Abstract
Glucagon-like peptide-1 (GLP-1)–based therapy improves glycaemic control through multiple mechanisms, with a low risk of hypoglycaemia and the additional benefit of clinically relevant weight loss. Since Starling and Bayliss first proposed the existence of intestinal secretions that stimulate the pancreas, tremendous progress has been made in the area of incretins. As a number of GLP-1 receptor agonists (GLP-1 RAs) continue to become available, physicians will soon face the challenge of selecting the right option customized to their patient's needs. The following discussion, derived from an extensive literature search using the PubMed database, applying the terms incretin, GLP-1, exenatide, liraglutide, albiglutide, dulaglutide, lixisenatide, semaglutide, and taspoglutide, provides a comprehensive review of existing and upcoming molecules in the GLP-1 RA class in terms of their structure, pharmacological profiles, efficacy, safety, and convenience. Search Methodology: A literature search was conducted using the PubMed database, applying the terms incretin, GLP-1, exenatide, liraglutide, albiglutide, dulaglutide, lixisenatide, semaglutide, and taspoglutide. Relevant articles were those that discussed structural, pharmacokinetic and pharmacodynamic differences, classification, long-acting and short-acting GLP-1 RAs, phase 3 trials, and expert opinions. Additional targeted searches were conducted on diabetes treatment guidelines and reviews on safety, as well as the American Diabetes Association/European Society for Study of Diabetes (ADA/EASD) statement on pancreatic safety.
Keywords: Beyond glycaemic control, comparison of glucagon-like peptide-1 receptor agonists, efficacy, glucagon-like peptide-1 receptor agonists, type 2 diabetes mellitus
INTRODUCTION
Although incretin-based therapies have only appeared on the market within the last decade following the regulatory approval of exenatide in 2005, the concept of incretins is at least a century old. In 1902, Bayliss and Starling proposed that intestinal mucosa produced a chemical that stimulated the pancreas to produce secretions.[1] Interestingly, this chemical did not earn its name until 30 years later when, in 1932, La Barre called it “incretin.”[2] Availability of radioimmunoassays for insulin accelerated research in this area, and in 1960s, different groups independently demonstrated that orally given glucose showed a better insulin response than intravenous glucose injections.[3,4] Glucose-dependent insulinotropic peptide was the first incretin isolated and its insulinotropic properties identified, although it was initially called gastric inhibitory polypeptide as its administration inhibited gastric acid secretion in dogs.[2] In 1985, a glucagon-like peptide-1 (GLP-1), GLP-1 (7–36)amide was characterized and shown to have insulinotropic properties[2] and, in the following year, Nauck et al. reported reduced incretin effects in patients with type 2 diabetes.[5] In 1993, they demonstrated that, in patients with poorly controlled type 2 diabetes, a single exogenous infusion of GLP-1 increased insulin levels in a glucose-dependent manner normalizing fasting hyperglycemia.[6] It was subsequently recognized that GLP-1 is degraded by the ubiquitous protease dipeptidyl peptidase-4 (DPP-4), and thus that GLP-1 not only stimulates glucose-mediated insulin secretion but also has inhibitory effects on glucagon secretion, gastric emptying, and enhancing satiety.[2] Hence, current therapeutic approaches have utilized either DPP-4-resistant mimetics of GLP-1 or inhibitors of the DPP-4 enzyme to enhance the activity of the endogenously secreted GLP-1 hormone.
Over the years, GLP-1 based therapies have become integral in many treatment guidelines, such as the American Diabetes Association (ADA)/European Society for Study of Diabetes, the American Association of Clinical Endocrinologists, and the International Diabetes Federation.[7,8,9]
This review article aims to describe current and future GLP-1 receptor agonists (RAs) with respect to structure, pharmacological profiles, efficacy, safety, and convenience.
MECHANISM OF ACTION
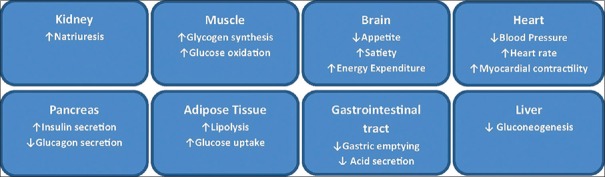
This class of injectable antihyperglycemic agents acts in a glucose-dependent manner and reduces both fasting and postprandial blood glucose levels. When given along with metformin, they do not pose the increased risk of hypoglycemia and weight gain typically seen with other antihyperglycemic agents such as sulfonylureas and insulins. In fact, they are associated with a modest weight loss in most patients. GLP-1 RAs improve glucose homeostasis through multifaceted action [Figure 1].[11,13] They enhance glucose-dependent insulin secretion, suppress inappropriately elevated glucagon levels, both in fasting and postprandial states, and slow gastric emptying. Slowing of gastric motility plays an important role in reducing postprandial glycemic excursions. They are also known to potentially enhance β-cell proliferation and have anti-apoptotic effects on these cells, inducing insulin biosynthesis.[10,11] The most frequently reported adverse events associated with this class are nausea, vomiting, and diarrhea, the causes of which are thought to be the effects of GLP-1 RAs on gastric emptying or involving the central nervous system. Some issues remain controversial, such as an association with pancreatitis and pancreatic and/or thyroid neoplasms and, despite the well-published data related to glucose-lowering efficacy, the use of GLP-1 RA is often limited to obese diabetic patients.[11]
Figure 1.
Mode of action of glucagon-like peptide-1 receptor agonist Data from: Meier JJ. Nat Rev Endocrinol 2012;8:728-42; Madsbad S, et al. Diabetes Obes Metab 2011;13:394-407
CLASSIFICATION OF GLUCAGON-LIKE PEPTIDE-1 RECEPTOR AGONISTS
GLP-1 RAs have been classified according to their basic structure and pharmacokinetic properties. Structurally, one group exploits the native GLP-1 with some amino-acid alterations which make it resistant to degradation by the DPP-4 enzyme. The other group was synthetically developed by replicating the structure of a naturally occurring protein, exendin-4 (Ex-4), (originally isolated from the saliva of the lizard Heloderma suspectum or Gila monster), with substantial homology to native GLP-1. Like native GLP-1, this protein has GLP-1R-activating properties, and is naturally resistant to degradation by the DPP-4 enzyme.[12]
Apart from structural classification, these drugs can also be classified based on the duration of their action [Figure 2],[11,13] i.e., short-acting and long-acting GLP-1 RAs.[11,13] Although even short-acting GLP-1 RAs are DPP-4 resistant due to structural modifications at their second and third N-terminal amino acids, they are eliminated via the renal tract, thus requiring either a once-daily (QD) or twice-daily (BID) dosing (e.g., exenatide and lixisenatide). Longer acting molecules have undergone some structural modifications to enhance their duration of action, while retaining their ability to act on the GLP-1 receptors (exenatide once weekly [EQW], dulaglutide, albiglutide, liraglutide). Short-acting compounds lead to fluctuations in plasma peptide levels for some time after administration. On the other hand, long-acting compounds tend to have a more consistent supraphysiological level of exogenous GLP-1 in the body. Some of the strategies used to prolong the life of these compounds include:
Figure 2.
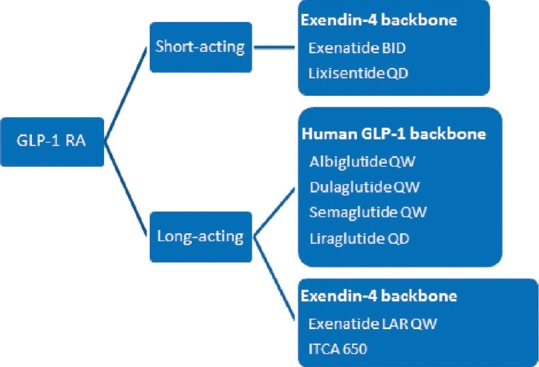
Classification of glucagon-like peptide-1 receptor agonists Data from: Meier JJ. Nat Rev Endocrinol 2012;8:728-42; Madsbad S, et al. Diabetes Obes Metab 2011;13:394-407
Albumin binding to prevent renal filtration, e.g., liraglutide, albiglutide, semaglutide
Conjugation to a modified IgG4 Fc fragment, e.g., dulaglutide, and
Coupling of the molecule to microspheres to delay absorption from the subcutaneous site, e.g., EQW [Figure 1].[11]
ITCA 650, a once-yearly therapy in development, provides continuous subcutaneous delivery of exenatide via a miniature pump embedded sub-dermally.[14] Apart from the dosing regimen, these differences also have implications for pharmacological parameters, as well as efficacy and tolerability.[11] While the classification based on duration of action clearly differentiates the long- and short-acting molecules in terms of mode of action, clinical profile and tolerability profile, some newer molecules offer overlapping features that, going forward, may limit the use of this classification. We will review the differences in efficacy and safety after briefly reviewing each individual molecule. The efficacy and safety data of the molecules from key trials have been tabulated in this review article. It is important to note that, unless specified, these are not head-to-head comparisons, and it may not be prudent to determine comparable efficacy through these tables. However, these tables provide a simple contextualization of the relevant results of the different studies.
REVIEW OF CURRENT AND EMERGING GLUCAGON-LIKE PEPTIDE-1 RECEPTOR AGONISTS
Reviewed below are the currently available GLP-1 RAs, some of which are available on the Indian market, while others have completed their development programs and are under review by the regulatory agencies.
Exenatide twice-daily
Introduction
Exenatide, the first GLP-1 RA, was introduced to the Indian market in 2007. Launched in the USA in 2005 and in Europe in 2006, it was discovered in a search for biologically active peptides in the venom of the Gila monster.[15]
Pharmacology and posology
Exenatide is a synthetic version of Ex-4, and bears 53% homology to the native GLP-1 molecule.[15] It is available in a prefilled pen device and is administered subcutaneously BID within 60 min before two major meals. The starting dose is 5 µg BID which, if well tolerated by the patient, may be titrated to 10 µg BID after a month. The mean terminal half-life after administration is 2.4 h and it remains detectable in plasma for approximately 10 h after a single dose.[16]
Efficacy
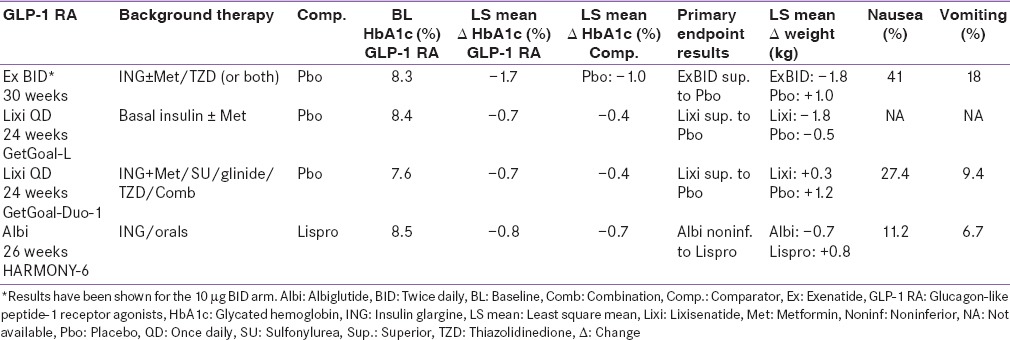
Three pivotal 30-week, phase 3, clinical trials, referred to as the AC2993 Diabetes Management for Improving Glucose Outcomes, investigated the efficacy and safety of exenatide BID (ExBID) in patients treated with metformin alone, patients treated with a sulfonylurea alone, and patients treated with both metformin and a sulfonylurea.[17,18,19] Reduction in glycated hemoglobin (Hb) in these trials with 10 µg of ExBID ranged from approximately 0.8% to 0.9% and decrease in weight from baseline observed was up to about 2.8 kg.[16,17,18,19] The durability of glycemic effect was noted for up to 3 years.[20] In other comparator studies, glycosylated Hb A1c (HbA1c) reduction with adjunctive ExBID was generally similar to that of basal insulin,[21] sulfonylureas,[22,23] and lixisenatide;[24] less than that of liraglutide,[25] and EQW.[26,27] The key studies have been summarized in Tables 1-7.[18,19,21,28,29,30,34,35,36,37,40,41,42,43,44,45,50,51,60,61,62,63,69,70,71,72,73,74] The slowing of gastric emptying has the likely effect of reducing postprandial glucose (PPG) excursions in some of these trials.[19] However, the short half-life may not be able to cover the postprandial surge postlunch, and the fasting plasma glucose (FPG) reductions are less when compared to the longer-acting members in the class.[25]
Table 1.
Monotherapy studies
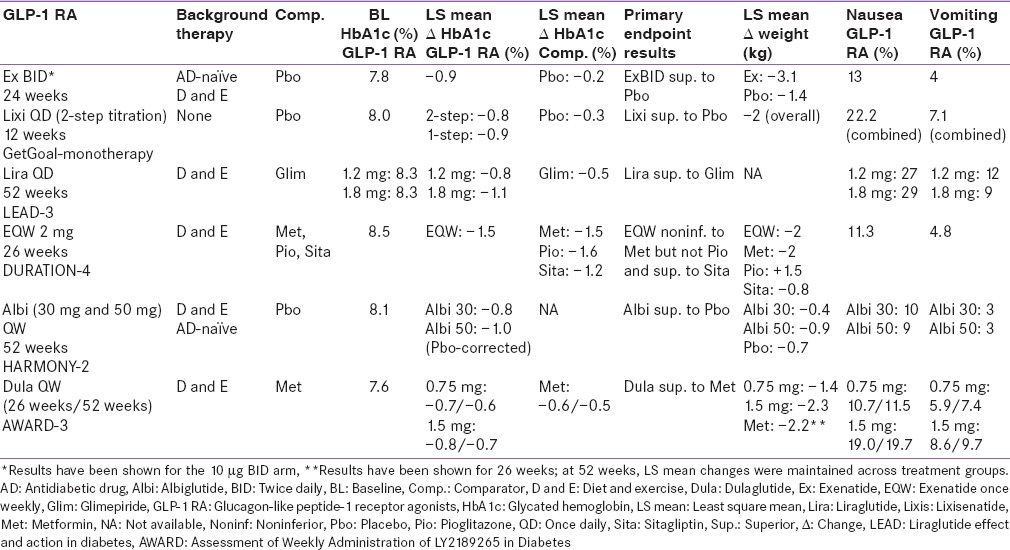
Table 7.
Comparison trials between GLP-1 RA
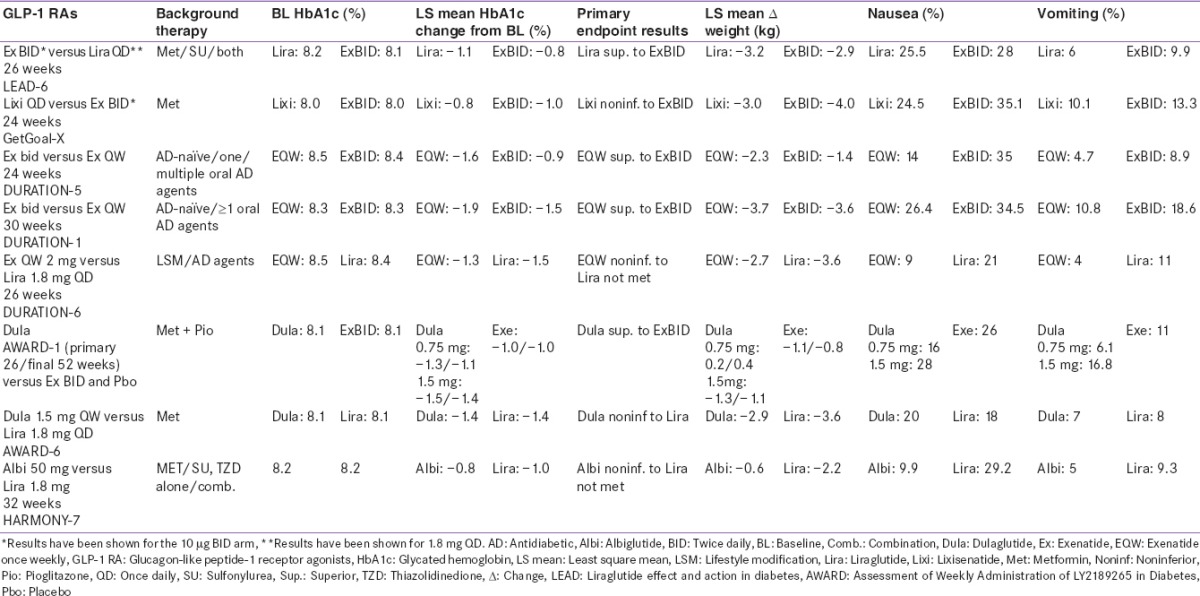
Table 2.
Add-on to metformin studies
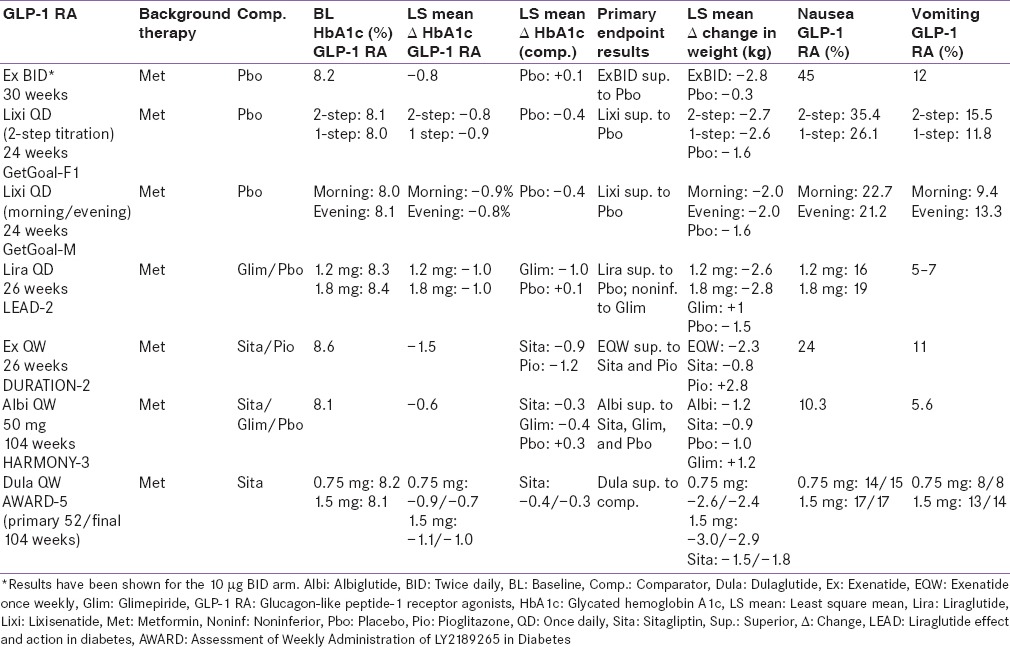
Table 3.
Add-on to metformin + sulfonylurea studies
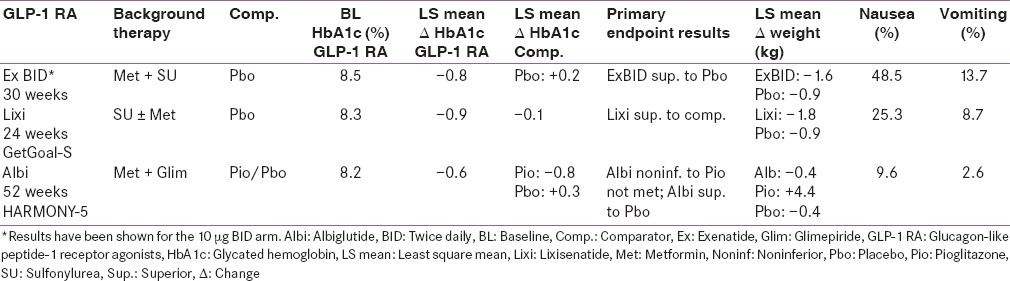
Table 4.
Add-on to metformin + thiazolidinedione studies
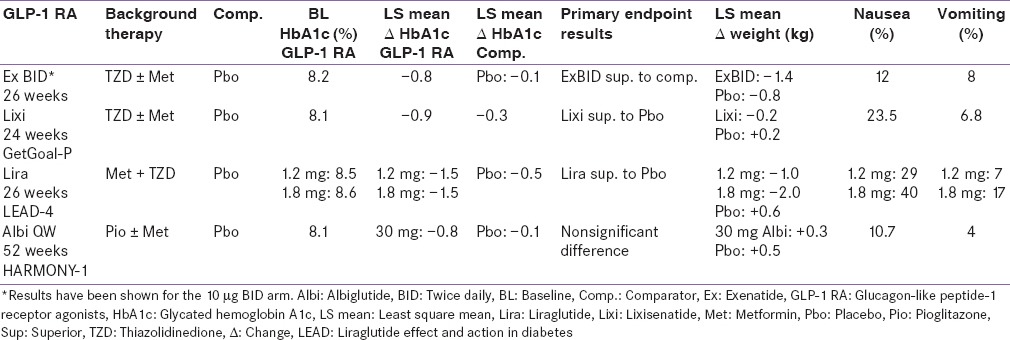
Table 5.
Comparisons with basal insulin
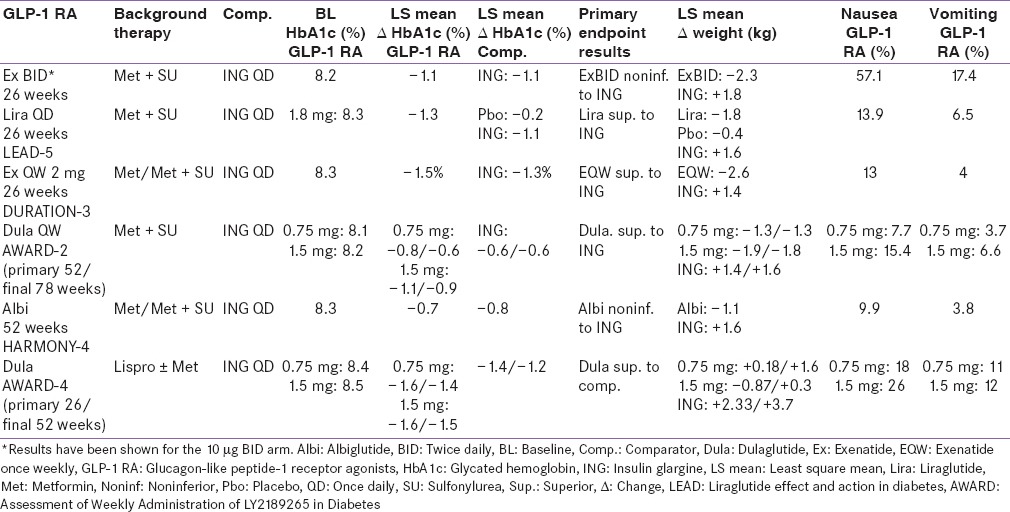
Table 6.
Add-on to background insulin therapy
Safety
The most common adverse effect was mild to moderate nausea and vomiting which decreased with time.[16] The incidence of hypoglycemia was low when not given alongside concomitant sulfonylurea or insulin therapy.[16] ExBID is not recommended for use in patients with end-stage renal disease (ESRD) or severe renal impairment.[16] In patients with moderate renal impairment, dose escalation from 5 to 10 µg should proceed conservatively.[16] Formation of anti-exenatide antibodies has been reported relatively frequently, which may be a result of only 53% homology to the native GLP-1 molecule and its nonhuman origin. In the three placebo-controlled trials (n = 963), 38% of patients had low titer anti-exenatide antibodies at 30 weeks. The presence of these antibodies generally did not impact the glycemic response in these patients. An additional 6% had a high titer of antibodies. About half of these had an attenuated glycemic response to exenatide.[16]
Liraglutide
Introduction
Liraglutide is an analog of human GLP-1 produced by recombinant DNA technology. It was approved for clinical use in Europe in 2009 and in the USA in 2010[12] and has been available in India since 2010.
Pharmacology and posology
This engineered peptide shares 97% homology to native human GLP-1.[31] There is no structural modification to render it resistant to DPP-4, but the slow absorption from the site after subcutaneous administration can be attributed to self-association leading to the formation of heptamers at the injection site.[31] Once in plasma, 99% remains bound to plasma albumin, with the bound molecule having a half-life of 13 h, making it suitable for QD administration. Liraglutide should be initiated with a dose of 0.6 mg QD for 1 week.[31,32] This low starting dose helps reduce gastrointestinal symptoms during initial titration but is not effective for glycemic control. After 1 week, the dose should be increased to 1.2 mg QD. If the 1.2-mg dose does not result in acceptable glycemic control, the dose can be further increased to 1.8 mg QD. Liraglutide is available in disposable, prefilled, and multi-dose pens.[12,32]
Efficacy
Liraglutide has been investigated in a clinical development program called Liraglutide Effect and Action in Diabetes (LEAD™)[25,33,34,35,36,37] which compared the two licensed doses, 1.2 mg and 1.8 mg of liraglutide, with glimepiride, rosiglitazone, and insulin glargine, as well as a direct comparison (LEAD™ 6) between liraglutide 1.8 mg and ExBID.[31] The duration of the studies varied from 26 to 52 weeks. The HbA1c reduction from baseline in the LEAD trials varied between 0.8% and 1.5% for the 1.2-mg arm and 1–1.5% for the 1.8-mg arm.[31] Change in weight observed with liraglutide in the LEAD trials varied from +0.3 kg to −1.2 kg for the 1.2-mg arm and −0.2 kg to − 2.6 kg for the 1.8-mg arm [Tables 1-7].[32] Liraglutide alone or in combination with oral antihyperglycemic agents was shown to be superior to placebo and active comparators in all LEAD trials except LEAD 2 where both doses were noninferior to glimepiride for HbA1c lowering.[31] Liraglutide had a modest effect on the reduction of gastric emptying, thus the reduction in PPG excursions can be mainly attributed to a reduction in preprandial glucose levels.[12] In a head-to-head trial with ExBID, there was a greater reduction in FPG with liraglutide, while better PPG reduction was seen with ExBID after breakfast and dinner.[31]
Safety
The most common adverse events were gastrointestinal in nature and tended to decrease over a period of time. Overall, the rate of hypoglycemia was low, except when liraglutide was used in combination with sulfonylureas when there was an increased risk of hypoglycemia. Anti-liraglutide antibodies were detected in 8.6% of liraglutide-treated patients, relatively lower than that seen with exenatide owing to high sequence identity with native GLP-1. In general, the presence of antibodies did not impact the clinical efficacy. No dose adjustment is recommended for patients with mild renal impairment. Liraglutide is not recommended in patients with severe renal impairment including those with ESRD.[32]
Lixisenatide
Introduction
Lixisenatide has been approved for the treatment of adults with type 2 diabetes in various countries including Europe, Mexico, Australia, and Japan. In September 2013, the manufacturers announced their decision to withdraw the lixisenatide New Drug Application in the USA, citing their reason as a request from the United States Food and Drug Administration (USFDA) to review the interim results from the ongoing cardiovascular (CV) outcomes trial, Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA). Publishing interim data could have impacted the integrity of the ongoing trial.[38] Lixisenatide is not yet available on the Indian market.
Pharmacology and posology
Lixisenatide is a QD GLP-1 RA which, like exenatide, has an Ex-4 backbone, but with some alterations, leading to a half-life of 2–3 h. Although the short half-life suggested a need for multiple daily dosing, initial dose-finding studies did not show much difference in efficacy between QD or BID dosing. Hence, QD dosing was selected for further evaluation.[12]
Efficacy
Lixisenatide 20 µg QD has been clinically evaluated in the GetGoal Program which included monotherapy, add-on therapy to metformin, sulfonylurea or pioglitazone, and in combination with basal insulin. Apart from GetGoalX, which had an active comparator (ExBID), all these trials compared lixisenatide to placebo.[24,39,40,41,42,43,44,45] Lixisenatide showed a significant decrease in HbA1c from baseline in these trials, ranging from 0.7% to 0.94% with an accompanying weight loss of 0.2–2.8 kg [Tables 1-7].[46] Lixisenatide has a moderate effect on FPG and a predominant effect on PPG, attributed to a reduction in gastric emptying.[12]
Safety
Like the other GLP-1 RAs, the main adverse effects are gastrointestinal in nature. Lixisenatide is potentially immunogenic like other protein/peptide-based products, and 69.8% of patients had a positive-antibody status at the end of the 24-week placebo-controlled studies. The change in HbA1c from baseline was similar, irrespective of antibody status (positive or negative). Lixisenatide is mainly eliminated through the kidneys and should be used with caution in patients with moderate renal impairment. Use is not recommended in patients with severe renal impairment or ESRD.[46]
Exenatide once weekly
Introduction
EQW was approved in Europe in 2011 and the USA in 2012.[12] It is not yet available in India.
Pharmacology and posology
In this once-weekly formulation, the exenatide molecule is embedded within poly (lactic-co-glycolic acid) microspheres, which, once injected subcutaneously, allows a steady and constant release of exenatide through diffusion and erosion of these microspheres, leading to a gradual rise in plasma concentrations. EQW is supplied in a single use, single dose pen.[47]
Efficacy
EQW has been examined in the clinical-trial program “Diabetes therapy Utilization: Researching changes in HbA1c weight and other factors Through Intervention with exenatide Once-weekly” (DURATION) using metformin, sitagliptin, pioglitazone, insulin glargine, liraglutide, and ExBID as active comparators.[26,27,48,49,50,51,52] The HbA1c reduction seen in the DURATION trials ranged from 1.3% to 1.9%. The reduction in weight ranged from 2 kg to 3.7 kg [Tables 1-7]. EQW demonstrated superior FPG reductions compared to ExBID in the two head-to-head studies, whereas ExBID showed greater reductions in the PPG excursions. In the DURATION program, EQW showed superiority or noninferiority to active comparators, except liraglutide, and pioglitazone.[26,27,48,49,50,51,52] It failed to demonstrate noninferiority to liraglutide for HbA1c lowering. Weight loss was also better for liraglutide.[52]
Safety
Gastrointestinal side effects were common with rates ranging from 11% to 26.4% across all studies,[26,27,48,49,50,51,52] although less than that seen with the BID regimen. In the head-to-head study against liraglutide 1.8 mg, EQW demonstrated a better tolerability profile in terms of nausea and vomiting.[52] EQW is not recommended for use in patients with ESRD and should be used with caution in moderate renal impairment. Small, asymptomatic subcutaneous injection-site nodules can be seen with the use of EQW, consistent with the known properties of microspheres.[47] About 49% of patients on EQW during the five active comparator-controlled trials had anti-exenatide antibodies during the trial; however, this did not seem to affect glycemic response except when it occurred in high titers.[47]
Dulaglutide
Introduction
Dulaglutide received USFDA approval in September 2014 and European agency approval in November 2014.[53,54] It was approved for use in India in December 2014.[55]
Pharmacology and posology
Dulaglutide is a long-acting GLP-1 analog covalently linked to human IgG Fc fragment, modified to increase the duration of pharmacodynamic activity, reduce DPP-4 inactivation, increase solubility, and reduce immunogenicity. The increased duration of pharmacodynamic activity can be attributed to reduced renal clearance; resulting in a plasma half-life of approximately 5 days, allowing for once-weekly dosing.[56,57,58]
Efficacy
The regulatory approvals of dulaglutide were based on the Assessment of Weekly Administration of LY2189265 in Diabetes (AWARD) 1-5 trials.[56] Data from six AWARD studies have been disclosed to date.[58,59,60,61,62,63,64] In these trials, two doses of dulaglutide once weekly, 0.75 mg and 1.5 mg, were compared to placebo, ExBID, insulin glargine, metformin, and sitagliptin, and dulaglutide 1.5 mg was compared to liraglutide 1.8 mg.[58,59,60,61,62,63,64] The duration of these trials ranged from 26 to 104 weeks.[58,59,60,61,62,63,64] Dulaglutide met its primary end-point in all six AWARD studies. The 1.5-mg dose further demonstrated superiority to its active comparators in all five registration trials and noninferiority to liraglutide 1.8 mg in the AWARD-6 trial. In various AWARD studies, HbA1c reduction with the 1.5-mg dose ranged from 0.8% to 1.6%. Similarly, weight loss of up to 3.2 kg was seen.[58,59,60,61,62,63,64] In comparison to placebo, dulaglutide showed clinically significant reductions in FPG levels. Interestingly, in the head-to-head study with ExBID, although classified as a long-acting molecule, dulaglutide demonstrated significantly greater reduction in PPG in addition to premeal plasma glucose values compared with ExBID.[59] Dulaglutide is the only GLP-1 RA so far to achieve noninferiority to liraglutide 1.8 mg as demonstrated in a head-to-head study (1.42% dulaglutide vs. 1.36% liraglutide, P < 0.001). Tolerability profile was similar for both medications.[64]
Safety
The most common adverse events were gastrointestinal in nature. Dulaglutide exhibited a relatively low immunogenicity with 1.6% of treated patients in the clinical trials developing anti-drug antibodies.[56]
Albiglutide
Introduction
Albiglutide is a long-acting GLP-1 RA approved in the USA as well as Europe, but not yet available on the Indian market.[65,66]
Pharmacology and posology
Albiglutide was developed by fusing a GLP-1 dimer to a recombinant human albumin; the resulting large size making it somewhat resistant to renal filtration, leading to a half-life of approximately 5 days and justifying a once-weekly dosing.[67] Albiglutide is approved as prefilled pens containing 30 mg or 50 mg of albiglutide and a solvent for reconstitution. The recommended dose is 30 mg once weekly, to be increased to 50 mg if required.[68]
Efficacy
The phase 3 trials for albiglutide, termed the HARMONY[67,69,70,71,72,73,74,75] program, consisted of eight randomized, controlled trials and compared albiglutide to placebo and active comparators such as insulin glargine, sitagliptin, pioglitazone, glimepiride, insulin lispro, and liraglutide. In the HARMONY trials, active comparators such as pioglitazone and liraglutide demonstrated significantly better glycemic control than albiglutide.[72,74] Albiglutide demonstrated a mean weight loss of 1.05 kg,[67] depending on the background therapy used.
Safety
Gastrointestinal adverse events were present, though less significant than those witnessed with liraglutide.[74] In the pool of placebo-controlled trials, injection-site reactions occurred more frequently with albiglutide (18%) than with placebo (8%).[76] Clinical trials reported development of antibodies to albiglutide in 4% of patients, but this did not appear to affect its efficacy.[68]
Semaglutide
Semaglutide is a long-acting GLP-1 RA being developed for once-weekly dosing. Semaglutide has some structural similarities to liraglutide with modifications to increase albumin binding and DPP-4 stability. A phase 2 trial has shown significant HbA1c reductions at high doses (≥0.8 mg) compared to liraglutide 1.8 mg. It is currently being evaluated in the phase 3 program SUSTAIN, which was initiated in 2013.[12]
Insulin degludec and liraglutide
A fixed-ratio combination of the basal insulin analog insulin degludec and liraglutide is being evaluated and developed as a QD injection (DUAL program). Recently published results from a 26-week study in patients with type 2 diabetes inadequately controlled with oral hypoglycemic drugs showed significant lowering in HbA1c.[77]
COMPARISON OF THE GLUCAGON-LIKE PEPTIDE-1 RECEPTOR AGONISTS
Overall efficacy
All the GLP-1 RAs that were developed after ExBID have been compared in head-to-head comparison trials with previously existing members in this class [Table 7]. In a study that compared two short-acting GLP-1 RAs, lixisenatide and ExBID, the glycemic efficacy was similar, with slightly better weight reduction seen with ExBID.[24] Studies comparing long- and short-acting GLP-1 RAs typically showed superior HbA1c reduction with long-acting members.[25,26,48,59] In the AWARD-1 study, both doses of once-weekly dulaglutide demonstrated superior glycemic control in terms of HbA1c and fasting glucose versus ExBID.[59]
Differential effect of fasting and postprandial glucose
Differences in molecular structure of these molecules results in different pharmacokinetic/pharmacodynamics profiles which reflect in their efficacy, safety, and tolerability. While one important benefit of long-acting over short-acting molecules is that they have more stable plasma levels over 24 h, with a predominant effect on FPG, the effect on delay of gastric emptying is lost in a few hours via modulation of vagal activity. While this may mean less gastrointestinal side effects, it also reflects in the better PPG efficacy of short-acting molecules.[11,12,78] However, this may not always hold true. For example, dulaglutide, however, exhibited clinically relevant postprandial glycemic benefits when compared with ExBID, as described above, somewhat limiting the duration of action-based classification.[59]
Immunogenicity
Consistent with the immunogenic properties of protein and peptide pharmaceuticals, individuals exposed to GLP-1 RAs may develop an immune response, including anti-drug antibodies. The molecular structure and homology to the native GLP-1 molecule may also impact the immunogenicity. Exenatide, which has 53% homology to the native GLP-1 molecule,[79] frequently results in formation of anti-exenatide antibodies (up to 43%).[31] The clinical relevance of these antibodies is not certain, but in the majority of patients their presence does not seem to impair the efficacy. In patients with high antibody titers, however, the exenatide-induced reduction in HbA1c level was significantly smaller than in patients with low titers of antibodies.[16] The proportion of patients developing antibodies against liraglutide is lower at 8.6%.[32] In clinical studies, treatment with dulaglutide was associated with a 1.6% incidence of treatment emergent dulaglutide anti-drug antibodies, suggesting that the structural modifications in the GLP-1 and modified IgG4 parts of the dulaglutide molecule, together with high homology with native GLP-1 and native IgG4, minimize the risk of immune response against dulaglutide.[80]
Effect on weight
Improved glycemic benefit does not always correlate with improved weight loss results. Despite EQW demonstrating superior glycemic control to ExBID in the DURATION 1 and 5 trials, weight loss was similar for both molecules in both trials. Similar results were seen in AWARD-1, with dulaglutide demonstrating superior efficacy but similar weight loss to ExBID [Table 7]. This variability in clinical trial results shows that we are unable to determine the pharmacodynamic action of these drugs from their duration of action. While we may classify drugs according to whether they are long- or short-acting, this does not fully determine their clinical characteristics or mode of action.
Gastrointestinal effects
Mild to moderate nausea, vomiting, and diarrhea, which decline over time, are the most frequent side effects with this class of drugs. While longer-acting molecules may be given independent of meal timing, shorter-acting molecules, like ExBID, should be administered at least an hour before meals to reduce gastrointestinal symptoms. To avoid discontinuation due to these events, appropriate counseling and expectation-setting around gastrointestinal symptoms, weight loss, and decreased appetite is critical at the time of prescription. These agents should be avoided in cases of severe gastrointestinal disease, such as gastroparesis.[16,32,46,47,56,76]
CHOOSING THE APPROPRIATE GLUCAGON-LIKE PEPTIDE-1 RECEPTOR AGONIST
With the introduction of more GLP-1 RA options to the market, physicians will face the challenge of selecting the most appropriate molecule for their patients. Kalra has described a bio-psychosocial model for such a selection. This model contains both biomedical factors (including efficacy, safety, tolerability, and versatility in combination with insulin), and psychosocial factors (such as the ability of the patient to self-inject, adherence to therapy, frequency of contact with physician, and meal patterns) that govern the choice of GLP-1 RA.[81] Another interesting concept by Kalra and Gupta is directly observed therapy (DOT) for diabetes, as already exists for tuberculosis.[82] Enabling patients to self-inject in front of a diabetes educator, DOT could be advantageous for both patient and practitioner, supporting patients while concurrently allowing for early observation of adverse effects. Pharmacological developments and the availability of much longer-acting molecules might facilitate long-term DOT in diabetology.[82]
Appropriate medication counseling is mandatory with GLP-1 RA prescription.[83]
BEYOND GLYCEMIC CONTROL
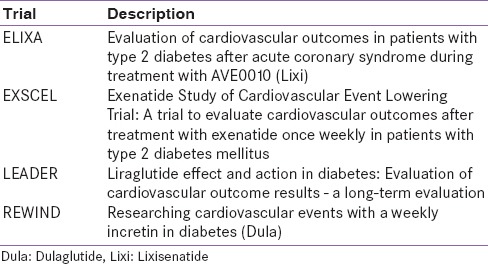
The wide distribution of GLP-1 receptors leads to the suggestion that, despite the principal effect of regulating glycemia, their other effects are varied and multifocal. Clinical studies have demonstrated that the weight loss potential of this class of drugs provides additional benefits over many other anti-hyperglycemic agents available. One of the most promising effects of this class of drugs is their potential to prevent beta cell apoptosis and promote beta cell regeneration, although most data toward this are preclinical. However, this encouraging data do indicate the potential to halt or reverse the progression of disease when used early.[84] There is also preclinical and clinical data indicating possible improvement in CV risk markers, including improvement in blood pressure, lipid profiles, and endothelial or myocardial dysfunction. The translation of such improvements in CV risk markers are being investigated in long-term CV outcome trials [Table 8].[85] Thus far, the only completed CV outcome trial, the ELIXA trial, was disclosed at ADA 2015. The results did not show a benefit on CV outcomes in the 6000 high-risk patients with diabetes.[86] The expression of GLP-1 receptors in the central nervous system has also led to some observations and evaluations regarding the neuroprotective/neurotrophic function of GLP-1. Some interesting data show potential benefit in the early treatment of Alzheimer's disease, by central GLP-1 RA stimulation with a long-acting agonist, such as Ex-4.[87]
Table 8.
Cardiovascular outcome trials
THE DEBATED ISSUES
While there is no doubt that this class of antihyperglycemic agents has clinically relevant efficacy, certain long-term adverse consequences continue to stimulate debate and cloud the visible benefits. These adverse events include the suggested association of the incretin-based therapies with pancreatitis, preneoplastic changes, pancreatic cancer, and thyroid carcinoma. Some recent publications have provoked wide discussion across scientific associations, regulatory agencies, and industry; however, diabetes experts and the US and EU regulatory agencies generally believe that the association of these events with GLP-1 RAs is not of sufficient significance to influence current treatment recommendations. In their opinion, potential risks call for long-term investigation but not avoidance of the therapies’ usage.[88,89,90]
CONCLUSION
Our understanding of diabetes is constantly evolving and GLP-1 RAs represent an important facet of this understanding. The GLP-1 RA class improves glycemic control through multiple mechanisms with a low risk of hypoglycemia and facilitates clinically relevant weight loss. Globally, this class is being recognized as an important therapy in the management of type 2 diabetes. Although there is comparatively less real-world experience of using these drugs in our country compared to the USA and Europe, there has been significant interest among Indian clinicians, as evidenced by recent publications and deliberations in academic forums. Although India and Asia have contributed patients to the clinical development programs of most of these molecules, a sub-group analysis that includes these patients is relevant, but is beyond the scope of this review. Any initial resistance for adopting these therapies could be attributed to fear of daily injections; however, the emerging once-weekly alternatives have the potential for more convenience and greater acceptance by patients, with better efficacy and tolerability. These once-weekly therapies may provide another therapeutic alternative when oral antihyperglycemic medications are unable to provide adequate glycemic control. Nevertheless, the enormous progress in this field has paved the way for other therapeutic options for tailored management of patients with type 2 diabetes. It is expected that this review, with its concise and comprehensive analysis of the existing and emerging molecules, and their differences in structure, pharmacology, clinical efficacy, and safety, will help our clinicians choose the right therapy for their patients.
Financial support and sponsorship
Nil.
Conflicts of interest
Kalra has received speaker fees/honoraria from Astra Zeneca, B-D, Boehringer-Ingelheim, Eli Lilly, NovoNordisk and Sanofi in the recent past. Baruah has received speaker honorarium and consultancy fees from Eli Lilly, Novo Nordisk, Sanofi, Boehringer-Ingelheim, Astra Zeneca, Novartis, MSD and USV. Unnikrishnan has been a speaker for Astra Zeneca, Boehringer-Ingelheim, Eli Lilly, NovoNordisk and Sanofi in the past. Adetunji is an employee of Eli Lilly and Company.
REFERENCES
- 1.Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol. 1902;28:325–53. doi: 10.1113/jphysiol.1902.sp000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60:470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elrick H, Stimmler L, Hlad CJ, Jr, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076–82. doi: 10.1210/jcem-24-10-1076. [DOI] [PubMed] [Google Scholar]
- 4.Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: Studies in normal and diabetic sujbjects. J Clin Invest. 1967;46:1954–62. doi: 10.1172/JCI105685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 6.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–4. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Standards of medical care in diabetes-2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 8.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. American Association of Clinical Endocrinologists’ comprehensive diabetes management algorithm 2013 consensus statement – Executive summary. Endocr Pract. 2013;19:536–57. doi: 10.4158/EP13176.CS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brussels, Belgium: International Diabetes Federation; 2012. International Diabetes Federation. Clinical Guidelines Task Force. Global Guideline for Type 2 Diabetes. [Google Scholar]
- 10.Nauck MA, Vilsbøll T, Gallwitz B, Garber A, Madsbad S. Incretin-based therapies: Viewpoints on the way to consensus. Diabetes Care. 2009;32(Suppl 2):S223–31. doi: 10.2337/dc09-S315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728–42. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 12.Lund A, Knop FK, Vilsbøll T. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: Differences and similarities. Eur J Intern Med. 2014;25:407–14. doi: 10.1016/j.ejim.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Madsbad S, Kielgast U, Asmar M, Deacon CF, Torekov SS, Holst JJ. An overview of once-weekly glucagon-like peptide-1 receptor agonists – Available efficacy and safety data and perspectives for the future. Diabetes Obes Metab. 2011;13:394–407. doi: 10.1111/j.1463-1326.2011.01357.x. [DOI] [PubMed] [Google Scholar]
- 14.Henry RR, Rosenstock J, Logan DK, Alessi TR, Luskey K, Baron MA. Randomized trial of continuous subcutaneous delivery of exenatide by ITCA 650 versus twice-daily exenatide injections in metformin-treated type 2 diabetes. Diabetes Care. 2013;36:2559–65. doi: 10.2337/dc12-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCormack PL. Exenatide twice daily: A review of its use in the management of patients with type 2 diabetes mellitus. Drugs. 2014;74:325–51. doi: 10.1007/s40265-013-0172-6. [DOI] [PubMed] [Google Scholar]
- 16.Princeton, NJ: Bristol-Myers Squibb Company; 2014. Byetta [package insert]. US Prescribing Information. [Google Scholar]
- 17.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Exenatide- Clinical Study Group. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–35. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 19.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–91. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 20.Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275–86. doi: 10.1185/030079908x253870. [DOI] [PubMed] [Google Scholar]
- 21.Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG. GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: A randomized trial. Ann Intern Med. 2005;143:559–69. doi: 10.7326/0003-4819-143-8-200510180-00006. [DOI] [PubMed] [Google Scholar]
- 22.Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Ragonesi PD, Querci F, et al. Exenatide versus glibenclamide in patients with diabetes. Diabetes Technol Ther. 2010;12:233–40. doi: 10.1089/dia.2009.0141. [DOI] [PubMed] [Google Scholar]
- 23.Derosa G, Putignano P, Bossi AC, Bonaventura A, Querci F, Franzetti IG, et al. Exenatide or glimepiride added to metformin on metabolic control and on insulin resistance in type 2 diabetic patients. Eur J Pharmacol. 2011;666:251–6. doi: 10.1016/j.ejphar.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 24.Rosenstock J, Raccah D, Korányi L, Maffei L, Boka G, Miossec P, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: A 24-week, randomized, open-label, active-controlled study (GetGoal-X) Diabetes Care. 2013;36:2945–51. doi: 10.2337/dc12-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: A 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 26.Blevins T, Pullman J, Malloy J, Yan P, Taylor K, Schulteis C, et al. DURATION-5: Exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1301–10. doi: 10.1210/jc.2010-2081. [DOI] [PubMed] [Google Scholar]
- 27.Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: A randomised, open-label, non-inferiority study. Lancet. 2008;372:1240–50. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 28.Moretto TJ, Milton DR, Ridge TD, Macconell LA, Okerson T, Wolka AM, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30:1448–60. doi: 10.1016/j.clinthera.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Liutkus J, Rosas Guzman J, Norwood P, Pop L, Northrup J, Cao D, et al. A placebo-controlled trial of exenatide twice-daily added to thiazolidinediones alone or in combination with metformin. Diabetes Obes Metab. 2010;12:1058–65. doi: 10.1111/j.1463-1326.2010.01251.x. [DOI] [PubMed] [Google Scholar]
- 30.Buse JB, Bergenstal RM, Glass LC, Heilmann CR, Lewis MS, Kwan AY, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: A randomized, controlled trial. Ann Intern Med. 2011;154:103–12. doi: 10.7326/0003-4819-154-2-201101180-00300. [DOI] [PubMed] [Google Scholar]
- 31.Rigato M, Fadini GP. Comparative effectiveness of liraglutide in the treatment of type 2 diabetes. Diabetes Metab Syndr Obes. 2014;7:107–20. doi: 10.2147/DMSO.S37644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bagsvaerd, Denmark: Novo Nordisk A/S; 2013. Victoza [package insert]. US Prescribing Information. [Google Scholar]
- 33.Marre M, Shaw J, Brändle M, Bebakar WM, Kamaruddin NA, Strand J, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26:268–78. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: The LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): A randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–81. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 36.Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD) Diabetes Care. 2009;32:1224–30. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): A randomised controlled trial. Diabetologia. 2009;52:2046–55. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanofi Press Release. [Last cited on 2014 Jun 20]. Available from: http://www.ensanofi.com/Images/33756_20130912_lixisenatide_en.pdf .
- 39.Fonseca VA, Alvarado-Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE. EFC GetGoal-Mono Study Investigators. Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: A randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono) Diabetes Care. 2012;35:1225–31. doi: 10.2337/dc11-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahrén B, Leguizamo Dimas A, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M) Diabetes Care. 2013;36:2543–50. doi: 10.2337/dc12-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolli GB, Munteanu M, Dotsenko S, Niemoeller E, Boka G, Wu Y, et al. Efficacy and safety of lixisenatide once daily vs. placebo in people with type 2 diabetes insufficiently controlled on metformin (GetGoal-F1) Diabet Med. 2014;31:176–84. doi: 10.1111/dme.12328. [DOI] [PubMed] [Google Scholar]
- 42.Rosenstock J, Hanefeld M, Shamanna P, Min KW, Boka G, Miossec P, et al. Beneficial effects of once-daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal-S) J Diabetes Complications. 2014;28:386–92. doi: 10.1016/j.jdiacomp.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Pinget M, Goldenberg R, Niemoeller E, Muehlen-Bartmer I, Guo H, Aronson R. Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P) Diabetes Obes Metab. 2013;15:1000–7. doi: 10.1111/dom.12121. [DOI] [PubMed] [Google Scholar]
- 44.Riddle MC, Aronson R, Home P, Marre M, Niemoeller E, Miossec P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: A 24-week, randomized, placebo-controlled comparison (GetGoal-L) Diabetes Care. 2013;36:2489–96. doi: 10.2337/dc12-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riddle MC, Forst T, Aronson R, Sauque-Reyna L, Souhami E, Silvestre L, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1) Diabetes Care. 2013;36:2497–503. doi: 10.2337/dc12-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paris, France: Sanofi-Aventis; 2013. Lyxumia [package insert] [Google Scholar]
- 47.Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2014. Bydureon [package insert]. US Prescribing Information. [Google Scholar]
- 48.Buse JB, Drucker DJ, Taylor KL, Kim T, Walsh B, Hu H, et al. DURATION-1: Exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care. 2010;33:1255–61. doi: 10.2337/dc09-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): A randomised trial. Lancet. 2010;376:431–9. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- 50.Diamant M, Van Gaal L, Stranks S, Northrup J, Cao D, Taylor K, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): An open-label randomised trial. Lancet. 2010;375:2234–43. doi: 10.1016/S0140-6736(10)60406-0. [DOI] [PubMed] [Google Scholar]
- 51.Russell-Jones D, Cuddihy RM, Hanefeld M, Kumar A, González JG, Chan M, et al. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): A 26-week double-blind study. Diabetes Care. 2012;35:252–8. doi: 10.2337/dc11-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buse JB, Nauck M, Forst T, Sheu WH, Shenouda SK, Heilmann CR, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): A randomised, open-label study. Lancet. 2013;381:117–24. doi: 10.1016/S0140-6736(12)61267-7. [DOI] [PubMed] [Google Scholar]
- 53.U.S. Food and Drug Administration. Dulaglutide Press Release. 2014. Sep 18, [Last cited on 2014 Nov 17]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm415180.htm .
- 54.Eli Lilly and Company, Indianapolis, USA. Dulaglutide Press Release. 2014. [Last accessed on 2015 Dec 16]. Available from: https://investor.lilly.com/releasedetailcfmreleaseid=871658 .
- 55.Central Drugs Standard Control Organization, Government of India. Dulaglutide Approval Letter. 2014 [Google Scholar]
- 56.Indianapolis, IN: Eli Lilly and Company; 2014. Trulicity [package insert]. US Prescribing Information. [Google Scholar]
- 57.Barrington P, Chien JY, Showalter HD, Schneck K, Cui S, Tibaldi F, et al. A 5-week study of the pharmacokinetics and pharmacodynamics of LY2189265, a novel, long-acting glucagon-like peptide-1 analogue, in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:426–33. doi: 10.1111/j.1463-1326.2011.01364.x. [DOI] [PubMed] [Google Scholar]
- 58.Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5) Diabetes Care. 2014;37:2149–58. doi: 10.2337/dc13-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wysham C, Blevins T, Arakaki R, Colon G, Garcia P, Atisso C, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1) Diabetes Care. 2014;37:2159–67. doi: 10.2337/dc13-2760. [DOI] [PubMed] [Google Scholar]
- 60.Georgino F, Benroubi M, Sun JH, Zimmerman AG, Pechtner V. Efficacy and Safety of Once Weekly Dulaglutide Versus Insulin Glargine in Combination with Metformin and Glimepiride in Type 2 Diabetes Patients (AWARD-2) Oral Presentation, ADA. 2014 doi: 10.2337/dc14-1625. [DOI] [PubMed] [Google Scholar]
- 61.Umpierrez G, Tofé Povedano S, Pérez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3) Diabetes Care. 2014;37:2168–76. doi: 10.2337/dc13-2759. [DOI] [PubMed] [Google Scholar]
- 62.Blonde L, Jendle J, Gross J, Woo V, Jiang H, Fahrbach JL, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): A randomised, open-label, phase 3, non-inferiority study. Lancet. 2015;385:2057–66. doi: 10.1016/S0140-6736(15)60936-9. [DOI] [PubMed] [Google Scholar]
- 63.Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): A randomized, phase III study. Diabetes Obes Metab. 2015;17:849–58. doi: 10.1111/dom.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dungan KM, Povedano ST, Forst T, González JG, Atisso C, Sealls W, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): A randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384:1349–57. doi: 10.1016/S0140-6736(14)60976-4. [DOI] [PubMed] [Google Scholar]
- 65.U.S Food and Drug Administration. Albiglutide Press Release. 2014. Apr 15, [Last cited on 2014 Nov 17]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm393289.htm .
- 66.European Medicines Agency. Albiglutide Press Release; January 23. 2014. [Last cited on 2014 Nov 17]. Available from: http://www.ema.europa.eu/ema/indexjsp?curl=pages/medicines/human/medicines/002735/smops/Positive/human_smop_000634jsp and mid=WC0b01ac058001d127 .
- 67.Weissman PN, Carr MC, Ye J, Cirkel DT, Stewart M, Perry C, et al. HARMONY 4: Randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57:2475–84. doi: 10.1007/s00125-014-3360-3. [DOI] [PubMed] [Google Scholar]
- 68.Poole RM, Nowlan ML. Albiglutide: First global approval. Drugs. 2014;74:929–38. doi: 10.1007/s40265-014-0228-2. [DOI] [PubMed] [Google Scholar]
- 69.Reusch J, Stewart M, Perkins C, Ordronneau P, June YE, Perry C, et al. HARMONY 1 Week 52 Results: Albiglutide vs. Placebo in Patients with Type 2 Diabetes Mellitus Not Controlled on Pioglitazone ± Metformin. [Presentation.] American Diabetes Association 73rd Scientific Sessions. Chicago, Illinois; June 21-25. 2013 [Google Scholar]
- 70.Nauck M, Stewart M, Perkins C, Jones-Leone A, Yang F, Perry C, et al. HARMONY 2 Wk 52 Results: Albiglutide Monotherapy in Drug Naïve Patients with Type 2 Diabetes Mellitus [abstract] American Diabetes Association, Late Breaking Abstracts. 2013;62(suppl 1A) Abstract 55. [Google Scholar]
- 71.Ahrén B, Johnson SL, Stewart M, Cirkel DT, Yang F, Perry C, et al. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37:2141–8. doi: 10.2337/dc14-0024. [DOI] [PubMed] [Google Scholar]
- 72.Home P, Stewart M, Yang F, Perry C, Carr MC. 52-Week Efficacy of Albiglutide vs. Placebo and vs. Pioglitazone in Triple Therapy (Background Metformin and Glimepiride) in People with Type 2 Diabetes: HARMONY 5 Study. [Oral Presentation.] American Diabetes Association 73rd Scientific Sessions; June 21-25. 2013 [Google Scholar]
- 73.Rosenstock J, Fonseca VA, Gross JL, Ratner RE, Ahrén B, Chow FC, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: A comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37:2317–25. doi: 10.2337/dc14-0001. [DOI] [PubMed] [Google Scholar]
- 74.Pratley RE, Nauck MA, Barnett AH, Feinglos MN, Ovalle F, Harman-Boehm I, et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): A randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2:289–97. doi: 10.1016/S2213-8587(13)70214-6. [DOI] [PubMed] [Google Scholar]
- 75.Leiter L, Carr MC, Stewart M, Jones-Leone M, Yang F, Handelsman Y. HARMONY 8: Once-weekly Glucagon-like Peptide 1 Receptor Agonist Albiglutide vs. Sitagliptin for Patients with Type 2 Diabetes with Renal Impairment: Week 26 Results. 49 EASD Barcelona 2013 Presentation Number: 906. 2013 [Google Scholar]
- 76.Wilmington, DE: GlaxoSmithKline LLC; 2014. Tanzeum (albiglutide) [package insert] [Google Scholar]
- 77.Gough SC, Bode B, Woo V, Rodbard HW, Linjawi S, Poulsen P, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: Results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2:885–93. doi: 10.1016/S2213-8587(14)70174-3. [DOI] [PubMed] [Google Scholar]
- 78.Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. 2011;60:1561–5. doi: 10.2337/db10-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dungan K, Buse JB. Glucagon-like peptide 1-based therapies for type 2 diabetes: A focus on exenatide. Clin Diabetes. 2005;23:56–62. [Google Scholar]
- 80.Eli Lilly and Company; 2014. [Last accesed on 2015 Dec 16]. Trulicity European Summary of Product Characteristics. Available from: http://ec.europa.eu/health/documents/community-register/2014/20141121130063/anx_130063_en.pdf . [Google Scholar]
- 81.Kalra S. Choosing appropriate glucagon-like peptide 1 receptor agonists: A patient-centered approach. Diabetes Ther. 2014;5:333–40. doi: 10.1007/s13300-014-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kalra S, Gupta Y. DOT (directly observed therapy) in diabetes: Current thought, future reality. Aust J Rural Health. 2014;22:206. doi: 10.1111/ajr.12123. [DOI] [PubMed] [Google Scholar]
- 83.Kalra S, Kalra B. Counselling patients for GLP-1 analogue therapy: Comparing GLP-1 analogue with insulin counselling. N Am J Med Sci. 2012;4:638–40. doi: 10.4103/1947-2714.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mudaliar S, Henry RR. The incretin hormones: From scientific discovery to practical therapeutics. Diabetologia. 2012;55:1865–8. doi: 10.1007/s00125-012-2561-x. [DOI] [PubMed] [Google Scholar]
- 85.Carty DM, Drummond R, Fisher M. Cardiovascular safety and GLP-1 receptor agonists. Pract Diabetes. 2013;30:242–5. [Google Scholar]
- 86.Sanofi Press Release. [Last cited on 2015 Sep 08]. Available from: http://www.en.sanofi.com/NasdaQ_OMX/local/press_releases/sanofis_lyxumia_lixisenatide_d_1926874_08-06-2015!18_16_00.aspx .
- 87.Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J Alzheimers Dis. 2010;19:1205–19. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: Are the GLP-1 therapies safe? Diabetes Care. 2013;36:2118–25. doi: 10.2337/dc12-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nauck MA. A critical analysis of the clinical use of incretin-based therapies: The benefits by far outweigh the potential risks. Diabetes Care. 2013;36:2126–32. doi: 10.2337/dc12-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.American Diabetes Association. ADA/EASD Statement Concerning the Use of Incretin Therapy and Pancreatic Disease. [Last cited on 2014 Dec 10]. Available from: http://www.diabetes.org/newsroom/press-releases/2013/recommendations-for.html .