Abstract
A facile method was developed to purify 2-[18F]fluoroethyl azide ([18F]FEA) using a C18 cartridge and an Oasis® HLB cartridge in series, in which [18F]FEA was exclusively trapped on the HLB cartridge. [18F]FEA can be eluted for reactions in solution; alternatively click labeling can be carried out on the HLB cartridge itself by loading an alkyne substrate and copper (I) catalyst dissolved in DMF onto the cartridge. This solid phase extraction methodology for purification and click labeling with [18F]FEA, either in solution or on the cartridge, is safe, simple, reproducible in high yield, and compatible with automated synthesis of 18F-labeled PET tracers.
Keywords: Fluorine-18, Radiolabeling, Click chemistry, 2-Fluoroethyl azide, Positron emission tomography, Solid phase extraction
Synthesis of positron emission tomography (PET) radiopharmaceuticals requires not only short reaction times, but also reliable and reproducible techniques, simple manipulations, and facile purification, due to the short half-lives of the radionuclides. Implementation of automated synthesis of PET tracers for clinical studies minimizes personnel exposure in compliance with ALARA (as low as reasonably achievable) exposure guidelines. In addition, the preparation of PET radiopharmaceuticals having high chemical and radiochemical purities as well as high specific activity (SA) and effective SA is critical for PET imaging, especially for imaging low abundance targets such as some neuroreceptors.1
Copper (I)-catalyzed azide–alkyne cycloaddition (CuAAC),2 due to its specificity, robustness, mild reaction conditions, and high yielding, is ideal for the synthesis of PET radiotracers containing short-lived positron emitting radionuclides such as 18F (t1/2 = 109.8 min). Among the many 18F labeled azide and alkyne moieties,3 2-[18F]fluoroethyl azide ([18F]FEA) ([18F]2)4 has been used the most for click labeling of small molecules and peptides for PET. However, the high volatility of [18F]2 increases radiation safety risk and can create challenges in click labeling manipulations.
A flow-and-trap method was originally reported for the isolation of [18F]2 from the reaction mixture. While effective, this method provided modest yields and required relatively large volumes of solvent.4 Although one-pot click labeling can be readily adapted to automated production, for some compounds the requirement for larger amounts of alkyne precursors and the alkaline reaction conditions lead to difficulties in purification and promote side reactions.5,6 A vacuum trap-to-trap method has been used to efficiently isolate [18F]2, but the low temperature trap is not compatible with automated modules.7 The increasing use of and demand for automated production of PET tracers including [18F]ICMT-11,8 [18F]AFTEP,9 and [18F]FluorApotrace (WC-4-116),10 have led us to develop a solid phase method for click labeling which can be readily incorporated in the automated production of these tracers using [18F]2.
Solid phase extraction (SPE) is widely used in radiochemistry for purification and formulation of PET radiopharmaceuticals. Although we have not seen prior reports of click-labeling reactions on the SPE cartridge itself, click labeling on a SPE cartridge is highly advantageous: click labeling always employs a radiolabeled moiety which typically needs to be purified or extracted by SPE after radiosynthesis and before use in the click reaction. In this Letter, a novel method is described both to purify [18F]2 with SPE cartridges in series and to carry out a click reaction of [18F]2 on the SPE cartridge itself.
2-[18F]Fluoroethyl azide ([18F]2) was synthesized from 2-azidoethyl-4-toluenesulfonate (1) in up to 90% radiochemical yield following the reported procedure.4,7 Purification of [18F]2 was carried out using standard SPE procedures, as shown in Scheme 1, followed by diluting the reaction mixture with water and passing the diluted reaction mixture through a silica-based C18 cartridge and a Waters Oasis® HLB hydrophilic–lipophilic-balanced polymer-based reverse-phase cartridge in series, then rinsing with water. The C18 cartridge was used to trap unreacted precursor as well as polar compounds observed at the HPLC solvent front (Fig. S1) from the aqueous reaction mixture. The Waters HLB cartridge was used to trap the radiolabeled product, [18F]2. K2CO3 and K222 pass through both cartridges into waste during the aqueous elution and water rinse. The radioactivity associated with [18F]2 was well retained (>95%) in the HLB cartridge, which can be easily shielded. This purification method was validated by HPLC analysis of a nonradioactive control reaction, in which the major UV peak retained by the HLB cartridge is the vinyl azide 3, previously confirmed by NMR analysis (Fig. S2).7
Scheme 1.
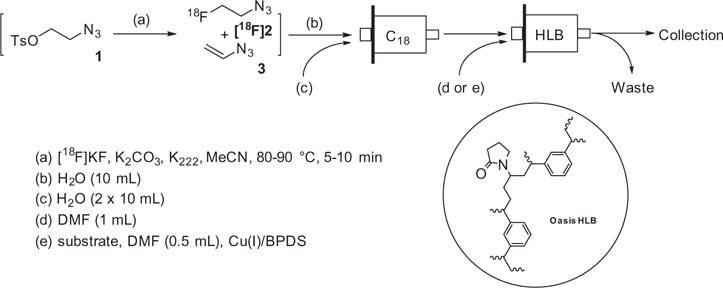
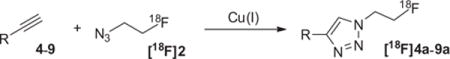
The removal of 1, acetonitrile, and K2CO3, etc. facilitates successful click labeling using [18F]2 and purification of the final products. In prior work, use of acetonitrile as a solvent was detrimental to the click reaction with [18F]2.7 DMF is a more compatible solvent for click labeling with [18F]2, but the radiosynthesis of [18F]2 in DMF afforded low yields. K2CO3 may promote decomposition of base-labile alkyne substrates and other potential side reactions.11 Reaction mixtures of [18F]2 with low quantities of alkyne substrates were analyzed by HPLC, and it was estimated that the clickable azide after SPE purification is about 0.25–0.3 equiv to the starting material 1 used. This reduction in ‘clickable’ substrate is significant, considering that only a small amount of alkyne substrate is needed to complete the click reaction with [18F]2.
It is worthwhile to note that the cycloaddition rate of 2 to an alkyne substrate appeared to far exceed that of the precursor 1 in a chelator-accelerated one-pot reaction,5 carried out in an aqueous acetonitrile solution. This promising observation led us to explore the potential of using this rate difference in click labeling to achieve selective reaction with 2. Regrettably, under our labeling conditions, the presence of excess of 1 or 3 in the click labeling of 7 (Table 1) with 2 either reduced the radiochemical yield or completely blocked the cycloaddition reaction. The observed difference may be specific for the alkyne substrates used or due to solvent or catalyst effect. These phenomena are well worth further investigation.
Table 1.
Summary of click labeling using 2-[18F]fluoroethyl azide ([18F]2) and different alkyne substrates in solution and using HLB SPE methodology
| |||||
|---|---|---|---|---|---|
| Entry | Substrate | Quantity (mg) | Reaction conditionsa (media/temperature/time) | [18F] 2 (%) | RCYb (%) |
| 1 | Control | SPE/RT/10 min | 100 | – | |
| 2 |
|
1 | DMF/RT/10 min SPE/RT/10 min |
1 1 |
97 95 |
| 3 |
|
1.5 | DMF/RT/10min SPE/RT/10min DMF/83 °C/10 min SPE/83 °C/10min |
0 1 0 0 |
95 90 95 95 |
| 4 |
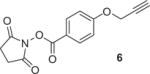
|
1.5 | DMF/acetonitrile/RT/5 min SPE/RT/5 min |
0 3 |
90c 90 |
| 5 |
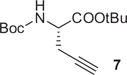
|
1.3 | DMF/RT/10 min SPE/RT/10 min |
0 1 |
90 90 |
| 6 |
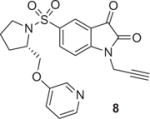
|
1.3 | SPE/RT/10 min | 1 | 60 |
| 7 |
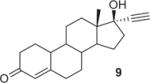
|
1.3 | DMF/RT/10min SPE/RT/10min DMF/83 °C/10 min SPE/83 °C/10min |
76 80 4.7 9 |
24 20 95.3 91 |
Solution method: in DMF (0.5 mL); SPE: DMF in Waters Oasis® HLB plus.
Radiochemical yield: determined by analytical HPLC.
Ref. 7.
[18F]2 was simply eluted with DMF for subsequent click labeling in solution using either copper sulfate/sodium ascorbate or bathophenanthrolinedisulfonate (BPDS) as a co-catalyst for the Cu(I) source (Table 1). The click labeling of [18F]2 with 5 (Table 1) directly on the HLB cartridge was first attempted using a mixture of copper sulfate/sodium ascorbate as the Cu(I) source. However, because this mixture is a suspension in DMF, it did not readily disseminate through the HLB cartridge and resulted in a poor yield of click labeling products. When BPDS, a Cu(I) chelator used in CuAAC to stabilize Cu(I) and to accelerate the cycloaddition reaction,12 was added to the mixture, it formed a homogenous solution in DMF; this dark solution evenly distributed through the HLB cartridge, significantly improving completion therefore increasing the yield of the click reaction.
The solution of alkyne substrate and the Cu(I) source in DMF was slowly loaded onto the HLB cartridge, allowing solvent exchange and even distribution of the reaction mixture. Once complete, the product can be simply eluted with acetonitrile and water for HPLC purification. The results of our click labeling of [18F]2 with model compounds and established radiopharmaceuticals are shown in Table 1. The click reaction behaved similarly in solution and in SPE. Except for substrate 9, [18F]2 was consumed within 10 min at room temperature to afford the desired compound as the major radioactive product. The reduced yield for 8 was due to the instability of the product formed. The click reaction with substrate 9 was slow at room temperature, but raising the reaction temperature to 83 °C increased the yield to over 90%. Because the reactivity of alkynes varies considerably depending on the electronic and steric environment of the substrate, consideration should be given to those factors during a labeling of new tracers. Using this method, we were able to make [18F]AFTEP (7a)6 using only 1–1.5 mg substrate and establish the automated production of 8a (FluorApotrace).
Click labeling using [18F]2 on a polymer-based SPE cartridge is a novel method. We believe that the following factors contribute to the successful click labeling using 2 in the HLB cartridge: high specificity and fast reaction between the alkyne and azide groups catalyzed by Cu(I)/BPDS; large excess of alkyne substrate relative to [18F]2 after SPE purification; good distribution of the reaction mixture throughout the cartridge; inertness of the HLB copolymer (absence of silanol functions), and stability of the HLB sorbent even when heated at 80–90 °C. This method has potential applications in labeling (e.g., click reaction) and purification (e.g., 4-[18F]fluoro-but-1-yne). Several other commercially available polymeric SPE cartridges (Phenomenex Strata-X™, Agilent Bond Elut Plexa, and EMD Millipore LiChrolut® EN) were also tested, the first two afforded results comparable to those obtained with the Waters HLB cartridge.
In conclusion, a novel method using SPE cartridges was developed for purification of and click labeling with [18F]2. This method is safe, simple, reproducible, high yielding, and can be readily adapted for automated syntheses.
Supplementary Material
Acknowledgments
This study was sponsored by DOE DE-SC0005434 and DE-SC0002032. We thank Robert Dennett and Brian Wingbermuehle for the production of [18F]fluoride.
Footnotes
Supplementary data
Supplementary data (These data include general information, experimental details and HPLC chromatographs of click reactions) associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.tetlet.2014.10.126.
Contributor Information
Dong Zhou, Email: zhoud@mir.wustl.edu.
Wenhua Chu, Email: chuw@mir.wustl.edu.
Xin Peng, Email: xpeng@go.wustl.edu.
Jonathan McConathy, Email: mcconathyj@mir.wustl.edu.
Robert H. Mach, Email: rmach@mail.med.upenn.edu.
John A. Katzenellenbogen, Email: jkatzene@illinois.edu.
References and notes
- 1.Lapi SE, Welch MJ. Nucl Med Biol. 2013;40:314. doi: 10.1016/j.nucmedbio.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Meldal M, Tornoe CW. Chem Rev. 2008;108:2952. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 3.Glaser M, Robins EG. J Labelled Compd Radiopharm. 2009;52:407. [Google Scholar]
- 4.Glaser M, Arstad E. Bioconjug Chem. 2007;18:989. doi: 10.1021/bc060301j. [DOI] [PubMed] [Google Scholar]
- 5.Galante E, Schoultz BW, Koepp M, Arstad E. Molecules. 2013;18:5335. doi: 10.3390/molecules18055335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McConathy J, Zhou D, Shockley SE, Jones LA, Griffin EA, Lee H, Adams SJ, Mach RH. Mol Imaging. 2010;9:329. [PubMed] [Google Scholar]
- 7.Zhou D, Chu W, Dence CS, Mach RH, Welch MJ. Nucl Med Biol. 2012;39:1175. doi: 10.1016/j.nucmedbio.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen QD, Smith G, Glaser M, Perumal M, Arstad E, Aboagye EO. Proc Natl Acad Sci USA. 2009;106:16375. doi: 10.1073/pnas.0901310106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solingapuram Sai KK, Huang C, Yuan L, Zhou D, Piwnica-Worms D, Garbow JR, Engelbach JA, Mach RH, Rich KM, McConathy J. J Nucl Med. 2013;54:1120. doi: 10.2967/jnumed.112.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu W, Rothfuss J, Zhou D, Mach RH. Bioorg Med Chem Lett. 2011;21:2192. doi: 10.1016/j.bmcl.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angell Y, Burgess K. Angew Chem, Int Ed. 2007;46:3649. doi: 10.1002/anie.200700399. [DOI] [PubMed] [Google Scholar]
- 12.Lewis WG, Magallon FG, Fokin VV, Finn MG. J Am Chem Soc. 2004;126:9152. doi: 10.1021/ja048425z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


