Abstract
Cell surface transmembrane protein IGFS8 (herein called EWI-2) negatively regulates melanoma TGF-β signaling and is well positioned to control the transition in TGF-β signaling from cytostatic (in early melanoma stages) to pro-invasion/metastasis (in later stages). EWI-2 functions by sequestering the tetraspanin proteins CD9 and CD81, thereby making them unavailable to support the association of TGFβ receptor 1 with TGFβ receptor 2.
Keywords: CD81, CD9, EWI-2/IGSF8, melanoma, TGF-β1, tetraspanins
EWI-2 Negatively Regulates TGF-β Signaling
Transforming growth factor-β (TGF-β) plays a dual role in cancer. In early stages of tumor growth TGF-β is cytostatic, but TGF-β–induced cytostasis is subsequently diminished thus allowing primary tumors to grow. At a later stage TGF-β signaling can reappear in support of epithelial-mesenchymal-transition (EMT), invasion, and metastasis in melanoma1 and other cancers.2 Transitions between these paradoxical tumor suppressor and promoter roles for TGF-β have been linked to a wide variety of molecular events, including changes in extracellular signal-regulated kinase (ERK) signaling, TGF-β receptor cleavage, upregulation of inhibitory Smad7, and recruitment of phosphatase PP2A.3 A recent report now introduces the cell surface transmembrane protein IGSF8 (herein called EWI-2)4 as a potentially important modulator of TGF-β signaling transitions. EWI-2 was discovered to be a negative regulator of TGF-β signaling based on a combination of EWI-2 knockdown and overexpression experiments, soluble TGF-β titration experiments, TGF-β inhibition experiments, and unbiased gene expression analyses in melanoma cells.5 In a key experiment illustrating this point, ablation of EWI-2 in SK-Mel-28 cells markedly enhanced phosphorylation of Smads 2 and/or 3, major downstream mediators of canonical TGF-β signaling.6 EWI-2 is well positioned to play a key role in orchestrating TGF-β signaling transitions in melanoma cells.5 EWI-2 expression is absent from primary melanocytes (which are growth-inhibited by TGF-β), present in primary tumors (which have diminished TGF-β signaling), and then diminished in metastatic melanomas (in which TGF-β signaling has reappeared) [Fig. 1A]. Consistent with EWI-2 serving as a temporary repressor of TGF-β signaling on primary tumor cells, ablation of EWI-2 caused (i) increased TGF-β-dependent cytostasis and (ii) increased TGF-β–dependent invasion, metastasis, and EMT-like changes in melanoma cells.5 The signaling pathways and transcriptional events responsible for selective upregulation of EWI-2 expression on primary melanoma cells remain to be determined.
Figure 1.
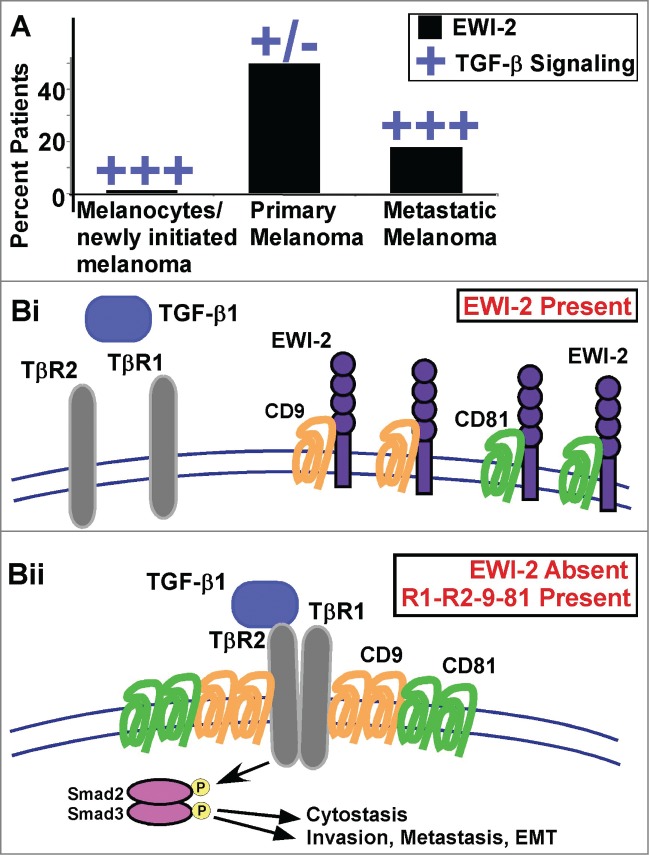
Impact of EWI-2 on TGF-β signaling. (A) Expression of IGSF8 (herein called EWI-2) is elevated in primary melanoma, diminished in metastatic melanoma, and absent in melanocytes.5 Conversely, TGF-β signaling is elevated in newly initiated melanoma, diminished in primary melanoma, but again elevated in metastatic melanoma. (Bi) When EWI-2 is present, tetraspanin proteins CD9 and CD81 are sequestered into hetero-oligomer complexes with EWI-2. As a result, CD9 and CD81 are unavailable to assist the association of TGFβ receptor 1 (TβR1) with TβR2. (Bii) In the absence of EWI-2, more CD9 and CD81 homo-oligomers are present, and together they aid in the association of TβR1 with TβR2 within an “R1-R2-9-81” complex. Consequently, TGF-β1 signaling is upregulated, leading to Smad2/3 activation and stimulation of melanoma epithelial-mesenchymal transition (EMT), invasion, and metastasis.
Hidden Contributions of CD9 and CD81
A key initial step in TGF-β signaling is the TGF-β-induced formation of complexes between TGFβ receptor 1 (TβR1) and TGFβ receptor 2 (TβR2).6 To understand how EWI-2 negatively regulates TGF-β signaling at this early step, it is necessary to consider the critical roles of the tetraspanin proteins CD9 and CD81. When EWI-2 is ablated or constitutively diminished in melanoma cells, both CD9 and CD81 become available to make major contributions to enhanced TβR1–TβR2 association (Fig. 1Bii). Furthermore, in the absence of EWI-2, CD9 becomes free to associate with TβR2 in a CD81-dependent manner.5 Thus, CD9 (and likely also CD81) is well-positioned to support TβR1–TβR2 association and subsequent downstream Smad2/3 activation, leading to cytostasis, invasion, EMT, and metastasis. Conversely, when EWI-2 is present, nearly all (˜80%) of it is associated directly with CD9 and CD81.4,5,7 This direct EWI-2 sequestration of CD9 and CD81 makes them unavailable to support TGF-β–initiated TβR1–TβR2 receptor association (Fig. 1Bi). Consistent with the model presented in Figure 1B, for all TGF-β–dependent functions that are increased as a consequence of EWI-2 ablation (e.g., TGF-β-dependent signaling, EMT, invasion, metastasis), the extent of the increase is entirely dependent on the presence of both CD9 and CD81.5 Notably, CD9 and CD81 may associate with several other partner proteins (e.g., claudin-1, CD44, MHC class I, CD224, EpCAM, integrins),8,9 but these interactions do not appear to be of sufficient proximity and stoichiometry to mimic the sequestering effects of EWI-2 indicated in Figure 1Bi.
Prognostic and Therapeutic Implications
The model in Figure 1B has prognostic implications regarding EWI-2 and TGF-β–dependent tumor functions. In this regard, tissue from EWI-2–ablated SK-Mel-28 xenograft tumors in mice showed significantly upregulated Smad2/3 phosphorylation. Exhibiting a similar trend, tissue samples from human melanoma patients with progressively lower EWI-2 expression showed inversely elevated phospho-Smad2/3.5 Consequently, we predict that progression to metastasis should be diminished in the 50% EWI-2Hi subset of primary melanomas, and patient survival should be improved in the ˜20% EWI-2Hi metastatic melanoma subset. However, these expected inverse correlations between EWI-2 expression and (i) melanoma progression to metastasis and (ii) patient survival remain to be established experimentally. The model presented in Figure 1B also has therapeutic implications. For example, key molecular associations (e.g., CD9-TβR2 in EWI-2–deficient cells) could be targeted in a screen to yield inhibitory agents. Such agents should selectively impair TGF-β signaling in EWI-2–negative invasive and metastatic melanoma cells and in EWI-2–negative aggressive primary melanomas, but not in EWI-2–positive primary melanomas. However, since TGF-β signaling has potent cytostatic effects in the early stages of melanoma, treatment with such agents would have to be limited to later stages of melanoma.
Broader Applications
Finally, it remains to be determined whether the negative regulatory effects of EWI-2 and the positive contributions of CD9 and CD81 to TGF-β signaling are limited to melanoma. In this regard, EWI-2 gene expression is upregulated in melanoma to a greater extent than in any other cancer type tested (see ref. 10 and the Oncomine database). Nonetheless, we have seen evidence that EWI-2 negatively regulates TGF-β signaling in at least 2 non-melanoma tumor types (unpublished results). Also, it remains untested whether EWI-2, CD9, or CD81 will affect other key TGF-β signaling functions, for example on cancer stem cells, or during tissue fibrosis, angiogenesis, or evasion of immunosurveillance.3
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Institutes of Health (CA42368 to MEH).
References
- 1.Perrot CY, Javelaud D, Mauviel A.. Insights into the transforming growth factor-beta signaling pathway in cutaneous melanoma. Ann Dermatol 2013; 25: 135–44; PMID:23717002; http://dx.doi.org/ 10.5021/ad.2013.25.2.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Padua D, Massague J. Roles of TGFbeta in metastasis. Cell Res 2009; 19: 89–102; PMID:19050696; http://dx.doi.org/ 10.1038/cr.2008.316 [DOI] [PubMed] [Google Scholar]
- 3.Principe DR, Doll JA, Bauer J, Jung B, Munshi HG, Bartholin L, Pasche B, Lee C, Grippo PJ. TGF-beta: duality of function between tumor prevention and carcinogenesis. J Natl Cancer Inst 2014; 106: djt369; PMID:24511106; http://dx.doi.org/ 10.1093/jnci/djt369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stipp CS, Kolesnikova TV, Hemler ME. EWI-2 is a major CD9 and CD81 partner, and member of a novel Ig protein subfamily. J Biol Chem 2001; 276: 40545–54; PMID:11504738; http://dx.doi.org/ 10.1074/jbc.M107338200 [DOI] [PubMed] [Google Scholar]
- 5.Wang HX, Sharma C, Knoblich K, Granter SR, Hemler ME. EWI-2 negatively regulates TGF-beta signaling leading to altered melanoma growth and metastasis. Cell Res 2015; 25: 370–85; PMID:25656846; http://dx.doi.org/ 10.1038/cr.2015.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov 2012; 11: 790–811; PMID:23000686; http://dx.doi.org/ 10.1038/nrd3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charrin S, Le Naour F, Labas V, Billard M, Le Caer JP, Emile JF, Petit MA, Boucheix C, Rubinstein E. EWI-2 is a new component of the tetraspanin web in hepatocytes and lymphoid cells. Biochem J 2003; 373(Pt 2): 409–21; PMID:12708969; http://dx.doi.org/ 10.1042/BJ20030343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovalenko OV, Yang XH, Hemler ME. A novel cysteine cross-linking method reveals a direct association between claudin-1 and tetraspanin CD9. Mol Cell Proteomics 2007; 6: 1855–67; PMID:17644758; http://dx.doi.org/ 10.1074/mcp.M700183-MCP200 [DOI] [PubMed] [Google Scholar]
- 9.Le Naour F, Andre M, Greco C, Billard M, Sordat B, Emile JF, Lanza F, Boucheix C, Rubinstein E. Profiling of the tetraspanin web of human colon cancer cells. Mol Cell Proteomics 2006; 5: 845–57; PMID:16467180; http://dx.doi.org/ 10.1074/mcp.M500330-MCP200 [DOI] [PubMed] [Google Scholar]
- 10.Scherf U, Ross DT, Waltham M, Smith LH, Lee JK, Tanabe L, Kohn KW, Reinhold WC, Myers TG, Andrews DT, et al.. A gene expression database for the molecular pharmacology of cancer. Nat Genet 2000; 24: 236–44; PMID:10700175; http://dx.doi.org/ 10.1038/73439 [DOI] [PubMed] [Google Scholar]


