Abstract
Type 2 inflammatory responses can be elicited by diverse stimuli, including toxins, venoms, allergens and infectious agents and play critical roles in resistance and tolerance associated with infection, wound healing, tissue repair and tumor development. Emerging data suggest that in addition to characteristic type 2-associated cytokines, the Epidermal Growth Factor (EGF)-like molecule Amphiregulin (AREG) may be a critical component of type 2-mediated resistance and tolerance. Notably, numerous studies demonstrate that in addition to the established role of epithelial- and mesenchymal-derived AREG, multiple leukocyte populations including mast cells, basophils, group 2 innate lymphoid cells (ILC2) and a subset of tissue-resident regulatory CD4+ T cells can express AREG. In this review, we discuss recent advances in our understanding of the AREG-EGF receptor pathway and its involvement in infection and inflammation, and propose a model for the function of this pathway in the context of resistance and tissue tolerance.
Introduction
The immune system is composed of a complex network of leukocytes interacting with other physiological systems to protect the host from pathogen invasion and tumorigenesis. Host protection can manifest itself in two ways: as resistance, the ability to eradicate invading pathogens or other foreign stimuli; or as tolerance, the capacity to decrease pathogen- or tumor-induced damage without affecting pathogen or tumor burden (Medzhitov et al., 2012; Schneider and Ayres, 2008). Immune-mediated resistance is tailored to match the type of invading pathogen, allowing for the production of polarized factors that specifically target distinct pathogens. For example, protective immunity to many viruses and bacteria is coordinated by the type 1 or type 17 immune response, which can include increases in interferon-γ (IFN-γ) and IL-17, while multicellular parasites elicit type 2 responses, associated with increases in interleukin-4 (IL-4), IL-5, IL-9 and IL-13 (Allen and Wynn, 2011; Gause et al., 2013; Palm et al., 2012; Pulendran and Artis, 2012). In contrast, tolerance mechanisms that mitigate damage through control of harmful inflammation and enhanced tissue repair are more general, and similar mechanisms of tolerance are utilized in response to a wide array of pathogens and stimuli. As such, the pathways involved in tolerance often include components of the type 2 immune response and can be activated by diverse stimuli, including toxins, venoms, allergens and infectious agents such as helminths, bacteria and viruses (Allen and Wynn, 2011; Gause et al., 2013; Palm et al., 2012; Pulendran and Artis, 2012).
While a wide array of factors contribute to the host response to pathogen infection or other foreign antigens, the Epidermal Growth Factor (EGF)-like molecule, Amphiregulin (AREG), has recently been shown to play a central role in orchestrating both host resistance and tolerance mechanisms. Although AREG and other EGF family members are originally described as epithelial cell-derived factors, recent data show that AREG can be expressed by multiple populations of activated immune cells in a variety of inflammatory conditions. At the current time, the immune cells described to have AREG-expressing capacity are primarily associated with type 2 responses (Table 1 and associated references). Recent studies indicate that AREG is a pivotal factor that can contribute to host resistance (Zaiss et al., 2006), but is primarily a key factor that induces tolerance by promoting the restoration of tissue integrity following damage associated with acute or chronic inflammation (Burzyn et al., 2013; Jamieson et al., 2013; Monticelli et al., 2011). In this review, we will summarize recent developments regarding the distribution and regulation of expression of AREG in the steady-state and during inflammation. We will then discuss the role of AREG in resistance and tolerance to a variety of pathogens, in promotion of tissue repair and homeostasis and in the regulation of inflammation and tumor progression.
Table 1.
Innate and adaptive immune cell populations as sources of Amphiregulin
| Arm of Immune system | Cell type | Host | Inflammatory setting | Reference |
|---|---|---|---|---|
| Innate | Basophils | Human | Allergy | Qi et al. JACI 2010 |
| Mouse | Contact hypersenstivity | Meulenbroeks et al. J Inv Derm 2014 | ||
| Eosinophils | Human | Ex vivo GMCSF stimulation | Matsumoto et al. Int Arch Allergy Immunol 2009 | |
| Mast Cells | Human | Ex vivo IgE cross-linking | Wang et al. JACI 2005 | |
| Human | Ex vivo FcεRI aggregation | Okumura et al. JACI 2005 | ||
| Mouse | Dermatitis, T cell transfer colitis, cancer | Zaiss et al. Immunity 2013 | ||
| Neutrophils | Human | Cystic fibrosis | Adib-Conquy et al. Mol Med 2008 | |
| Group 2 innate lymphoid cells (ILC2) | Human | Atoptic dermatitis | Salimi et al. J Exp Med 2013 | |
| Mouse | Influenza | Monticelli et al. Nature Immunol 2011 | ||
| Dendritic cells | Human & mouse (in vitro) | ATP stimulation | Bles et al. Blood 2010 | |
| Adaptive | CD4+ T cells | Human | Ex vivo TCR stimulations | Qi et al. PloS ONE 2012 |
| Mouse | Trichuris muris | Zaiss et al. Science 2006 | ||
| Regulatory CD4+ T cells (subset) | Mouse | Muscle injury | Burzyn et al. Cell 2013 | |
| Tumor infiltrating CD8+ T cells | Mouse | Chemical carcinogenesis | Kwong et al. J. Invest. Derm. 2010 |
Distribution and regulation of amphiregulin expression
AREG, a member of the epidermal growth factor (EGF) family, is constitutively expressed by a number of epithelial and mesenchymal cell types during development and homeostasis (Berasain and Avila, 2014). In addition to being implicated in a variety of physiologic processes, including regulation of lung morphogenesis (Schuger et al., 1996), keratinocyte proliferation (Cook et al., 1991) and mammary gland development (Li et al., 1992), studies demonstrating that wounded keratinocytes rapidly induce robust AREG expression (Kennedy-Crispin et al., 2012) have contributed to the hypothesis that epithelial-derived AREG can act to promote tissue repair and integrity. Importantly, AREG-gene deficient mice display very few abnormalities under homeostatic conditions (Luetteke et al., 1999), but resolution of a variety of inflammatory challenges is impaired in these mice (Berasain et al., 2005; Meulenbroeks et al., 2015; Perugorria et al., 2008; Zaiss et al., 2006), supporting the hypothesis that AREG plays a critical role in restoring tissue integrity following infection or injury.
As our appreciation for the dialogue between tissue-resident immune cells and the epithelial cells they interact with grows, accumulating data suggest that the immune system may also play a critical role in AREG-epidermal growth factor receptor (EGFR) signaling. For example, in the context of various inflammatory stimuli, basophils (Qi et al., 2010), eosinophils (Matsumoto et al., 2009), mast cells (Okumura et al., 2005; Wang et al., 2005; Zaiss et al., 2013), group 2 innate lymphoid cells (ILC2) (Monticelli et al., 2011; Salimi et al., 2013), dendritic cells (DCs) (Bles et al., 2010) and activated CD4+ T cells (Qi et al., 2012; Zaiss et al., 2006) have all been shown to express AREG (Table 1). In addition, AREG expression has also been reported in neutrophils in the sputum of cystic fibrosis (CF) patients (Adib-Conquy et al., 2008), in a subset of tumour-infiltrating CD8+ T cells (Kwong et al., 2010) and in a phenotypically and functionally distinct subtype of FoxP3-expressing CD4+ regulatory T cells (Tregs) that accumulates in injured muscles (Burzyn et al., 2013) or the inflamed colon of mice (Schiering et al., 2014). These Treg cell populations have a distinct T cell receptor (TCR) repertoire, express the IL-33R (Burzyn et al., 2013; Schiering et al., 2014) and are distinguished from other Treg cell populations in that they express AREG (Burzyn et al., 2013). While it is possible that other leukocytes may express AREG in a variety of contexts, the cell types currently described to express AREG and their pattern of activation suggest that immune-derived AREG is associated with type 2 immune-mediated resistance and tolerance mechanisms.
Furthermore, multiple immune mediators including prostaglandin E2 (Berasain et al., 2005; Qi et al., 2012; Shao et al., 2003), cAMP (Johansson et al., 2004), insulin-like growth factor-1 (IGF-1) (Rodland et al., 2008) and transforming growth factor-β (TGF-β) (Bennett et al., 1992; Zhou et al., 2012) can induce AREG expression, suggesting that AREG production may be regulated by the activation of multiple cell types during an immune response.
Diverse stimuli have been associated with induction of AREG expression in leukocytes. For example, IL-33 exposure stimulates AREG expression in ILC2 (Monticelli et al., 2011; Salimi et al., 2013) but not in multipotent progenitor type 2 cells (Saenz et al., 2013). Basophils express high quantities of AREG following exposure to IL-3. However, human basophils produce substantially less AREG in response to IgE-cross-linking than to IL-3 exposure (Qi et al., 2010), which is consistent with evidence from mouse models suggesting that basophils are heterogeneous in their development and effector functions (Siracusa et al., 2011). In humans, prostaglandin E2 has been shown to be a potent inducer of AREG expression in a variety of CD4+ T cell subsets (Qi et al., 2012). In murine T cells, AREG is expressed in a subset of tissue-resident CD4+ Treg cells (Burzyn et al., 2013) and could be induced by TCR stimulation in T helper-2 (Th2) but not Th1 cells (Zaiss et al., 2006), suggesting that AREG expression is restricted within subsets of CD4+ T cells. Consistent with this, while type 2 inflammation can be initiated independently of AREG in some contexts (Kajiwara et al., 2010; Yagami et al., 2010), there are a growing number of disease states linking AREG and type 2 inflammation (see below).
While the findings outlined above demonstrate that immune cells can be induced to express AREG, numerous open questions remain related to the biology of AREG responses. For example, further work is needed to determine how hematopoietic AREG expression and function is regulated in vivo. In addition, how the signals that direct AREG expression from distinct cell types during development differ from those that induce AREG expression in response to infection, inflammation or injury remains poorly understood. Importantly, the distinct roles of hematopoietic- and non-hematopoietic cell-derived AREG remain unclear. AREG is expressed as a membrane-bound protein, and activation of AREG on non-hematopoietic cells is regulated largely at the level of release from the cell membrane. However, the ability of hematopoietic cells to migrate to the site of inflammation and locally up-regulate AREG expression can substantially influence local concentrations of this growth factor. Thus, hematopoietic and non-hematopoietic cells may play different roles in contributing to AREG responses. Finally, how other pathways regulate AREG responsiveness is incompletely defined. For instance, AREG requires heparin sulfate expression on target cells, which is tightly controlled during inflammation (Simon Davis and Parish, 2013), in order to efficiently signal via the EGFR (Johnson and Wong, 1994). These data suggest that a combination of mechanisms may allow for a highly regulated spatio-temporal accumulation of AREG at the site of epithelial damage and inflammation. Further investigation into the regulation of AREG expression, the significance of hematopoietic- versus non-hematopoietic-derived AREG and the mechanisms that regulate AREG responsiveness will require the development of new genetic tools that permit the manipulation of AREG expression and responsiveness on a cell lineage-specific basis.
Taken together, these findings demonstrate that AREG expression is elicited by diverse stimuli yet is primarily associated with immune cell populations activated in type 2 immune responses, wound repair and the resolution of inflammation. Below, we will discuss data that suggest critical roles for hematopoietic-derived sources of AREG during infection, inflammation and tissue homeostasis, with particular focus on recent developments in our understanding of how AREG contributes to the pleiotropic aspects of type 2 immunity.
Amphiregulin–mediated resistance and tolerance to infection
Helminths trigger potent type-2 immune responses in both mice and humans (Allen and Wynn, 2011; Gause et al., 2013; Pulendran and Artis, 2012). Helminth infections can cause severe tissue damage and often cannot be cleared by the host. The immune system therefore must develop strategies to tolerate the persisting pathogen and simultaneously mitigate any associated tissue injury. As a result, type 2 immunity can not only mediate clearance of pathogens but also has additional functions, such as rapid repair of tissue damage and promoting homeostasis of infected tissues, which includes the suppression of local inflammation (Allen and Wynn, 2011; Gause et al., 2013; Pulendran and Artis, 2012).
In the context of helminth infection, where parasite invasion and the resultant infection-induced inflammatory response can damage the epithelial layer, enhanced epithelial cell proliferation has a number of beneficial effects, including repair of damaged epithelial cells and helminth expulsion via the `epithelial escalator' (Cliffe et al., 2005). Since distinct helminth parasites utilize various routes to infect the host, a wide range of immune defense mechanisms have evolved to provide protection while limiting pathological inflammation (Anthony et al., 2007; Maizels et al., 2012). For the expulsion of gut-dwelling helminths, the host immune response induces several different effector mechanisms. In addition to signals that activate the `epithelial escalator', the type 2 cytokines IL-4, IL-5, IL-9 and IL-13 contribute to increased muscle contractility, diarrhea and changes to the mucus that lines the gastrointestinal tract (Cliffe and Grencis, 2004; Cliffe et al., 2005; Hasnain et al., 2011; Herbert et al., 2009; Patel et al., 2009). However, the signals that directly regulate epithelial cell proliferation during helminth infection remain less well understood.
AREG is a well-characterized mitogenic stimuli in epithelial cell layers, making it a potential candidate that could contribute to epithelial cell responses following helminth infection. Consistent with this, AREG gene-deficient mice exhibit delayed clearance of the intestinal parasite Trichuris muris, which correlated with diminished proliferation of colonic epithelial cells compared to infected control mice, suggesting a key role for AREG in promoting tissue protective epithelial responses in the intestine (Zaiss et al., 2006). Although the cellular sources of AREG remain undefined in this setting, enhanced proliferation of the epithelial cell layer in infected wild-type mice is dependent on CD4+ T cells, further highlighting the importance of the crosstalk between immune and epithelial cells in the regulation of the AREG-EGFR pathway.
Recent data have demonstrated important roles for AREG in the homeostasis of inflamed tissues and epithelial integrity during non-helminth lung infections (Monticelli et al., 2011; Turner et al., 2013 101). Although type 1 cytokine responses are necessary for resistance to Influenza virus infection, a variety of type 2-associated cell types populate the lungs following pathogen clearance to repair and resolve infection-induced tissue damage and inflammation. For example, following influenza infection of Rag1−/− mice, AREG promotes the regeneration of bronchial epithelium, which enhances tissue integrity and survival of infected mice (Monticelli et al., 2011). Similarly, in the context of bacterial-viral co-infections, mice that had been previously infected with Influenza rapidly succumb to a consecutive bacterial infection in the lungs (Jamieson et al., 2013). This effect is not due to an overwhelming pathogen burden but is instead associated with impaired tissue integrity and immunopathology. Co-infected mice exhibit increased necrosis in the airway epithelial cell layer and genes involved with tissue repair are significantly downregulated in comparison to single-infected mice. However, co-infected mice can be rescued by administration of AREG into the lungs, with no associated influence on pathogen burden. Finally, fungal infection of Dectin-1 gene deficient mice induces severe pulmonary pathology compared to wild-type controls despite similar pathogen burdens, and treatment with recombinant AREG promotes epithelial repair and increased survival of Dectin-1-deficient hosts (Branzk et al., 2014). These data indicate that AREG is an important growth factor that promotes host tolerance by sustaining lung tissue integrity and homeostasis following infection-induced tissue damage. The cellular sources and signaling pathways that regulate AREG induction and its tissue protective effects outside the context of helminth infection require further investigation.
Amphiregulin orchestrates tissue homeostasis
An important component of type 2 immune responses is the limitation and repair of acute tissue damage (Chen et al., 2012). Helminths migrating through vital organs, such as the lung and liver, can cause severe, acute tissue damage to the host. In a number of different settings, type 2 immune responses support tissue repair, such as in the lungs (Chen et al., 2012; Loke et al., 2007), the gut (Seno et al., 2009) and the skin (Lucas et al., 2010). Helminth-induced type 2 inflammation is characterized by the activation of a number of innate immune effector cell types, including ILC2, alternatively activated (M2) macrophages and eosinophils. Although these populations have important roles in pathogen resistance, emerging data suggest they may also play important roles in tissue repair. Importantly, tissue damage itself can provide activation signals for these cell types. Following cellular stress or tissue damage, adenosine is released at the site of inflammation, which can induce up-regulation of the alarmin IL-33 and enhance the development of tissue-protective M2 macrophages and eosinophils at the site of parasite infection (Patel et al., 2014). IL-33 is a potent activator of ILC2, an innate source of AREG, and contributes to M2 differentiation. It has been suggested that M2 macrophages, which can limit acute inflammation and directly promote tissue repair, can mitigate acute lung injury during worm infections through expression of Arginase and the epithelial mitogen IGF-1 (Chen et al., 2012). Notably, the mitogenic activity of IGF-1 is context-dependent in that IGF-1 alone does not induce epithelial cell proliferation, but can enhance EGFR signaling and proliferative responses in the presence of EGF family members (Worster et al., 2012). Furthermore, IGF-1 can induce AREG expression in keratinocytes that then contributes to the mitogenic effect of IGF-1 (Rodland et al., 2008; Vardy et al., 1995). Collectively, these data indicate the possibility that the observed potent mitogenic stimulus for epithelial cells at the site of infection may be induced by coordinated expression of AREG and IGF-1. Thus, immune cells activated by tissue damage and the type 2 immune response may influence tissue repair and homeostasis through AREG-dependent pathways. The extent to which this induced mitogenic stimulus is a direct effect of IGF-1 or in part mediated by IGF-1 induced AREG in vivo is undefined and warrants further investigation.
In the context of muscle injury, a similar reparative role of innate type 2 immune responses and AREG has recently been shown (Burzyn et al., 2013; Heredia et al., 2013). Upon muscle injury, eosinophils rapidly infiltrate the wound and eosinophil-derived IL-4 induces the proliferation of fibro/adipogenic progenitors (FAPs), thereby preventing their differentiation into adipocytes. In this way, FAPs supported myogenesis and the regeneration of skeletal muscle after injury (Heredia et al., 2013). Further, eosinophil influx is associated with accumulation of a distinct subpopulation of CD4+ Treg cells at the site of muscle injury (Burzyn et al., 2013). These muscle-resident Treg cells have a characteristic gene expression pattern and a restricted TCR repertoire that distinguishes them from circulating Treg cells. Furthermore, these muscle-resident Treg cells express AREG, which can enhance the myogenic differentiation of skeletal muscle satellite cells in vitro. In vivo, muscle healing could be enhanced by injection of recombinant AREG into the injured muscle, while healing was inhibited by Treg cell depletion (Burzyn et al., 2013). These data indicate that innate immune responses as well as Treg cell-derived AREG play an important role through various mechanisms in muscle regeneration after injury.
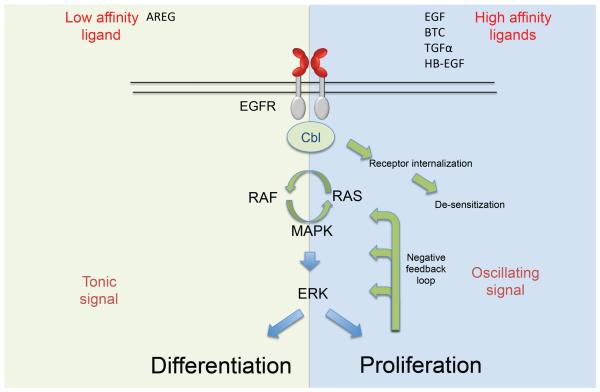
AREG is well-suited for these diverse roles in tissue repair, as it is distinct from most other EGF-like growth factors in that it can not only induce a mitogenic signal but can also induce cell differentiation. This unexpected activity of a “growth factor” is the basis for the name “Amphi”-regulin, as this factor induces proliferation in some cell lines, while inducing growth arrest and differentiation in others (Shoyab et al., 1988). While EGFR signaling mechanisms have been reviewed comprehensively elsewhere (Arteaga and Engelman, 2014; Avraham and Yarden, 2011), how AREG may mediate differential effects of EGFR signaling to promote tissue homeostasis is a key area of investigation. The demonstration that AREG binds the EGFR with unusually low affinity (Jones et al., 1999) and displays differential binding capacity for the EGFR compared to other EGFR ligands (Macdonald-Obermann and Pike, 2014) helps to explain how AREG can mediate distinct biological outcomes compared to other EFG-like molecules (Figure 1). Due to a single amino difference in its receptor-binding domain compared to other EGF-family members, AREG forms an unstable interaction with the receptor (Adam et al., 1995) and fails to induce receptor internalization (Stern et al., 2008), leading to sustained downstream signaling. This is in contrast to other EGFR ligands which bind EGFR with high affinity and thereby induce a rapid internalization and degradation of the EGFR, and associated negative feedback loops within the MAP-kinase signaling cascade (Avraham and Yarden, 2011).
Figure 1. Amphiregulin is a bi-functional growth factor that may induce either cell proliferation or differentiation.
The EGFR is a growth factor receptor well known for its capacity to induce mitogenic stimuli. Nevertheless, it is also well established that the EGFR is important for the differentiation of specific cell types. In recent years it has been revealed that binding characteristics of EGF ligands and qualitative differences in signaling downstream of the receptor dictate the dynamics of gene expression and the fate of recipient cells. Sustained signals downstream of the receptor induces stable ERK activation and leads to growth arrest and cell differentiation, while an oscillating signal downstream of the receptor induces a repetitive activation and inactivation of ERK, leading to proliferation of the target cell. AREG is distinct from most other EGF-like growth factors in that it can not only induce a mitogenic signal but can also induce cell differentiation. This is explained by a single amino acid difference in the receptor-binding domain, which decreases the stability of the AREG-EGFR. Such interaction fails to induce receptor internalization and enables AREG to induce a sustained signal downstream of the receptor. In contrast, most other EGFR ligands, such as EGF, TGFα or HB-EGF, bind EGFR with high affinity and thereby induce internalization and degradation of the receptor, and induce negative feedback loops within the MAP-kinase signaling cascade. This activation and termination of an induced signal by the high-affinity ligands of the EGFR in effect lead to a ligand-specific oscillation of the MAP-kinase signaling pathway and thus a mitogenic signal in the recipient cell.
This activation and termination of an induced signal by the high-affinity ligands of the EGFR in effect leads to a ligand-specific oscillation of the MAP-kinase signaling pathway (Shankaran and Wiley, 2010). Such a qualitative difference in signaling directly dictates the dynamics of gene expression (Purvis and Lahav, 2013). In fibroblasts, the frequency of the EGFR-induced oscillation of ERK directly correlates with the strength of the induced proliferation signal: the higher the ligand concentration, the higher the pulse frequency and therefore the higher the induced proliferation (Albeck et al., 2013). However, low-affinity binding by AREG fails to induce this oscillating signal, and sustained, stable activation of ERK is not growth-inducing, but induces growth arrest and cell differentiation (Imayoshi et al., 2013; Marshall, 1995; Pasic et al., 2011; Traverse et al., 1992). As a consequence, AREG induces differentiation in a variety of different cell lines. Neuronal PC12 cells, for instance, are induced to proliferate in the presence of EGF and to differentiate in the presence of AREG (Kimura and Schubert, 1992). Notably, kidney-derived MDCK cells (Chung et al., 2005a), mammary epithelial cells (Mukhopadhyay et al., 2013) and myoepithelial cells (Pasic et al., 2011) follow a similar pattern, suggesting conservation of this signaling pathway across multiple cell types. Since leukocyte-derived AREG can be rapidly expressed at the site of tissue damage by infiltrating leukocytes, the capacity of AREG to induce differentiation of tissue resident precursor cells, such as satellite cells in the muscle, may substantially contribute to tissue repair in a wide range of different organs.
Amphiregulin contributes to tissue repair and fibrosis
A critical aspect of wound repair is the deposition of connective tissue proteins, such as collagen and fibronectin, in and around an open wound. Several different cell types, including peripheral blood circulating fibrocytes, contribute to the deposition of connective tissue proteins into the wound (Suga et al., 2014). As a result, a layer of differentiated myofibroblasts can quickly develop at localized sites of injury where they produce collagen in order to stabilize and contract the wound, allowing for efficient healing. However, in situations of chronic inflammation, uncontrolled wound repair mechanisms, including unrestrained myofibroblast differentiation and activation and excessive collagen deposition can lead to fibrosis and impaired organ function (Allen and Wynn, 2011; Gause et al., 2013).
Accumulating evidence suggests that dysregulation of the dialogue between the immune system and EGFR signaling pathways can contribute to pathological fibrosis in the context of chronic inflammation. The immune-derived cytokines IL-13 and TGF-β have been demonstrated to potentiate collagen deposition and fibrosis in the lung, liver, skin and intestine (Ask et al., 2008; Chiaramonte et al., 1999; Desmouliere et al., 1993; Hamid et al., 1994; Zhou et al., 2012), and attempts to therapeutically target these signaling pathways have revealed TGF-β-dependent and -independent mechanisms of disease (Kaviratne et al., 2004; Lee et al., 2001). For example, IL-13 normally signals through a heterodimeric receptor composed of the IL-4Rα and IL-13Rα1 subunits, and attempts to inhibit IL-13 signaling using a soluble high-affinity IL-13Rα2 fusion protein as a decoy receptor have achieved promising results in diminishing hepatic and pulmonary fibrosis (Chiaramonte et al., 2003; Zheng et al., 2008). However, recent studies have demonstrated that IL-13 signaling through cell surface-expressed IL-13Rα2 on macrophages can stimulate TGF-β expression to promote myofibroblast differentiation and fibrosis (Brunner et al., 2013; Fichtner-Feigl et al., 2006). Notably, TGF-β can regulate AREG expression (Bennett et al., 1992), and AREG has been ascribed a critical role in the development of TGF-β-induced pulmonary fibrosis (Zhou et al., 2012). Whether IL-13 signaling can lead directly to AREG induction through TGF-β remains to be addressed. Collectively, these data indicate that immune pathways regulate multiple profibrotic mechanisms and provoke the hypothesis that this may be achieved at least in part through AREG-EGFR-mediated effects on myofibroblasts.
Pathological fibrosis can be considered an unrestrained wound healing response and can involve activation of immune cells typically associated with type 2 immune responses including ILC2 and M2 macrophages. ILC2 have been identified as major drivers of liver, skin and pulmonary fibrosis (Chang et al., 2011; Hams et al., 2014; McHedlidze et al., 2013; Salimi et al., 2013), and ILC2 express both IL-13 and AREG (Monticelli et al., 2011), suggesting that ILC2-derived factors may also mediate induction or progression of inflammation-associated fibrosis. Indeed, the recent demonstration that a lipid mediator, Maresin1, can limit ILC2-derived IL-13 and pulmonary inflammation supports this hypothesis (Krishnamoorthy et al., 2014). While ILC2-derived AREG may substantially contribute to protective tissue remodeling following influenza-induced damage, its role in fibrosis under chronic inflammatory conditions remains undefined. Notably, EGFR inhibitors can ameliorate symptoms of fibrotic disease in mice (Le Cras et al., 2011; Liu et al., 2012; Vargaftig and Singer, 2003). Identification of the EGFR ligands that support fibrosis, and defining the role of immune-derived AREG in this process, remains an area of ongoing investigation that may reveal new targets for therapeutic intervention.
Amphiregulin and Tregs can suppress local inflammation
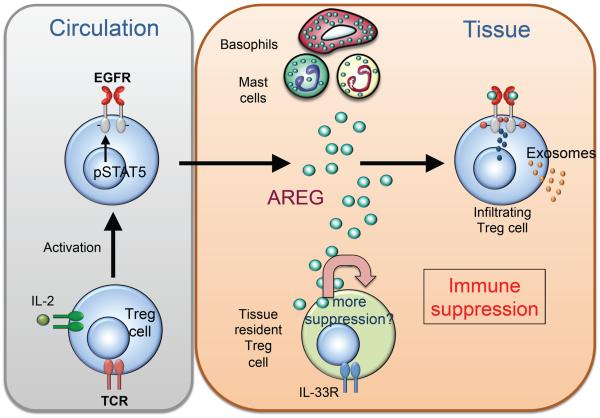
Successful wound repair requires efficient resolution of local inflammation (Martin and Leibovich, 2005) as dysregulation of pathogen-elicited immune responses carries the risk of inducing collateral tissue damage. Thus, efficient resolution of local inflammation is necessary to prevent excessive wound healing responses and tissue fibrosis. An important cell type associated with type 2 immune responses that contributes to local immune suppression is the mast cell (de Vries and Noelle, 2010). Mast cell-derived IL-10, for example, has a prominent immune regulatory function in a number of different settings (Grimbaldeston et al., 2007; Leveson-Gower et al., 2013). Furthermore, efficient induction of immune tolerance can involve collaboration between mast cells and Treg cells (Lu et al., 2006), potentially due to the role of mast cell-derived AREG in supporting Treg cell function (Zaiss et al., 2013). In the absence of mast cell-derived AREG, Treg cells fail to suppress the development of colitis in a T cell transfer induced mouse colitis model, are unable to induce an immunosuppressive environment in a mouse tumor vaccination model and fail to suppress dermal inflammation (Zaiss et al., 2013). Furthermore, the development of dermatitis could be reversed by the cotransfer of bone marrow derived mast cells differentiated from wild-type but not AREG-deficient bone marrow (Zaiss et al., 2013). Together, these data demonstrate that mast cells can be a critical source of AREG and can promote CD4+ Treg cell-mediated suppression of localized immune responses (Figure 2).
Figure 2. Amphiregulin enhances regulatory T cell function.
AREG has been shown to be of critical importance for efficient CD4+ regulatory T (Treg) cell function, and in the absence of AREG, CD4+ Treg cells cannot suppress local inflammation. As CD4+ Treg cells become activated they up-regulate the EGFR, in part via STAT5 mediated signaling. These activated regulatory T cells migrate to the site of inflammation, where they are exposed to AREG, which enhances their suppressive capacity, possibly through secretion of immunosuppressive exosomes. Depending on the type and the site of inflammation, CD4+ Treg cells are dependent on different cell types for AREG; in chronic inflammation mast cell derived Amphiregulin enhances CD4+ Treg cell function, while in an acute inflammatory response basophils are described as the main source of AREG. In addition, some organs contain tissue-specific CD4+ Treg cells. In response to inflammation, these tissue-resident Treg cells can produce Amphiregulin that may act in an autocrine fashion to enhance their suppressive capacity. The extent that AREG derived from these different cell types contributes to immune regulation within inflamed tissues is an ongoing area of study.
Recent studies have revealed a potential molecular mechanism through which AREG promotes Treg cell function (Okoye et al., 2014). Treg cells can secrete exosomes that transfer immunosuppressive micro-RNAs (miRNAs) from Treg cells to effector T cells, and AREG enhances the secretion of such exosomes (Okoye et al., 2014). The source of AREG that enhances Treg cell function appears to be dependent on the site and the type of the immune response. In addition to mast cells, basophils can also be an important source of AREG. In the context of contact hypersensitivity reactions in the skin, basophil-derived and not mast cell-derived AREG was essential for enhanced Treg cell function (Meulenbroeks et al., 2015). Taken together, these findings reveal a mechanism by which mobilized cells of the innate immune system use AREG to enhance the suppressive capacity of Treg cells to dampen local immune responses and to induce peripheral tolerance. These data also raise the possibility that the specific tissue microenvironment where inflammation is localized may determine the source of AREG and influence its function.
The recent finding that tissue-resident Treg cell populations express AREG themselves (Burzyn et al., 2013) suggests that these unique Treg cell populations might possess an autocrine positive feedback loop. Such a mechanism would release tissue-resident Treg cells from the delay associated with leukocyte-derived AREG that is dependent on the influx of cells into the site of tissue damage. The degree to which Treg cell-derived AREG directly contributes to wound healing and indirectly contributes to resolution of local inflammation remains undefined.
Amphiregulin promotes immune suppression in the tumor microenvironment
Immune-mediated tolerance mechanisms such as limitation of inflammation and tissue repair extend beyond mediating the host response to pathogens. Tumor antigens also elicit immune responses that promote tolerance, which can allow tumors to grow unchecked but can also limit inflammation and collateral damage to healthy tissues surrounding the tumor microenvironment. For instance, a number of factors have been associated with the progression of epithelial-associated solid tumors, including local accumulation of CD4+ Th2 cells, M2 macrophages and expression of IL-13 and TGF-β (Jovanovic et al., 2014; Palucka and Banchereau, 2012; Palucka et al., 2013). Epithelial-associated solid tumors also very commonly express the EGFR and are associated with local expression of EGF ligands, including AREG (Ciardiello and Tortora, 2008; Herbst et al., 2001; Shao et al., 2003; Yamada et al., 2008). While the role of AREG in anti-tumor immunity and tolerance remains unclear, the wide clinical use of EGFR antagonists for the treatment of a number of metastatic epithelial cancers suggests that AREG may play a critical role in orchestrating responses to tumors.
Despite the underlying idea that many epithelial tumors are dependent on EGFR-mediated signals for their growth, treatment of cancer patients with EGFR antagonists is associated with multiple side effects, such as skin rashes, fever, stomatitis and diarrhea (Ciardiello and Tortora, 2008), suggesting the involvement of off-target effects. Since the EGFR is of central importance for the homeostasis and integrity of the skin, inhibition of this pathway may underlie the loss of dermal barrier function (Lichtenberger et al., 2013; Mascia et al., 2013). However, the severe skin reactions may also be associated with a general dysregulation of the immune system. In particular, the skin appears very sensitive to immune dysregulation (Dudda et al., 2008), and Treg cell dysfunction in IPEX children normally first manifests itself in skin rashes (Halabi-Tawil et al., 2009). Thus, it is possible that EGFR antagonists could function in part via the suppression of local Treg cell populations.
The significance of Treg cell function in down-regulating immune defenses against tumors has been demonstrated in mice (Li et al., 2010). The underlying mechanism of CTLA-4 blocking antibodies in enhancing tumor immune responses in cancer patients remains controversial, as inhibition of CTLA-4 may both enhance anti-tumor effector T cell function as well as impair Treg cell function and thereby limit local immunosuppression. However, recent studies suggest that CTLA-4 blocking antibodies function mainly via the depletion of Treg cells within the tumor microenvironment (Simpson et al., 2013). These data indicate that in cancer patients, sustained Treg cell activity suppresses anti-tumor immunity. Thus, an effect on Treg cell function could also explain some of the inflammatory side effects reported by patients treated with EGFR antagonists. Such a mechanism may also explain a number of somewhat enigmatic clinical observations associated with EGFR antagonist treatment. A striking observation in patients treated with EGFR antagonists is that tumor regression rates did not correlate with the expression of the targeted molecule (i.e. EGFR) on the responsive tumor cells (Burtness et al., 2005; Kim et al., 2008). The EGFR is upregulated in a feedback loop, leading to the expectation that tumors overexpressing the EGFR would be particularly vulnerable to EGFR antagonism and growth factor starvation (Herbst et al., 2001). However, in some cases, even tumors that fail to express detectable amounts of the EGFR respond in clinically objective ways to monoclonal antibody therapy (Chung et al., 2005b). Collectively, these data suggest that EGFR antagonists may restore the function of anti-tumor immune responses by interfering with local immunosuppressive EGFR-responsive Treg cells, although this hypothesis remains to be formally tested.
Recent experimental data in a murine model support the hypothesis that EGFR inhibitors can relieve local Treg cell immunosuppression and activate secondary antitumor immunity following tumor immunization (Zaiss et al., 2013). Immunization of B16 tumor bearing mice with DCs loaded with a peptide derived from the immunogenic tumor antigen TRP2 induces a CD8+ T cell response and tumor rejection in AREG gene-deficient mice but not in C57BL/6 wild-type mice. Treatment of tumor-bearing mice with EGFR blocking antibody following immunization with peptide-pulsed DCs also induces tumor rejection in wild-type mice. Notably, B16 melanoma cells do not express the EGFR but are dependent on Treg cell function for the induction of a tumor-intrinsic immunosuppressive environment (Sutmuller et al., 2001). Together, these data suggest that the absence of AREG-EGFR signaling enhances immunization-induced tumor specific CD8+ T cells through relief of Treg cell-mediated suppression (Zaiss et al., 2013). These experimental findings provoke the hypothesis that Treg cell function might also be diminished in cancer patients undergoing EGFR antagonist treatment. Conservation of this pathway is suggested by the finding that in a murine model of Hepatitis B-virus infection, elevated hepatic AREG expression is associated with the presence of hyper-suppressive EGFR+ CD4+ Treg cells that could limit antiviral CD8+ T cell function (Dai et al., 2014). Further research examining the extent to which EGFR antagonists can inhibit the function of local CD4+ Treg cell populations could impact therapeutic treatment of multiple disease states, including chronic viral infection and cancer.
Concluding remarks and future perspectives
The immune system contributes to homeostasis by promoting host protective mechanisms against a variety of harmful insults ranging from toxins, venoms and allergens to cancerous cells and infectious agents. Immune-mediated resistance and tolerance mechanisms, the latter involving both the control of harmful inflammation and direct contributions to enhanced wound healing and tissue repair, are key components of the host-protective response. It is becoming clear that in many cases the same cell populations and even the same specific molecules can simultaneously contribute to the immune functions that control resistance and tolerance as well as development or progression of fibrosis and cancer. In the context of type 2 inflammation elicited by heterogeneous stimuli, AREG is one such molecule (Figure 3). The ability of AREG to induce epithelial cell proliferation and differentiation can promote helminth expulsion in the context of a polarized type 2 response and enhance wound healing and tissue repair following infection or injury. The effects of AREG on Treg cells may also down-modulate harmful inflammation but at the same time potentiate tumor growth.
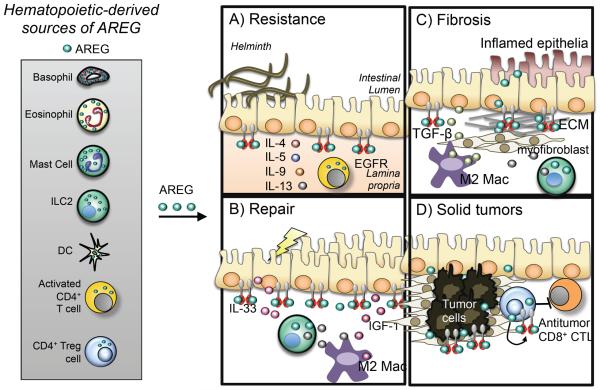
Figure 3. Leukocyte-derived Amphiregulin in health and disease.
Recent evidence implicates hematopoietic-derived AREG expression as an important component of pathogen resistance and tissue tolerance mechanisms. Type 2-associated immune cells of both the innate and adaptive lineages have been reported to express AREG. (a) In the context of helminth infection, CD4+ T cells and AREG promote epithelial cell turnover to expedite helminth eradication, although the specific cellular sources of AREG in this process remain undefined. (b) Tissue damage can cause up-regulation of the alarmin IL-33, a strong positive stimulus for ILC2 accumulation and cytokine production that can support tissue-protective M2 macrophage differentiation. Recent data suggest the possibility that ILC2, M2 MΦ, AREG and IGF-1 may collaborate to promote wound healing at mucosal barrier surfaces. (c) Uncontrolled wound healing responses can result in pathologic tissue fibrosis. It is possible that the same cells (ILC2 and M2 MΦ) and the same cytokines (IL-13 and AREG) that promote tissue repair can become dysregulated in the presence of chronic inflammation and an altered cytokine milieu to promote fibrotic responses, including myofibroblast differentiation, proliferation and ECM deposition. (d) Epithelial-associated solid tumor microenvironments can have high local concentrations of AREG and tumor infiltrating EGFR+ CD4+ Treg cells. Treatment with EGFR antagonists or Treg cell depleting agents can promote tumor regression. Tissue-resident CD4+ Treg cells can express and respond to AREG, suggesting the possibility that in addition to growth factor starvation of the tumor, EGFR antagonists may function in part to inhibit local CD4+ Treg cell function, thereby alleviating immunosuppression and enhancing antitumor immune responses, including the activity and function of tumor specific CD8+ cytotoxic T lymphocytes (CTLs). Much work remains to define the cellular sources of AREG and the mechanisms regulating its expression in inflamed tissues. Increased understanding of these pathways could reveal new therapeutic targets for multiple disease states.
The demonstration that immune cells can express AREG and contribute to tissue homeostasis suggests that this is an important component of the crosstalk between immune and epithelial cells. Continued investigation of the signals that elicit immune-derived AREG, the mechanisms regulating EGFR-mediated ligation and signaling and development of new genetic tools to determine the cellular sources of AREG in vivo will greatly increase our understanding of the role the AREG-EGFR pathway plays in the context of resistance to infection and in tissue tolerance. Addressing these gaps in knowledge has the potential to inform development of new therapeutic interventions for immunity, chronic inflammation and cancer.
Acknowledgements
We thank Alice Sijts, as well as members of the Zaiss and of the Artis lab, for critical appraisal of the manuscript. We apologize to colleagues whose work could not be directly quoted due to space constraints. Research in the Zaiss lab is supported by the European Union (CIG: “EGFR for Immunity”), the Medical Research Council (MR/M011755/1) and Asthma UK. The Artis lab is supported by the National Institutes of Health (AI061570, AI095608, AI074878, AI095466, AI106697, AI102942, AI097333), the Crohns and Colitis Foundation of America and the Burroughs Wellcome Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam R, Drummond DR, Solic N, Holt SJ, Sharma RP, Chamberlin SG, Davies DE. Modulation of the receptor binding affinity of amphiregulin by modification of its carboxyl terminal tail. Biochim Biophys Acta. 1995;1266:83–90. doi: 10.1016/0167-4889(94)00224-3. [DOI] [PubMed] [Google Scholar]
- Adib-Conquy M, Pedron T, Petit-Bertron AF, Tabary O, Corvol H, Jacquot J, Clement A, Cavaillon JM. Neutrophils in cystic fibrosis display a distinct gene expression pattern. Mol Med. 2008;14:36–44. doi: 10.2119/2007-00081.Adib-Conquy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albeck JG, Mills GB, Brugge JS. Frequency-modulated pulses of ERK activity transmit quantitative proliferation signals. Mol Cell. 2013;49:249–261. doi: 10.1016/j.molcel.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Rutitzky LI, Urban JF, Jr., Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nature reviews. Immunology. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer cell. 2014;25:282–303. doi: 10.1016/j.ccr.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ask K, Bonniaud P, Maass K, Eickelberg O, Margetts PJ, Warburton D, Groffen J, Gauldie J, Kolb M. Progressive pulmonary fibrosis is mediated by TGF-beta isoform 1 but not TGF-beta3. Int J Biochem Cell Biol. 2008;40:484–495. doi: 10.1016/j.biocel.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham R, Yarden Y. Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat Rev Mol Cell Biol. 2011;12:104–117. doi: 10.1038/nrm3048. [DOI] [PubMed] [Google Scholar]
- Bennett KL, Plowman GD, Buckley SD, Skonier J, Purchio AF. Regulation of amphiregulin mRNA by TGF-beta in the human lung adenocarcinoma cell line A549. Growth factors. 1992;7:207–213. doi: 10.3109/08977199209046925. [DOI] [PubMed] [Google Scholar]
- Berasain C, Avila MA. Amphiregulin. Seminars in cell & developmental biology. 2014;28:31–41. doi: 10.1016/j.semcdb.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Berasain C, Garcia-Trevijano ER, Castillo J, Erroba E, Lee DC, Prieto J, Avila MA. Amphiregulin: an early trigger of liver regeneration in mice. Gastroenterology. 2005;128:424–432. doi: 10.1053/j.gastro.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Bles N, Di Pietrantonio L, Boeynaems JM, Communi D. ATP confers tumorigenic properties to dendritic cells by inducing amphiregulin secretion. Blood. 2010;116:3219–3226. doi: 10.1182/blood-2010-01-265611. [DOI] [PubMed] [Google Scholar]
- Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nature immunology. 2014;15:1017–1025. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner SM, Schiechl G, Kesselring R, Martin M, Balam S, Schlitt HJ, Geissler EK, Fichtner-Feigl S. IL-13 signaling via IL-13Ralpha2 triggers TGF-beta1-dependent allograft fibrosis. Transplantation research. 2013;2:16. doi: 10.1186/2047-1440-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, Mathis D. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nature immunology. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF, Jr., Wynn TA, Gause WC. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. The Journal of clinical investigation. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaramonte MG, Mentink-Kane M, Jacobson BA, Cheever AW, Whitters MJ, Goad ME, Wong A, Collins M, Donaldson DD, Grusby MJ, Wynn TA. Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response. The Journal of experimental medicine. 2003;197:687–701. doi: 10.1084/jem.20020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Graves-Deal R, Franklin JL, Coffey RJ. Differential effects of amphiregulin and TGF-alpha on the morphology of MDCK cells. Exp Cell Res. 2005a;309:149–160. doi: 10.1016/j.yexcr.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005b;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- Cliffe LJ, Grencis RK. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv Parasitol. 2004;57:255–307. doi: 10.1016/S0065-308X(04)57004-5. [DOI] [PubMed] [Google Scholar]
- Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–1465. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- Cook PW, Mattox PA, Keeble WW, Pittelkow MR, Plowman GD, Shoyab M, Adelman JP, Shipley GD. A heparin sulfate-regulated human keratinocyte autocrine factor is similar or identical to amphiregulin. Mol Cell Biol. 1991;11:2547–2557. doi: 10.1128/mcb.11.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai K, Huang L, Chen J, Yang L, Gong Z. Amphiregulin promotes the immunosuppressive activity of intrahepatic CD4 regulatory T cells to impair CD8 T cell immunity against hepatitis B virus infection. Immunology. 2014 doi: 10.1111/imm.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries VC, Noelle RJ. Mast cell mediators in tolerance. Curr Opin Immunol. 2010;22:643–648. doi: 10.1016/j.coi.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudda JC, Perdue N, Bachtanian E, Campbell DJ. Foxp3+ regulatory T cells maintain immune homeostasis in the skin. The Journal of experimental medicine. 2008;205:1559–1565. doi: 10.1084/jem.20072594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nature reviews. Immunology. 2013;13:607–614. doi: 10.1038/nri3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nature immunology. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- Halabi-Tawil M, Ruemmele FM, Fraitag S, Rieux-Laucat F, Neven B, Brousse N, De Prost Y, Fischer A, Goulet O, Bodemer C. Cutaneous manifestations of immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. Br J Dermatol. 2009;160:645–651. doi: 10.1111/j.1365-2133.2008.08835.x. [DOI] [PubMed] [Google Scholar]
- Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. The Journal of clinical investigation. 1994;94:870–876. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hams E, Armstrong ME, Barlow JL, Saunders SP, Schwartz C, Cooke G, Fahy RJ, Crotty TB, Hirani N, Flynn RJ, et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:367–372. doi: 10.1073/pnas.1315854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, Dickey BF, Wilson MS, Wynn TA, Grencis RK, Thornton DJ. Muc5ac: a critical component mediating the rejection of enteric nematodes. The Journal of experimental medicine. 2011;208:893–900. doi: 10.1084/jem.20102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert DR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, Orekov T, Perkins C, Wang Q, Brombacher F, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. The Journal of experimental medicine. 2009;206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS, Kim ES, Harari PM. IMC-C225, an anti-epidermal growth factor receptor monoclonal antibody, for treatment of head and neck cancer. Expert opinion on biological therapy. 2001;1:719–732. doi: 10.1517/14712598.1.4.719. [DOI] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Isomura A, Harima Y, Kawaguchi K, Kori H, Miyachi H, Fujiwara T, Ishidate F, Kageyama R. Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science. 2013;342:1203–1208. doi: 10.1126/science.1242366. [DOI] [PubMed] [Google Scholar]
- Jamieson AM, Pasman L, Yu S, Gamradt P, Homer RJ, Decker T, Medzhitov R. Role of tissue protection in lethal respiratory viral-bacterial coinfection. Science. 2013;340:1230–1234. doi: 10.1126/science.1233632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CC, Yndestad A, Enserink JM, Ree AH, Aukrust P, Tasken K. The epidermal growth factor-like growth factor amphiregulin is strongly induced by the adenosine 3',5'-monophosphate pathway in various cell types. Endocrinology. 2004;145:5177–5184. doi: 10.1210/en.2004-0232. [DOI] [PubMed] [Google Scholar]
- Johnson GR, Wong L. Heparan sulfate is essential to amphiregulin-induced mitogenic signaling by the epidermal growth factor receptor. The Journal of biological chemistry. 1994;269:27149–27154. [PubMed] [Google Scholar]
- Jones JT, Akita RW, Sliwkowski MX. Binding specificities and affinities of egf domains for ErbB receptors. FEBS letters. 1999;447:227–231. doi: 10.1016/s0014-5793(99)00283-5. [DOI] [PubMed] [Google Scholar]
- Jovanovic K, Siebeck M, Gropp R. The route to pathologies in chronic inflammatory diseases characterized by T helper type 2 immune cells. Clinical and experimental immunology. 2014;178:201–211. doi: 10.1111/cei.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara N, Oboki K, Ohno T, Ishii A, Sunnarborg SW, Okumura K, Saito H, Nakae S. Amphiregulin is not essential for ovalbumin-induced acute airway inflammation in mice. Allergology international : official journal of the Japanese Society of Allergology. 2010;59:207–211. doi: 10.2332/allergolint.09-OA-0144. [DOI] [PubMed] [Google Scholar]
- Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, Wakefield LM, Letterio JJ, Wynn TA. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. Journal of immunology. 2004;173:4020–4029. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- Kennedy-Crispin M, Billick E, Mitsui H, Gulati N, Fujita H, Gilleaudeau P, Sullivan-Whalen M, Johnson-Huang LM, Suarez-Farinas M, Krueger JG. Human keratinocytes' response to injury upregulates CCL20 and other genes linking innate and adaptive immunity. The Journal of investigative dermatology. 2012;132:105–113. doi: 10.1038/jid.2011.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Grandis JR, Rinaldo A, Takes RP, Ferlito A. Emerging perspectives in epidermal growth factor receptor targeting in head and neck cancer. Head Neck. 2008;30:667–674. doi: 10.1002/hed.20859. [DOI] [PubMed] [Google Scholar]
- Kimura H, Schubert D. Schwannoma-derived growth factor promotes the neuronal differentiation and survival of PC12 cells. J Cell Biol. 1992;116:777–783. doi: 10.1083/jcb.116.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy N, Burkett PR, Dalli J, Abdulnour RE, Colas R, Ramon S, Phipps RP, Petasis NA, Kuchroo VK, Serhan CN, Levy BD. Cutting Edge: Maresin-1 Engages Regulatory T Cells To Limit Type 2 Innate Lymphoid Cell Activation and Promote Resolution of Lung Inflammation. Journal of immunology. 2014 doi: 10.4049/jimmunol.1402534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong BY, Roberts SJ, Silberzahn T, Filler RB, Neustadter JH, Galan A, Reddy S, Lin WM, Ellis PD, Langford CF, et al. Molecular analysis of tumor-promoting CD8+ T cells in two-stage cutaneous chemical carcinogenesis. The Journal of investigative dermatology. 2010;130:1726–1736. doi: 10.1038/jid.2009.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Cras TD, Acciani TH, Mushaben EM, Kramer EL, Pastura PA, Hardie WD, Korfhagen TR, Sivaprasad U, Ericksen M, Gibson AM, et al. Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma. American journal of physiology. Lung cellular and molecular physiology. 2011;300:L414–421. doi: 10.1152/ajplung.00346.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) The Journal of experimental medicine. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveson-Gower DB, Sega EI, Kalesnikoff J, Florek M, Pan Y, Pierini A, Galli SJ, Negrin RS. Mast cells suppress murine GVHD in a mechanism independent of CD4+CD25+ regulatory T cells. Blood. 2013;122:3659–3665. doi: 10.1182/blood-2013-08-519157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Plowman GD, Buckley SD, Shipley GD. Heparin inhibition of autonomous growth implicates amphiregulin as an autocrine growth factor for normal human mammary epithelial cells. J Cell Physiol. 1992;153:103–111. doi: 10.1002/jcp.1041530114. [DOI] [PubMed] [Google Scholar]
- Li X, Kostareli E, Suffner J, Garbi N, Hammerling GJ. Efficient Treg depletion induces T-cell infiltration and rejection of large tumors. Eur J Immunol. 2010;40:3325–3335. doi: 10.1002/eji.201041093. [DOI] [PubMed] [Google Scholar]
- Lichtenberger BM, Gerber PA, Holcmann M, Buhren BA, Amberg N, Smolle V, Schrumpf H, Boelke E, Ansari P, Mackenzie C, et al. Epidermal EGFR controls cutaneous host defense and prevents inflammation. Sci Transl Med. 2013;5:199ra111. doi: 10.1126/scitranslmed.3005886. [DOI] [PubMed] [Google Scholar]
- Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, Bayliss G, Dworkin LD, Yan H, Zhuang S. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol. 2012;23:854–867. doi: 10.1681/ASN.2011050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke P, Gallagher I, Nair MG, Zang X, Brombacher F, Mohrs M, Allison JP, Allen JE. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. Journal of immunology. 2007;179:3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. Journal of immunology. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–2750. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- Macdonald-Obermann JL, Pike LJ. Different epidermal growth factor (EGF) receptor ligands show distinct kinetics and biased or partial agonism for homodimer and heterodimer formation. The Journal of biological chemistry. 2014;289:26178–26188. doi: 10.1074/jbc.M114.586826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels RM, Hewitson JP, Smith KA. Susceptibility and immunity to helminth parasites. Curr Opin Immunol. 2012;24:459–466. doi: 10.1016/j.coi.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Mascia F, Lam G, Keith C, Garber C, Steinberg SM, Kohn E, Yuspa SH. Genetic ablation of epidermal EGFR reveals the dynamic origin of adverse effects of anti-EGFR therapy. Sci Transl Med. 2013;5:199ra110. doi: 10.1126/scitranslmed.3005773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Fukuda S, Nakamura Y, Saito H. Amphiregulin production by human eosinophils. Int Arch Allergy Immunol. 2009;149(Suppl 1):39–44. doi: 10.1159/000210652. [DOI] [PubMed] [Google Scholar]
- McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, Voehringer D, McKenzie AN, Neurath MF, Pflanz S, Wirtz S. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenbroeks C, van Weelden H, Schwartz C, Voehringer D, Redegeld F, Rutten VP, Willemse T, Sijts AJ, Zaiss DM. Basophil-Derived Amphiregulin is Essential for UVB Irradiation-Induced Immune Suppression. The Journal of investigative dermatology. 2015 doi: 10.1038/jid.2014.329. [DOI] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nature immunology. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay C, Zhao X, Maroni D, Band V, Naramura M. Distinct Effects of EGFR Ligands on Human Mammary Epithelial Cell Differentiation. PLoS One. 2013;8:e75907. doi: 10.1371/journal.pone.0075907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, Seabra MC, Wilson MS. MicroRNA-Containing T-Regulatory-Cell-Derived Exosomes Suppress Pathogenic T Helper 1 Cells. Immunity. 2014;41:89–103. doi: 10.1016/j.immuni.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura S, Sagara H, Fukuda T, Saito H, Okayama Y. FcepsilonRI-mediated amphiregulin production by human mast cells increases mucin gene expression in epithelial cells. J Allergy Clin Immunol. 2005;115:272–279. doi: 10.1016/j.jaci.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nature reviews. Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka K, Coussens LM, O'Shaughnessy J. Dendritic cells, inflammation, and breast cancer. Cancer journal. 2013;19:511–516. doi: 10.1097/PPO.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasic L, Eisinger-Mathason TS, Velayudhan BT, Moskaluk CA, Brenin DR, Macara IG, Lannigan DA. Sustained activation of the HER1-ERK1/2-RSK signaling pathway controls myoepithelial cell fate in human mammary tissue. Genes Dev. 2011;25:1641–1653. doi: 10.1101/gad.2025611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N, Kreider T, Urban JF, Jr., Gause WC. Characterisation of effector mechanisms at the host:parasite interface during the immune response to tissue-dwelling intestinal nematode parasites. Int J Parasitol. 2009;39:13–21. doi: 10.1016/j.ijpara.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N, Wu W, Mishra PK, Chen F, Millman A, Csoka B, Koscso B, Eltzschig HK, Hasko G, Gause WC. A2B adenosine receptor induces protective antihelminth type 2 immune responses. Cell Host Microbe. 2014;15:339–350. doi: 10.1016/j.chom.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Perugorria MJ, Latasa MU, Nicou A, Cartagena-Lirola H, Castillo J, Goni S, Vespasiani-Gentilucci U, Zagami MG, Lotersztajn S, Prieto J, et al. The epidermal growth factor receptor ligand amphiregulin participates in the development of mouse liver fibrosis. Hepatology. 2008;48:1251–1261. doi: 10.1002/hep.22437. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis JE, Lahav G. Encoding and decoding cellular information through signaling dynamics. Cell. 2013;152:945–956. doi: 10.1016/j.cell.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Operario DJ, Georas SN, Mosmann TR. The acute environment, rather than T cell subset pre-commitment, regulates expression of the human T cell cytokine amphiregulin. PLoS One. 2012;7:e39072. doi: 10.1371/journal.pone.0039072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Operario DJ, Oberholzer CM, Kobie JJ, Looney RJ, Georas SN, Mosmann TR. Human basophils express amphiregulin in response to T cell-derived IL-3. J Allergy Clin Immunol. 2010;126:1260–1266. e1264. doi: 10.1016/j.jaci.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodland KD, Bollinger N, Ippolito D, Opresko LK, Coffey RJ, Zangar R, Wiley HS. Multiple mechanisms are responsible for transactivation of the epidermal growth factor receptor in mammary epithelial cells. The Journal of biological chemistry. 2008;283:31477–31487. doi: 10.1074/jbc.M800456200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz SA, Siracusa MC, Monticelli LA, Ziegler CG, Kim BS, Brestoff JR, Peterson LW, Wherry EJ, Goldrath AW, Bhandoola A, Artis D. IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. The Journal of experimental medicine. 2013;210:1823–1837. doi: 10.1084/jem.20122332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST, McKenzie AN, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. The Journal of experimental medicine. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014 doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nature reviews. Immunology. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuger L, Johnson GR, Gilbride K, Plowman GD, Mandel R. Amphiregulin in lung branching morphogenesis: interaction with heparan sulfate proteoglycan modulates cell proliferation. Development. 1996;122:1759–1767. doi: 10.1242/dev.122.6.1759. [DOI] [PubMed] [Google Scholar]
- Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran H, Wiley HS. Oscillatory dynamics of the extracellular signal-regulated kinase pathway. Curr Opin Genet Dev. 2010;20:650–655. doi: 10.1016/j.gde.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Shao J, Lee SB, Guo H, Evers BM, Sheng H. Prostaglandin E2 stimulates the growth of colon cancer cells via induction of amphiregulin. Cancer Res. 2003;63:5218–5223. [PubMed] [Google Scholar]
- Shoyab M, McDonald VL, Bradley JG, Todaro GJ. Amphiregulin: a bifunctional growth-modulating glycoprotein produced by the phorbol 12-myristate 13-acetate-treated human breast adenocarcinoma cell line MCF-7. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:6528–6532. doi: 10.1073/pnas.85.17.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon Davis DA, Parish CR. Heparan sulfate: a ubiquitous glycosaminoglycan with multiple roles in immunity. Frontiers in immunology. 2013;4:470. doi: 10.3389/fimmu.2013.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. The Journal of experimental medicine. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, Wherry EJ, Jessup HK, Siegel LA, Kambayashi T, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern KA, Place TL, Lill NL. EGF and amphiregulin differentially regulate Cbl recruitment to endosomes and EGF receptor fate. Biochem J. 2008;410:585–594. doi: 10.1042/BJ20071505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga H, Rennert RC, Rodrigues M, Sorkin M, Glotzbach JP, Januszyk M, Fujiwara T, Longaker MT, Gurtner GC. Tracking the elusive fibrocyte: identification and characterization of collagen-producing hematopoietic lineage cells during murine wound healing. Stem Cells. 2014;32:1347–1360. doi: 10.1002/stem.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. The Journal of experimental medicine. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288(Pt 2):351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JE, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld JC, Panzer U, Helmby H, Stockinger B. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. The Journal of experimental medicine. 2013;210:2951–2965. doi: 10.1084/jem.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy DA, Kari C, Lazarus GS, Jensen PJ, Zilberstein A, Plowman GD, Rodeck U. Induction of autocrine epidermal growth factor receptor ligands in human keratinocytes by insulin/insulin-like growth factor-1. J Cell Physiol. 1995;163:257–265. doi: 10.1002/jcp.1041630206. [DOI] [PubMed] [Google Scholar]
- Vargaftig BB, Singer M. Leukotrienes mediate part of Ova-induced lung effects in mice via EGFR. American journal of physiology. Lung cellular and molecular physiology. 2003;285:L808–818. doi: 10.1152/ajplung.00377.2002. [DOI] [PubMed] [Google Scholar]
- Wang SW, Oh CK, Cho SH, Hu G, Martin R, Demissie-Sanders S, Li K, Moyle M, Yao Z. Amphiregulin expression in human mast cells and its effect on the primary human lung fibroblasts. J Allergy Clin Immunol. 2005;115:287–294. doi: 10.1016/j.jaci.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Worster DT, Schmelzle T, Solimini NL, Lightcap ES, Millard B, Mills GB, Brugge JS, Albeck JG. Akt and ERK control the proliferative response of mammary epithelial cells to the growth factors IGF-1 and EGF through the cell cycle inhibitor p57Kip2. Sci Signal. 2012;5:ra19. doi: 10.1126/scisignal.2001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagami A, Kajiwara N, Oboki K, Ohno T, Morita H, Sunnarborg SW, Okumura K, Ogawa H, Saito H, Nakae S. Amphiregulin is not essential for induction of contact hypersensitivity. Allergology international : official journal of the Japanese Society of Allergology. 2010;59:277–284. doi: 10.2332/allergolint.09-OA-0149. [DOI] [PubMed] [Google Scholar]
- Yamada M, Ichikawa Y, Yamagishi S, Momiyama N, Ota M, Fujii S, Tanaka K, Togo S, Ohki S, Shimada H. Amphiregulin is a promising prognostic marker for liver metastases of colorectal cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:2351–2356. doi: 10.1158/1078-0432.CCR-07-4499. [DOI] [PubMed] [Google Scholar]
- Zaiss DM, van Loosdregt J, Gorlani A, Bekker CP, Grone A, Sibilia M, van Bergen en Henegouwen PM, Roovers RC, Coffer PJ, Sijts AJ. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38:275–284. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss DM, Yang L, Shah PR, Kobie JJ, Urban JF, Mosmann TR. Amphiregulin, a TH2 cytokine enhancing resistance to nematodes. Science. 2006;314:1746. doi: 10.1126/science.1133715. [DOI] [PubMed] [Google Scholar]
- Zheng T, Liu W, Oh SY, Zhu Z, Hu B, Homer RJ, Cohn L, Grusby MJ, Elias JA. IL-13 receptor alpha2 selectively inhibits IL-13-induced responses in the murine lung. Journal of immunology. 2008;180:522–529. doi: 10.4049/jimmunol.180.1.522. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Lee JY, Lee CM, Cho WK, Kang MJ, Koff JL, Yoon PO, Chae J, Park HO, Elias JA, Lee CG. Amphiregulin, an epidermal growth factor receptor ligand, plays an essential role in the pathogenesis of transforming growth factor-beta-induced pulmonary fibrosis. The Journal of biological chemistry. 2012;287:41991–42000. doi: 10.1074/jbc.M112.356824. [DOI] [PMC free article] [PubMed] [Google Scholar]