremia. In a similar study done in Kenya, mortality was high, and all patients who died had altered mental status on admission.[15] In our study, the total prevalence of altered mental status on admission was 51.3%, and the mortality prevalence among the patients with altered mental status was 14.3%.
Development of renal failure was found to be the only independent predictor of mortality. Due to the small number of subjects with this primary outcome, this may have been a pure chance finding. A larger study would be necessary to study the predictors of mortality. Coma was not found to be associated with an increased risk of mortality.
The sample size was limited by the study duration. We, therefore, propose that a larger prospective study with active investigation for complications and full daily laboratory assessment to study the disease course during DKA admission should be done in future.
CONCLUSION
The mortality rate from DKA hospitalizations is high at UTH, Lusaka. Treatment noncompliance is the single highest identifiable precipitant of DKA. Aspiration, development of renal failure, altered sensorium, CVAs, and pneumonia at baseline are associated with an increased risk of mortality. Development of renal failure during admission is predictive of mortality. There is a need to sensitize DM patients to take their medications regularly, return for follow-up as required by their clinicians, and seek medical attention when they notice any diabetic complications and possible signs of infection. There is also a need for regular primary health care screening so as to avoid acute presentations such as DKA.
Financial support and sponsorship
This work is an output from a project funded by The Welcome Trust through Southern Africa Consortium for Research Excellence for capacity building for the benefit of developing countries.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ganie MA, Koul S, Razvi HA, Laway BA, Zargar AH. Hyperglycemic emergencies in Indian patients with diabetes mellitus on pilgrimage to Amarnathji yatra. Indian J Endocrinol Metab. 2012;16(Suppl 1):S87–90. doi: 10.4103/2230-8210.94267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anumah FO. Management of hyperglycaemic emergencies in the tropics. Ann Afr Med. 2007;6:45–50. doi: 10.4103/1596-3519.55718. [DOI] [PubMed] [Google Scholar]
- 3.Kitabchi AE, Umpierrez GE, Murphy MB, Barrett EJ, Kreisberg RA, Malone JI, et al. Hyperglycemic crises in diabetes. Diabetes Care. 2004;27(Suppl 1):S94–102. doi: 10.2337/diacare.27.2007.s94. [DOI] [PubMed] [Google Scholar]
- 4.Elmehdawi RR, Elmagerhei HM. Profile of diabetic ketoacidosis at a teaching hospital in Benghazi, Libyan Arab Jamahiriya. East Mediterr Health J. 2010;16:292–9. [PubMed] [Google Scholar]
- 5.Mbugua PK, Otieno CF, Kayima JK, Amayo AA, McLigeyo SO. Diabetic ketoacidosis: Clinical presentation and precipitating factors at Kenyatta National Hospital, Nairobi. East Afr Med J. 2005;82(12 Suppl):S191–6. doi: 10.4314/eamj.v82i12.9381. [DOI] [PubMed] [Google Scholar]
- 6.Zouvanis M, Pieterse AC, Seftel HC, Joffe BI. Clinical characteristics and outcome of hyperglycaemic emergencies in Johannesburg Africans. Diabet Med. 1997;14:603–6. doi: 10.1002/(SICI)1096-9136(199707)14:7<603::AID-DIA406>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 7.Russ R and the University of North Carolina at Chapel Hill. How many?. A dictionary of units of measurement. 2000 [Google Scholar]
- 8.Realsen J, Goettle H, Chase HP. Morbidity and mortality of diabetic ketoacidosis with and without insulin pump care. Diabetes Technol Ther. 2012;14:1149–54. doi: 10.1089/dia.2012.0161. [DOI] [PubMed] [Google Scholar]
- 9.Elmehdawi R, Ehmida M, Elmagrehi H. Incidence of Diabetic Ketoacidosis during Ramadan Fasting in Benghazi-Libya. Oman Med J. 2009;24:99–102. doi: 10.5001/omj.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogbera AO, Awobusuyi J, Unachukwu C, Fasanmade O. Clinical features, predictive factors and outcome of hyperglycaemic emergencies in a developing country. BMC Endocr Disord. 2009;9:9. doi: 10.1186/1472-6823-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welch BJ, Zib I. Case Study: Diabetic Ketoacidosis in Type 2 Diabetes: “Look Under the Sheets”. Clin Diabetes; 2004;22:198–200. [Google Scholar]
- 12.Umpierrez GE, Kitabchi AE. Diabetic ketoacidosis: Risk factors and management strategies. Treat Endocrinol. 2003;2:95–108. doi: 10.2165/00024677-200302020-00003. [DOI] [PubMed] [Google Scholar]
- 13.Jovanovic A, Stolic RV, Rasic DV, Markovic-Jovanovic SR, Peric VM. Stroke and diabetic ketoacidosis – Some diagnostic and therapeutic considerations. Vasc Health Risk Manag. 2014;8(10):201–4. doi: 10.2147/VHRM.S59593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Central Statistical Office. HIV prevalence. [Last accessed on 2015 Dec 01]. Available from: http://www.zamstats.gov.zm/surveys/zdhs.php .
- 15.Otieno CF, Kayima JK, Mbugua PK, Amayo AA, Mcligeyo SO. Prognostic factors in patients hospitalised with diabetic ketoacidosis at Kenyatta National Hospital, Nairobi. East Afr Med J. 2010;87:66–73. doi: 10.4314/eamj.v87i2.60600. [DOI] [PubMed] [Google Scholar]
- 16.Jang TB, Chauhan V, Morchi R, Najand H, Naunheim R, Kaji AH. Hypokalemia in diabetic ketoacidosis is less common than previously reported. Intern Emerg Med. 2015;10:177–80. doi: 10.1007/s11739-014-1146-8. [DOI] [PubMed] [Google Scholar]
- 17.Kakoma PK, Kadiebwe DM, Kayembe AM, Makonga PK, Bugeme M, Mukuku O. [Article in French] [Diabetic ketoacidosis in adults in Sendwe Hospital Lubumbashi: About 51 cases] Pan Afr Med J. 2014;1:17, 324. doi: 10.11604/pamj.2014.17.324.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekpebegh C, Longo-Mbenza B. Mortality in hyperglycemic crisis: A high association with infections and cerebrovascular disease. Minerva Endocrinol. 2013;38:187–93. [PubMed] [Google Scholar]
- 19.Elmehdawi RR, Ehmida M, Elmagrehi H, Alaysh A. Incidence and mortality of diabetic ketoacidosis in benghazi-libya in 2007. Oman Med J. 2013;28:178–83. doi: 10.5001/omj.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]


