Abstract
Background:
The survival of atraumatic restorative treatment (ART) restorations would be enhanced if near total elimination of cariogenic microorganisms could be done in the process of cavity cleaning before placing a restoration. Thus, use of disinfecting agents for achieving this goal could herald a new beginning in the field of contemporary dentistry.
Aim:
To assess and compare the cavity disinfection efficacy of APF gel, Brazilian Propolis, Diode Laser, and 2% chlorhexidine (CHX).
Materials and Methods:
The study was a randomized, single blinded, parallel grouped, active controlled trial. Eighty primary molars in 68 children with cavitated dentinal occlusal caries were randomly assigned into four groups (20 teeth each) Group I: APF gel; Group II: Propolis; Group III: Diode Laser, and Group IV: 2% CHX (control). After cavity preparation using ART procedure, dentinal samples collected before and after disinfection with respective agent of the group. These samples were subjected to microbiological evaluation, for total viable count (TVC) on blood agar, Streptococcus mutans on mutans-sanguis (MS) agar, and Lactobacilli (LB) on Rogosa agar.
Results:
Intragroup comparison (Wilcoxon signed rank test) showed significant reductions in TVC, MS, and LB counts in all the groups. Pairwise Mann–Whitney test showed APF gel had least bacterial reductions among the agents tested.
Conclusion:
This study illustrated the need for cavity disinfection. Diode Laser and Brazilian Propolis are equally effective as 2% CHX in cavity disinfection.
Keywords: APF gel, atraumatic restorative treatment, cavity disinfection, diode Laser and 2% chlorhexidine, Propolis
Introduction
The management of carious lesions has been changing dramatically due to improved understanding of the caries process. Atraumatic restorative treatment (ART) is one example of a contemporary approach that found to be conservative and advantageous for management of carious lesions in young children.[1] In contrast to the traditional approaches of complete caries removal, ART removes only infected dentin and allows the affected dentin to get remineralized.[2] The primary goal of ART intervention in both primary and permanent dentitions is to induce self-repair to arrest the caries process and thus maintain the pulp vitality.[3] Earlier studies have shown that caries process is not perpetuated in remaining affected dentin at the base of the cavity due to the absence of the substrate.[2]
However, clinical trials with long-term follow-up demonstrated cariogenic microorganisms persist under restorations and have an important role in the development of secondary caries.[4,5,6] To reduce the potential for residual caries, the use of an antibacterial agent was found to be beneficial in disinfection of dentin before restoring the cavity. Chlorhexidine (CHX) 2% has been in the use for this purpose and found to be effective in reducing cariogenic microflora residing in the remaining dental tissues.[7] Studies have also reported fluorides at high concentrations (>3040 ppm) had a potent bactericidal effect.[8]
Recently, there has been a growing trend to seek natural remedies as part of medical and dental therapeutics, which has been termed as “phytotherapeutics” or “ethnopharmacology.”[9] Propolis, a resinous wax-like substance extracted from bee-hive is gaining importance for its antibacterial, antifungal, and anti-inflammatory properties. Due to these properties, it has a wide range of applications in the field of medicine. In dentistry, it has been tried as root canal disinfectant,[10] direct pulp capping agent,[11] mouth rinse,[12] and in managing periodontal diseases.[13] These findings indicate Propolis can be tested as a cavity disinfectant.
A breakthrough for dental Laser systems came in the mid 1990s. Diode Laser systems quickly began establishing themselves as compact, competitively priced, and versatile additions to the dentist's repertoire.[14,15] Diode Laser can be used for a multitude of dental procedures, predominantly soft tissue procedures such as pulpotomy, frenectomy, gingivectomy,[14] and some hard tissue procedures such as root canal disinfection and tooth whitening.[15] Application of diode Laser in root canal disinfection showed promising results in effectively reducing the microbial load in infected root canals than that of chemical disinfectants.[16,17] Because of its effective use in root canal disinfection and no availability of reports till date in regard to cavity disinfection, it has been tested in this study. Hence, this study was designed to evaluate and compare the cavity disinfection efficacy of APF gel, Propolis, Diode Laser, and 2% CHX in primary teeth.
Materials and Methods
This study was a randomized, single blinded, parallel grouped, active controlled trial approved by the Institutional Review Board (VDC/RP/2012–63) and included 80 primary molars from 68 children attending the outpatient clinic of Department of Pedodontics from August 2013 to April 2014. A pilot study was conducted to check the feasibility and to obtain the sample size for the main study. Based on observations of the pilot study, a sample size of 20 was determined for each group. A letter providing all the information of the study was given to the parent/guardian, and they were considered after receiving the written consent. Teeth included in the study met the following criteria; primary molars with cavitated occlusal dentinal caries with no medical history; no clinical and radiographic signs of pulpal involvement; children not received antibiotic therapy since 4 weeks before sampling.
Group allocation
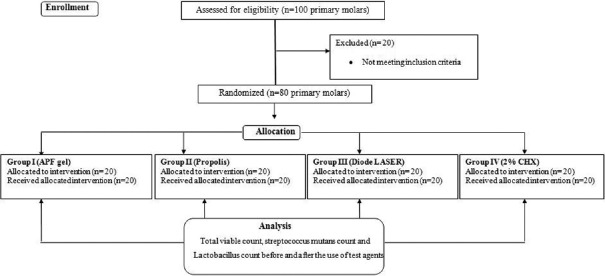
Eighty primary molars were randomly assigned (lottery method) into four groups by an assistant, and each group received one of the following agents [Table 1 and Figure 1].
Table 1.
Group allocation and test agents used for cavity disinfection
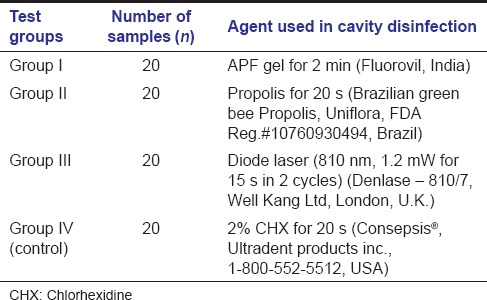
Figure 1.
Summary flow chart of study design
Dentinal sample collection and inoculation
After isolating with rubber dam, caries lesion was excavated by ART technique.[18] Based on color and consistency, the infected dentin was completely excavated using sterile spoon excavator (# 3000, API India), and the affected dentin was left behind. A dentinal sample was then collected from the base of the cavity using sterile spoon excavator (# 3000, API India) and after disinfection with test agents, cavity was rinsed sterile distilled water, and another dentinal sample was collected using a sterile excavator and transferred into Eppendorf tubes containing 0.5 ml of phosphate buffer solution.
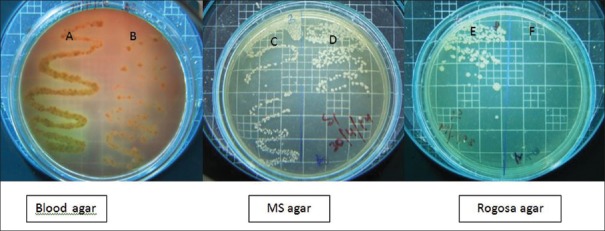
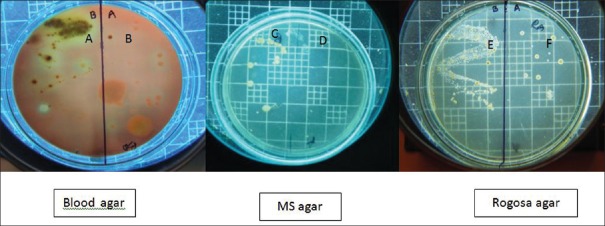
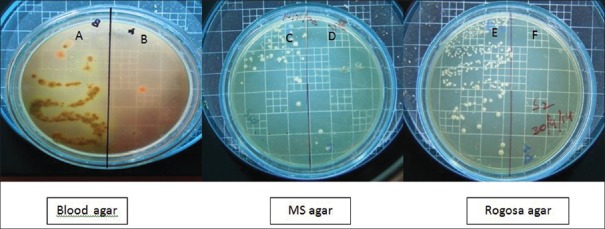
Two microliters of the samples collected before and after disinfection were inoculated on the two halves of blood agar (Himedia, India) for total viable count (TVC). Similarly, 2 µl of the samples collected before and after disinfection were inoculated on the mutans sanguis (MS) agar (M977; Himedia, India) a selective medium for Streptococcus mutans (MS), and Rogosa agar medium (M130; Himedia, India) for Lactobacilli (LB), respectively. The plates were incubated for 72 h in an atmosphere containing 5% CO2 and 95% N2 at 37°C. Colony forming units on the plates were counted using colony counter by a blinded assessor [Figures 2-5].
Figure 2.
(A) Total viable count before cavity disinfection in Group I; (B) total viable count after cavity disinfection in Group I; (C) mutans sanguis counts before cavity disinfection in Group I; (D) mutans sanguis counts after cavity disinfection in Group I; (E) Lactobacilli counts before cavity disinfection in Group I; and (F) Lactobacilli counts after cavity disinfection in Group I
Figure 5.
(A) Total viable count before cavity disinfection in Group IV; (B) total viable count after cavity disinfection in Group IV; (C) mutans sanguis counts before cavity disinfection in Group IV; (D) mutans sanguis counts after cavity disinfection in Group IV; (E) Lactobacilli counts before cavity disinfection in Group IV; and (F) Lactobacilli counts after cavity disinfection in Group IV
Figure 3.
(A) Total viable count before cavity disinfection in Group II; (B) total viable count after cavity disinfection in Group II; (C) mutans sanguis counts before cavity disinfection in Group II; (D) mutans sanguis counts after cavity disinfection in Group II; (E) Lactobacilli counts before cavity disinfection in Group II; and (F) Lactobacilli counts after cavity disinfection in Group II
Figure 4.
(A) Total viable count before cavity disinfection in Group III; (B) total viable count after cavity disinfection in Group III; (C) mutans sanguis counts before cavity disinfection in Group III; (D) mutans sanguis counts after cavity disinfection in Group III; (E) Lactobacilli counts before cavity disinfection in Group III; and (F) Lactobacilli counts after cavity disinfection in Group III
Results
The recorded data did not show normal distribution; therefore, nonparametric statistical tests were used. Wilcoxon signed-rank test for intragroup comparison, Kruskal–Wallis ANOVA and Mann–Whitney U-test for intergroup comparisons.
Intragroup comparisons
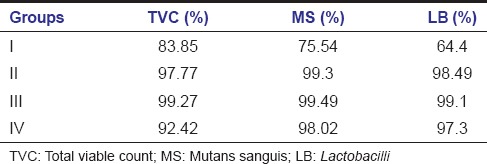
Wilcoxon signed-rank test demonstrated that there were reductions in TVC, MS, and LB counts after cavity disinfection with all the test agents and were statistically significant [Table 2]. However, there were differences in percentage reductions of bacterial counts with cavity disinfectants. Diode Laser showed greater percentage reductions followed by Propolis and 2% CHX. APF gel showed least percentage bacterial reductions among the test agents [Table 3].
Table 2.
Intragroup comparison of bacterial counts (colony forming units) in different groups before and after cavity disinfection (Wilcoxon signed-rank test)
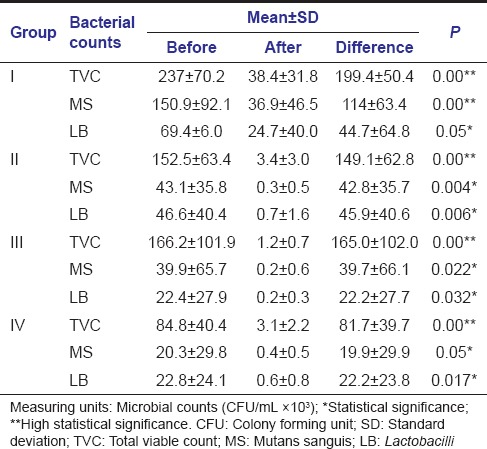
Table 3.
Percentage reductions of total viable count, mutans sanguis counts, and Lactobacilli counts after cavity disinfection with test agents
Intergroup comparisons
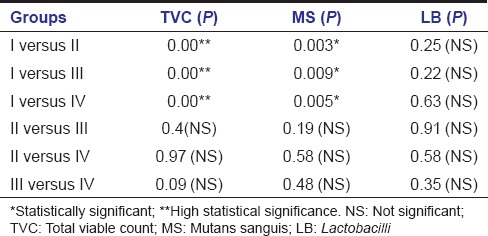
Kruskal–Wallis ANOVA demonstrated that when TVC and MS count reductions were compared between the groups, it was of statistical significance. Even though there was a difference in LB count reduction among the groups, it was not significant [Table 4]. To compare the cavity disinfection efficacy of the test agents on one to one basis (pairwise comparison), Mann–Whitney U-test was used and it showed statistical significance in TVC and MS count reductions when Group I (APF gel) was compared with other groups reflecting lesser amount of bacterial reductions with the use of APF gel when compared to other agents. However, the difference with LB count reductions was not significant [Table 5].
Table 4.
Intergroup comparison of bacterial counts (colony forming units) among different groups after cavity disinfection (Kruskal-Wallis ANOVA)
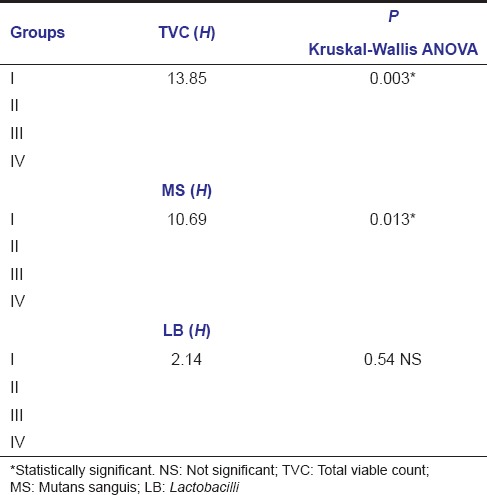
Table 5.
Pair-wise comparison of bacterial counts (colony forming units) among different groups after cavity disinfection (Mann-Whitney U-test)
Discussion
The principal objective of caries removal is to eliminate infected, necrotic hard tissues, and microorganisms that may cause persistent inflammation and treatment failure. Thus, thorough removal of the infected dentin has a direct influence and impact on the clinical success of the restoration.[19] The caries treatment procedures widely practiced currently does not eliminate all the microorganisms in the residual dental tissues.[20,21] Cavity disinfection is an adjunctive approach to reduce the bacteria in the residual dental tissues left after cavity preparation.
It is well-known that S. mutans and LB were the principal colonizers that are capable of producing dental caries in experimental animals and humans.[22] In this study, APF gel, Propolis, Diode Laser, and 2% CHX were tested against these cariogenic bacteria. A culture-dependent approach was used in this study as it is considered to be one of the most reliable methods for detecting the viable bacteria, particularly when samples were taken immediately after the antimicrobial treatment. Therefore, MS, LB, and TVC were evaluated from the samples that are collected before and after cavity disinfection with the test agents, and it demonstrated a significant bacterial count reduction in all the groups.
With the use of APF gel, greater percentage reduction of TVC was observed when compared to MS and LB counts, suggesting APF gel was more effective against facultative anaerobes than the principal cariogenic microorganisms. The probable mechanisms of antimicrobial activity of fluorides include fluoride increases the acquisition of protons by the cells and results in a reduction in tolerance of the oral bacteria to grow and metabolize in acidic environment.[8] The other mechanism is by inhibition of glycolysis.[8] Fluoride exerts this inhibitory effect by interfering with the uptake and degradation of polysaccharides by the bacterial cells and also by reducing the ability of the cell to maintain the homeostasis. Fluoride also interferes with the enzyme systems, namely, enolase and active proton transport ATPase systems regulating the metabolic activity of saccharolytic microorganisms. This results in significant reduction in the metabolic activity of the saccharolytic microorganisms.[8] Fluorides also have an advantage of remineralizing the affected dentin. However, on the overall comparison, APF gel was found to be least effective in cavity disinfection among the agents tested.
Various studies have demonstrated the bactericidal effects of Propolis against S. mutans.[23,24,25] This study showed that Propolis is also effective against LB and other cariogenic microorganisms. It was also observed that greater percentage of reductions in TVC, MS, and LB counts with Propolis when compared to 2% CHX (active control) in this study. The antimicrobial properties of Propolis were attributed to the flavonoids, phenolics, and various aromatic compounds. Flavonoids have antibacterial, antifungal, antiviral, antioxidant, and anti-inflammatory properties. Flavonoids including galangin, Pinocembrin, and pinostrobin are known as effective flavonoids against bacteria. Ferulic acid and caffeic acid present in the Propolis also contribute to the bactericidal action.[25]
The cavity disinfection with Diode Laser was found to be superior when compared to the other groups. The reduction of TVC, MS, and LB counts with the use of Laser was about 99.27%, 99.49%, and 99.1%, respectively, almost an absolute reduction. It was also observed that Diode Laser was equally effective against S. mutans and LB and other facultative anaerobes. The principle mechanisms involved in antibacterial activity of diode Laser include the thermal and photodisruptive effects.[26] It causes lethal damage to cells including the destruction of cell wall integrity and possibly denaturation of proteins. The damage of the cell wall ceases the cell growth and successive cell lysis. At the same time, cell proteins are highly sensitive to the thermal changes leading to denaturation of proteins and cell death. Laser also causes expansion of intratubular water and collapse of water vapor as deep as possible, which is capable of producing an acoustic wave strong enough to disrupt the intratubular bacteria.[26] The higher reductions in microbial counts can also be attributed to the greater depth of penetration of Laser radiation about 1 mm deep into the dentin, surpassing the effect range of chemical disinfectants.[15]
The conventional 2% CHX was considered as active control since it is commercially available for cavity disinfection. The observations of this study confirmed that 2% CHX was more effective than APF gel. However, it showed a less cavity disinfection ability when compared with the Diode Laser and Propolis. The disinfectant efficiency of CHX depends on the mechanism of adsorption onto the cell wall of microorganisms, causing leakage of intracellular components. At low concentrations, CHX has a bacteriostatic effect causing leakage of the small molecular weight substances from microorganisms. At higher concentrations, CHX has a bactericidal effect due to the cytoplasmic precipitation and/or coagulation that probably caused by protein cross-linkage. Furthermore, the cationic properties of the CHX enable their adsorption by dentin surface, making CHX molecules penetrate deeper inside the dentinal tubules and elongating its residual antimicrobial activity.[27]
Conclusion
This study demonstrated the importance of cavity disinfection in reducing the bacteria in residual dental tissues. The superior results obtained with Diode Laser suggest it as highly effective for cavity disinfection. It was also observed that both Diode Laser and Brazilian Propolis are equally effective as 2% CHX for cavity disinfection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Smales RJ, Yip HK. The atraumatic restorative treatment (ART) approach for primary teeth: Review of literature. Pediatr Dent. 2000;22:294–8. [PubMed] [Google Scholar]
- 2.Franzon R, Guimarães LF, Magalhães CE, Haas AN, Araujo FB. Outcomes of one-step incomplete and complete excavation in primary teeth: A 24-month randomized controlled trial. Caries Res. 2014;48:376–83. doi: 10.1159/000357628. [DOI] [PubMed] [Google Scholar]
- 3.Wicht MJ, Haak R, Schütt-Gerowitt H, Kneist S, Noack MJ. Suppression of caries-related microorganisms in dentine lesions after short-term chlorhexidine or antibiotic treatment. Caries Res. 2004;38:436–41. doi: 10.1159/000079624. [DOI] [PubMed] [Google Scholar]
- 4.Weerheijm KL, Kreulen CM, de Soet JJ, Groen HJ, van Amerongen WE. Bacterial counts in carious dentine under restorations: 2-year in vivo effects. Caries Res. 1999;33:130–4. doi: 10.1159/000016506. [DOI] [PubMed] [Google Scholar]
- 5.González-Cabezas C, Li Y, Gregory RL, Stookey GK. Distribution of three cariogenic bacteria in secondary carious lesions around amalgam restorations. Caries Res. 1999;33:357–65. doi: 10.1159/000016534. [DOI] [PubMed] [Google Scholar]
- 6.Bönecker M, Toi C, Cleaton-Jones P. Mutans streptococci and Lactobacilli in carious dentine before and after atraumatic restorative treatment. J Dent. 2003;31:423–8. doi: 10.1016/s0300-5712(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 7.Ersin NK, Uzel A, Aykut A, Candan U, Eronat C. Inhibition of cultivable bacteria by chlorhexidine treatment of dentin lesions treated with the ART technique. Caries Res. 2006;40:172–7. doi: 10.1159/000091120. [DOI] [PubMed] [Google Scholar]
- 8.Pediatrics: A Review of the Antibacterial Effect of Fluoride. [Last accessed on 2003 Jan 01]. Available from: http://www.oralhealthgroup.com/features/paediatrics-a-review-of-the-antibacterial-effect-of-fluoride/
- 9.Neelakantan P, Jagannathan N, Nazar N. Ethnopharmacological approach in endodontic treatment: A focused review. Int J Drug Res. 2011;3:68–77. [Google Scholar]
- 10.Verma MK, Pandey RK, Khanna R, Agarwal J. The antimicrobial effectiveness of 25% propolis extract in root canal irrigation of primary teeth. J Indian Soc Pedod Prev Dent. 2014;32:120–4. doi: 10.4103/0970-4388.130786. [DOI] [PubMed] [Google Scholar]
- 11.Parolia A, Kundabala M, Rao NN, Acharya SR, Agrawal P, Mohan M, et al. Acomparative histological analysis of human pulp following direct pulp capping with propolis, mineral trioxide aggregate and Dycal. Aust Dent J. 2010;55:59–64. doi: 10.1111/j.1834-7819.2009.01179.x. [DOI] [PubMed] [Google Scholar]
- 12.Dodwad V, Kukreja BJ. Propolis mouthwash: A new beginning. J Indian Soc Periodontol. 2011;15:121–5. doi: 10.4103/0972-124X.84379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebaraa EC, Pustiglioni AN, de Lima LA, Mayer MP. Propolis extract as an adjuvant to periodontal treatment. Oral Health Prev Dent. 2003;1:29–35. [PubMed] [Google Scholar]
- 14.Maturo P, Perugia C, Docimo R. Versatility of an 810 nm diode laser in pediatric dentistry. Int J Clin Dent. 2013;6:161–72. [Google Scholar]
- 15.Pirnat S. Versatility of an 810 nm diode laser in dentistry: An overview. J Laser Health Acad. 2007;4:1–9. [Google Scholar]
- 16.Moritz A, Gutknecht N, Schoop U, Goharkhay K, Doertbudak O, Sperr W. Irradiation of infected root canals with a diode laser in vivo: Results of microbiological examinations. Lasers Surg Med. 1997;21:221–6. doi: 10.1002/(sici)1096-9101(1997)21:3<221::aid-lsm1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 17.Gutknecht N, Alt T, Slaus G, Bottenbergd P, Rosseel P, Lauwers S, et al. A clinical comparison of the bactericidal effect of the diode laser and 5% sodium hypochlorite in necrotic root canals. J Oral Laser Appl. 2002;2:151–7. [Google Scholar]
- 18.Holmgren CJ, Frencken JE. Painting the future for ART. Community Dent Oral Epidemiol. 1999;27:449–53. doi: 10.1111/j.1600-0528.1999.tb02047.x. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee A, Watson TF, Kidd EA. Dentine caries excavation: A review of current clinical techniques. Br Dent J. 2000;188:476–82. doi: 10.1038/sj.bdj.4800515. [DOI] [PubMed] [Google Scholar]
- 20.Habeeb HM, AL-Mizraqchi AS, Ibraheem AF. Effect of ozonated water on adherent Mutans Streptococci (In vitro study) J Bagh Coll Dentistry. 2009;21:18–23. [Google Scholar]
- 21.de Almeida Neves A, Coutinho E, Cardoso MV, Lambrechts P, Van Meerbeek B. Current concepts and techniques for caries excavation and adhesion to residual dentin. J Adhes Dent. 2011;13:7–22. doi: 10.3290/j.jad.a18443. [DOI] [PubMed] [Google Scholar]
- 22.Cura F, Palmieri A, Girardi A, Martinelli M, Scapoli L, Carinci F. Lab-Test(®) 4: Dental caries and bacteriological analysis. Dent Res J (Isfahan) 2012;9(Suppl 2):S139–41. doi: 10.4103/1735-3327.109723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duailibe SA, Gonçalves AG, Ahid FJ. Effect of a propolis extract on Streptococcus mutans counts in vivo. J Appl Oral Sci. 2007;15:420–3. doi: 10.1590/S1678-77572007000500009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libério SA, Pereira AL, Araújo MJ, Dutra RP, Nascimento FR, Monteiro-Neto V, et al. The potential use of propolis as a cariostatic agent and its actions on mutans group streptococci. J Ethnopharmacol. 2009;125:1–9. doi: 10.1016/j.jep.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 25.Hegde KS, Bhat SS, Rao A, Sain S. Effect of propolis on Streptococcus mutans counts: An in vivo study. Int J Clin Pediatr Dent. 2013;6:22–5. doi: 10.5005/jp-journals-10005-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antibacterial Effects of Diode Laser and Chlorhixidine Gluconate on Streptococcus mutans in Coronal Cavity. [Last accessed on 2013 Aug 23]. Available from: https://www.webmedcentral.com/wmcpdf/ArticleWMC004179.pdf .
- 27.Ferraz CC, Gomes BP, Zaia AA, Teixeira FB, Souza-Filho FJ. Comparative study of the antimicrobial efficacy of chlorhexidine gel, chlorhexidine solution and sodium hypochlorite as endodontic irrigants. Braz Dent J. 2007;18:294–8. doi: 10.1590/s0103-64402007000400004. [DOI] [PubMed] [Google Scholar]