Abstract
BACKGROUND
Glaucoma is an important cause of irreversible blindness. This study describes the characteristics of a large, diverse group of glaucoma patients and evaluates associations between demographic and clinical characteristics and blindness.
METHODS
Data were gathered via retrospective chart review of patients (N = 1,454) who were seen between July 2007 and July 2010 by glaucoma service providers at Duke Eye Center. Visual acuity and visual field criteria were used to determine whether patients met the criteria for legal blindness. Descriptive and comparative statistical analyses were performed on the glaucoma patients who were not blind (n = 1,258) and those who were blind (n = 196). A subgroup analysis of only those patients with primary open-angle glaucoma was also performed.
RESULTS
In this tertiary care population, 13% (n = 196) of glaucoma patients met criteria for legal blindness, nearly one-half of whom (n = 94) were blind from glaucoma, and another one-third of whom (n = 69) had glaucoma-related blindness. The most common glaucoma diagnosis at all levels of vision was primary open-angle glaucoma. A larger proportion of black patients compared with white patients demonstrated vision loss; the odds ratio (OR) for blindness was 2.25 (95% CI, 1.6–3.2) for black patients compared with white patients. The use of systemic antihypertensive medications was higher among patients who were blind compared with patients who were not blind (OR = 2.1; 95% CI, 1.4–3.1). A subgroup analysis including only patients with primary open-angle glaucoma showed similar results for both black race and use of systemic antihypertensive medications. The relationship between use of systemic antihypertensive medications and blindness was not different between black patients and white patients (interaction P = .268).
LIMITATIONS
Data were based on chart review, and associations may be confounded by unmeasured factors.
CONCLUSIONS
Treated systemic hypertension may be correlated with blindness, and the cause cannot be explained solely by race. In addition, this study demonstrated that there is continued disparity between black patients and white patients with regards to blindness from glaucoma.
In 2002 in North Carolina, it was estimated that more than 66,000 people over age 40 years had primary open-angle glaucoma (POAG), the most common type of glaucoma [1]. Glaucoma is a group of blinding eye diseases characterized by progressive optic nerve degeneration and loss of visual field. An important risk factor is elevated eye pressure; thus, the goal for the treatment of all types of chronic glaucoma is lowering eye pressure to preserve functional vision for the patient's lifetime. Treatment prior to the onset of symptoms can prevent vision loss, since vision loss from glaucoma is irreversible. Approaches to treating glaucoma by lowering eye pressure include medications, incisional surgeries, and laser procedures. There is currently no cure for glaucoma, but recent decades have seen the advent of new classes of glaucoma medications (eg, prostaglandin analogues) and newer surgical devices (eg, glaucoma shunt implants), which give doctors more options to attempt to keep the disease under control.
Risk factors for glaucoma include older age, black race, family history of glaucoma, history of eye trauma, history of steroid use, systemic diseases such as hypertension and diabetes, and nearsightedness [2]. Risk factors for progressing to blindness from glaucoma include advanced visual field loss at presentation, noncompliance with treatment, age, and black race [3, 4]. Understanding the extent to which black race is a risk factor for glaucoma and for glaucoma-related blindness is of particular importance both in North Carolina, where 22% of the population is black, and more specifically in Durham County, where 39% of the population is black [5]. Understanding these risk factors in current glaucoma patients in North Carolina could help to prevent blindness.
Glaucoma Blindness
Despite progress in the development of novel medical and surgical approaches for treatment of glaucoma, open-angle glaucoma still stands as a leading cause of irreversible blindness, and it accounts for 12% of all cases of blindness worldwide [6, 7]. A 2003 study estimated that 15% of open-angle glaucoma patients lose vision in 1 eye within 15 years of their initial diagnosis, and 6.4% lose vision in both eyes [8].
The purpose of this study is to assess the characteristics of blindness in current glaucoma patients at a tertiary care facility with a broad referral base and a diverse patient population. We sought to compare glaucoma patients who are blind to those who are not blind, with regards to race, age, sex, glaucoma type, treatment history, coexisting ocular pathologies, and systemic medications. The goal is to provide an up-to-date perspective on the state of glaucoma blindness in North Carolina, in order to guide future efforts in continuing to improve care for the state's glaucoma patients.
Methods
Data Source and Participants
Duke University's institutional review board approved this retrospective chart review of patient records. Adult glaucoma patients were identified using the Duke Enterprise Data Unified Content Explorer (DEDUCE) to query the Duke University Health System coding record [9]. This study included patients with a glaucoma diagnosis code in the electronic medical record who were seen July 2007–July 2010 by glaucoma service providers at Duke Eye Center. The following ICD-9 glaucoma diagnosis codes were used: 365.1, 365.11, 365.12, 365.13, 365.14, 365.2, 365.23, 365.3, 365.4, 365.5, 365.52, 365.6, 365.62, 365.63, 365.65, and 365.89. A total of 4,707 unique patients were identified; data on age, race, sex, and state of residence were available from the DEDUCE search.
The electronic medical records of 1,629 patients were reviewed; these patients were selected in a randomized fashion using a random number generator. Demographic information, visual function metrics, and ocular and glaucoma history (including number and type of prior glaucoma surgeries and current glaucoma medications) were recorded; systemic medications listed in the ophthalmic note were reviewed; and antihypertensive and diabetic medications were recorded. The type of glaucoma was obtained from the medical record (either from the attending assessment or the ophthalmic history section). When multiple glaucoma diagnoses were listed, the more specific code was used for analysis. For example, if open-angle glaucoma and pseudoexfoliation glaucoma were both listed, then 365.52 (pseudoexfoliation) was used for analysis. Table 1 (online version only) lists the glaucoma diagnoses of the patients who were blind, monocular, driving restricted, or not blind.
TABLE 1.
Diagnoses of the Glaucoma Patients Who Are Blind, Monocular, Driving Restricted, and/or Not Blind
| Glaucoma diagnosis (ICD-9 code) | Blind (n = 163) | Not blind | ||
|---|---|---|---|---|
| Not blind (including those with some visual impairment) (n = 1,258) | With some visual impairment | |||
| Monocular (n = 352) | Driving restricted (n = 100) | |||
| Open-angle glaucoma (365.1) | 6 (4%) | 48 (4%) | 12 (3%) | 4 (4%) |
| Primary open-angle glaucoma (365.11) | 97 (60%) | 697 (55%) | 168 (48%) | 57 (57%) |
| Normal tension glaucoma (365.12) | 2 (1%) | 91 (7%) | 17 (5%) | 2 (2%) |
| Pigmentary glaucoma (365.13) | 0 (0%) | 33 (3%) | 4 (1%) | 0 (0%) |
| Juvenile open-angle glaucoma (365.14) | 3 (2%) | 17 (1%) | 6 (2%) | 2 (2%) |
| Primary angle closure glaucoma (365.2) | 6 (4%) | 67 (5%) | 12 (3%) | 3 (3%) |
| Steroid-induced glaucoma (365.3) | 0 (0%) | 13 (1%) | 3 (1%) | 2 (2%) |
| Glaucoma associated with congenital abnormalities (365.4) | 7 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Lens-associated glaucoma (365.5) | 0 (0%) | 10 (0.8%) | 6 (2%) | 1 (1%) |
| Exfoliation glaucoma (365.52) | 3 (2%) | 47 (4%) | 8 (2%) | 3 (3%) |
| Secondary glaucoma (365.6) | 12 (7%) | 51 (4%) | 30 (9%) | 6 (6%) |
| Uveitic glaucoma (365.62) | 6 (4%) | 76 (6%) | 29 (8%) | 11 (11%) |
| Neovascular glaucoma (365.63) | 14 (9%) | 35 (3%) | 26 (7%) | 5 (5%) |
| Traumatic glaucoma (365.65) | 0 (0%) | 47 (4%) | 20 (6%) | 1 (1%) |
| Mixed-mechanism glaucoma (365.89) | 7 (4%) | 26 (2%) | 11 (3%) | 3 (3%) |
For each patient, the best-corrected visual acuity at the most recent visit within the study period was noted. The most recent automated visual field assessment within the study period was also reviewed. Prior visual fields were reviewed if the most recent study was unreliable (defined as greater than 30% fixation losses, false negatives, or false positives) or if the study was a 10-2 test. If no reliable visual fields were available, the confrontational visual field results noted on the provider's exam were used. Patients were categorized as blind if they met the criteria for legal blindness: best-corrected visual acuity of 20/200 or worse in the better-seeing eye, or a constricted visual field less than 20 degrees in the better-seeing eye. Patients who met these criteria for only 1 eye were categorized as monocular. Patients were categorized as “driving restricted” if they met the following criteria, which define whether a person is eligible for an unrestricted North Carolina state driver's license: visual acuity worse than 20/50 in both eyes, or a constricted visual field less than 60 degrees in both eyes.
The chart review was completed by 2 of the authors; the first 1,069 records were reviewed by J.A.R., and the remaining 560 records were reviewed by J.S.S. and confirmed by J.A.R. To examine the internal consistency between reviewers, a duplicate review of 5% of the initial records was performed by J.S.S.; there was no difference between the datasets (data not shown). After the initial chart review, 175 patients were excluded for the following reasons: lack of electronic medical record during the study period (n = 135), lack of glaucoma diagnosis (n = 39), and not seen by the glaucoma service (n = 1). This left 1,454 patients remaining in the dataset.
To validate that the random sample of 1,454 patients (the overall sample, shown in Table 2) was representative of the entire population of 4,707 patients (the DEDUCE set), a comparative analysis was performed. This analysis showed no statistically significant differences between the 2 groups with regards to age, sex, race, or state of residence (data not shown).
TABLE 2.
Demographic Characteristics of the Overall Sample and of the Glaucoma Patients Who Are Blind, Monocular, Driving Restricted, and/or Not Blind
| Overall samplea (N = 1,454) | Blindb (n = 163) | Not blind | |||
|---|---|---|---|---|---|
| Not blind (including those with some visual impairment) (n = 1,258) | With some visual impairment | ||||
| Monocularc (n = 352) | Driving restrictedd (n = 100) | ||||
| Age | |||||
| P-valuee | .99 | .30 | < .001 | ||
| Mean ± SD (years) | 71.4 ± 15 | 71 ± 17 | 71 ± 15 | 72 ± 17 | 76 ± 18 |
| Median (years) | 73 | 73 | 73 | 74 | 80 |
| Sex | |||||
| P-valuef | .39 | .03 | .83 | ||
| Female, No. (%) | 811 (56%) | 85 (52%) | 701 (56%) | 179 (51%) | 54 (54%) |
| Male, No. (%) | 643 (44%) | 78 (48%) | 557 (44%) | 173 (49%) | 46 (46%) |
| Race | |||||
| P-valueg | < .001 | < .001 | .02 | ||
| White, No. (%) | 792 (54%) | 63 (39%) | 708 (56%) | 166 (47%) | 43 (43%) |
| Black, No. (%) | 574 (39%) | 94 (58%) | 469 (37%) | 162 (46%) | 51 (51%) |
| Asian, No. (%) | 29 (2%) | 1 (1%) | 27 (2%) | 4 (1%) | 1 (1%) |
| American Indian, No. (%) | 7 (.5%) | 0 (0%) | 7 (.6%) | 2 (.6%) | 0 (0%) |
| Multiracial, No. (%) | 1 (.1%) | 0 (0%) | 1 (.1%) | 1 (.3%) | 0 (0%) |
| All others, No. (%) | 32 (2%) | 1 (1%) | 31 (2%) | 14 (4%) | 3 (3%) |
| Declined to state race, No. (%) | 9 (.6%) | 2 (1%) | 7 (.6%) | 3 (1%) | 2 (2%) |
Note. SD, standard deviation.
Data for the random subset whose charts were reviewed (overall sample; n = 1,454 patients) were compared with a cohort of glaucoma patients identified by querying the Duke University Health System coding record using the Duke Enterprise Data Unified Content Explorer (N = 4,705 patients), and both groups were statistically similar. There was also racial similarity between the study population and the population of Durham County, based on 2012 estimates of Durham County and North Carolina state population demographics [5].
Patients who are blind are compared with patients who are not blind.
Monocular patients are compared with patients who are not blind and not monocular.
Patients in the driving restricted group are compared with patients who are not blind.
Wilcoxon rank-sum test.
Chi-square test.
Fisher's exact test.
Statistical Analyses
Statistical analyses were performed using SAS software version 9.2. A P-value less than .05 was considered statistically significant.
Visual impairment
Descriptive statistics were summarized for the groups of patients using means with standard deviations for age and using percentages for the categorical variables. A Wilcoxon rank-sum test was used to assess the significance of the difference between medians for age. A chi-square test was used to compare proportions for sex, and Fisher's exact test was used to compare proportions for race. For the comparisons, patients who were blind were compared to patients who were not blind (which included both monocular and driving restricted patients); the monocular group was compared to patients who were not blind and not monocular; and the driving restricted group was compared to the total group of patients who were not blind. Logistic regression analysis was performed to look for an association of black race with blindness; the covariates for this analysis were age, sex, and age and sex.
Glaucoma diagnosis and treatment
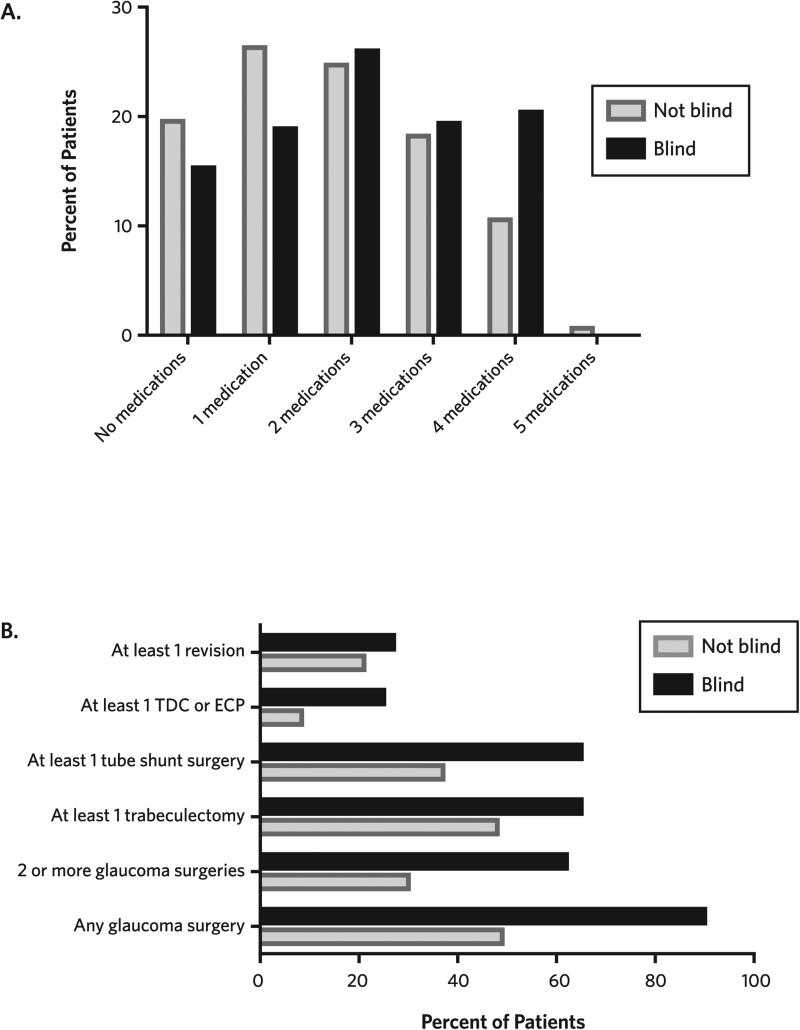
For the data on glaucoma diagnoses, proportions are described in the text. The Wilcoxon rank-sum test was used to compare the mean number of glaucoma medications used by patients who were blind with the mean for patients who were not blind. For the data on glaucoma medications, proportions of patients were compared for those who were blind and those who were not blind using 0, 1, 2, 3, 4, or 5 glaucoma medications; these results are summarized in the text. For the glaucoma surgery data described in the text, a chi-square test was used to compare proportions for the different types of glaucoma surgeries, comparing the group of patients who were blind with the group who were not blind (see Figure 1; online version only).
FIGURE 1. Glaucoma Medications and Surgeries in Patients Who Are Blind or Not Blind.
A. Percentages of glaucoma patients taking 0–5 types of glaucoma medications are depicted for patients who are blind and those who are not blind. On average, glaucoma patients who are not blind are taking fewer glaucoma medications (1.8) than glaucoma patients who are blind (2.1; P < .001).
B. Percentages of glaucoma patients who have had glaucoma procedures are depicted. A greater percentage of patients who are blind have undergone at least 1 or at least 2 glaucoma surgeries, trabeculectomy surgery, glaucoma tube implant surgery, or a cycloablative procedure.
Note. ECP, endoscopic cyclophotocoagulation; TDC, transscleral diode cyclophotocoagulation.
Ocular and systemic comorbidities
Descriptive statistics were summarized for the groups of patients using percentages for the categorical variables. A chi-square test was used to compare proportions for patients with comorbid ocular conditions: age-related macular degeneration (ARMD), diabetic retinopathy (background/mild, proliferative), and/or uveitis. A chi-square test was also used to compare proportions for patients with comorbid systemic conditions; the use of medications for hypertension or diabetes was used as a surrogate for diagnosis of these conditions. For the comparisons, patients who were blind were compared to patients who were not blind (which included the monocular and driving restricted patients); the monocular group was compared to patients who were not blind and not monocular; and the driving restricted group was compared to the total group of patients who were not blind.
Univariable odds ratio calculations
For the characteristics with statistically significant differences between groups, odds ratios were calculated along with 95% CIs, based on the estimates of relative risk for the group of patients who were blind compared with the group of patients who were not blind.
Subgroup analysis of POAG patients
Subgroup analyses including only patients with POAG, excluding the diagnoses known to be causally related to hypertension and diabetes, were performed to examine the association between blindness and the following variables: race, use of systemic antihypertensive medications, and use of systemic diabetes medications. Chi-square tests were used to determine whether the difference in proportions was significantly associated with blindness. Odds ratios were calculated along with 95% CIs, based on the estimates of relative risk of the group of POAG patients who were blind compared with the group of POAG patients who were not blind. Multivariable logistic regression analysis was carried out using the variables the univariable analyses found to be associated with blindness: black race and use of systemic antihypertensive medications. An interaction term was included to determine whether the relationship between use of antihypertensive medications and blindness was different for black patients versus white patients.
Results
Visual Impairment in Glaucoma Patients
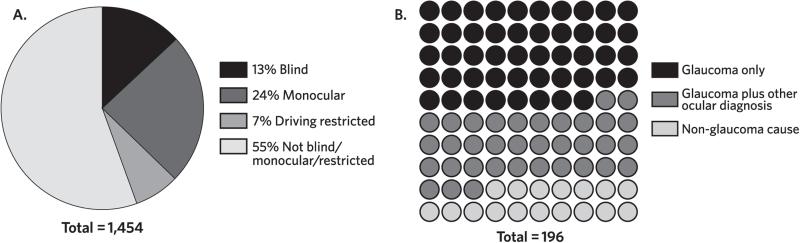
In this population of glaucoma patients, 196 of 1,454 (13%) met visual criteria for legal blindness (from all causes), 352 (24%) were monocular, and 100 (7%) were not legally blind but had levels of visual impairment that prohibited them from driving (see Figure 2A). Of the patients who were blind, about one-half (n = 94) were blind due to glaucoma, and one-third (n = 69) were blind due to glaucoma as well as at least 1 other cause (see Figure 2B). Thirty-three patients were blind due to a nonglaucomatous cause; these patients were not included in the subsequent analyses.
FIGURE 2. Visual Impairment and Causes in Glaucoma Patients.
A. Of the 1,454 patient records reviewed, 196 (13%) met criteria for legal blindness; 352 (24%) were monocular; and 100 (7%) were not legally blind but had levels of visual impairment that restricted their driving.
B. Of the 196 glaucoma patients who met criteria for legal blindness, 94 patients were blind from glaucoma (represented by black dots). Sixty-nine patients were blind from glaucoma as well as another ocular cause (for example, neovascular glaucoma due to advanced proliferative diabetic retinopathy, represented by grey dots), and 33 glaucoma patients were blind from non-glaucomatous causes (for example, age-related macular degeneration and retinal vascular occlusive disease, represented by light dots). The black and grey dots represent the patients with glaucoma-related blindness; these glaucoma patients are described in the tables as the “blind” group.
The patients who were blind and the patients who were monocular were similar in age to the patients who were not blind (mean ages: 71 years, 72 years, and 71 years, respectively), but patients in the driving restricted group were older (mean age, 76 years; P < .001; see Table 2). There was no statistically significant sex difference between the patients who were blind (P = .39) and those in the driving restricted group (P = .83) compared to the patients who were not blind; however there was an increase in the percentage of males in the monocular group (49%) compared with the other groups (44%; P = .03).
With regards to race, a larger proportion of black patients demonstrated vision loss. Specifically, there were higher percentages of black patients among those who were blind (P < .001), those who were monocular (P < .001), and those in the driving restricted group (P = .02) than in the group of patients who were not blind, with an odds ratio of 2.25 (95% CI, 1.6–3.2) for black race and blindness compared with white race (see Table 3). Logistic regression analyses showed no association between blindness and age (P = .88) or sex (P = .90).
TABLE 3.
Univariable Odds Ratios of Factors Potentially Associated with Glaucoma-Related Blindness
| Factor | Odds ratio (95% CI) |
|---|---|
| Black race compared with white racea | 2.25 (1.60-3.16) |
| Antihypertensive medication use | 2.09 (1.39-3.14) |
| Diabetes medication use | 1.73 (1.17-2.54) |
| Diagnosis of age-related macular degeneration | 0.55 (0.24-1.28) |
| Diagnosis of uveitis | 0.65 (0.34-1.23) |
The numbers of non-black and non-white patients were insufficient for a meaningful analysis of other races.
Glaucoma Diagnosis and Treatment
Half of glaucoma patients in the dataset were diagnosed with POAG; these patients comprised 55% of the patients who were not blind, 60% of patients who were blind, and 48% of monocular patients. Patients who were blind were more likely to have secondary glaucoma diagnoses than those who were not blind. Patients who were blind were more likely than those who were not blind to have glaucoma associated with congenital anomalies (4% versus 0%), secondary glaucoma (7% versus 4%), neovascular glaucoma (9% versus 3%), and mixed-mechanism glaucoma (4% versus 2%). Monocular patients were more likely to have secondary glaucoma (9%), neovascular glaucoma (7%), or traumatic glaucoma (6%) compared with those who were not blind (4%), and those who were blind (0%).
Patients who were blind had received more treatments. On average, patients who were blind were using 2.1 medications (standard deviation [SD], 1.4) compared to only 1.8 medications among patients who were not blind (SD, 1.3; P = .001). The proportion of patients taking 4 glaucoma medications was higher among patients who were blind; 20% of patients who were blind were taking 4 glaucoma medications compared to 11% of patients who were not blind. The number of incisional glaucoma surgeries and cycloablative therapies was also higher among visually impaired patients. Compared to patients who were not blind, patients who were blind were more likely to have had trabeculectomy (48% versus 65%; P < .001), tube shunt surgery (37% versus 65%; P < .001), or a cycloablative procedure (8% versus 25%; P < .001). Additionally, patients who were blind were more likely to have had 2 or more glaucoma-related procedures compared to patients who were not blind (62% versus 30%; P < .001). There was no statistically significant difference in surgical revisions for glaucoma procedures between patients who were blind and those who were not blind (27% versus 21%; P = .10).
Ocular Comorbidities and Visual Impairment
Ocular comorbidities of the glaucoma patients in this study included ARMD, diabetic retinopathy (background/mild or proliferative), and uveitis (see Table 4). There was no significant difference in the diagnosis of ARMD between patients who were not blind and those who were blind (P = .16) or monocular (P = .20); however, patients in the driving restricted group were significantly more likely to have a diagnosis of ARMD compared with patients who were not blind (P = .004). With regards to diabetic retinopathy, visually impaired patients were as likely to present with background or mild diabetic retinopathy as were patients who were not blind. However, all 3 groups of visually impaired patients (blind, monocular, and driving restricted) had a higher percentage of proliferative diabetic retinopathy (P = .001 for patients who were blind, P < .001 for those who were monocular, and P < .001 for those in the driving restricted group). There was no statistically significant difference in the diagnosis of uveitis between patients who were not blind and those who were blind (P = .18) or those in the driving restricted group (P = .32), although monocular patients were significantly more likely to have uveitis compared with patients who were not blind (P = .03).
TABLE 4.
Systemic and Ocular Comorbidities in Glaucoma Patients Who Are Blind, Monocular, Driving Restricted, and/or Not Blind
| Blinda (n = 150)b | Not blind |
|||
|---|---|---|---|---|
| Not blind (including those with some visual impairment) (n = 1,228)b |
With some visual impairment
|
|||
| Monocularc (n = 341) | Driving restrictedd (n = 97) | |||
| Ocular comorbidities | ||||
| Age-related macular degeneration, No. (%) | 6 (4% of patients who are blind) | 83 (7% of patients who are not blind) | 28 (8%) | 14 (15%) |
| P-value | .16 | .20 | .004 | |
| Diabetic retinopathy, No. (%) | ||||
| Background/mild | 5 (3%) | 42 (3%) | 15 (4%) | 3 (3%) |
| Proliferative | 17 (11%) | 50 (4%) | 28 (8%) | 12 (12%) |
| P-value | .001 | < .001 | < .001 | |
| Uveitis, No. (%) | 11 (7%) | 129 (10%) | 46 (13%) | 13 (14%) |
| P-value | .18 | .03 | .32 | |
| Systemic comorbidities | ||||
| Antihypertensive medication use, No. (%) | 118 (79%) | 784 (64%) | 242 (71%) | 68 (70%) |
| P-value | .003 | .001 | .101 | |
| Diabetes medication use, No. (%) | 42 (28%) | 226 (18%) | 86 (25%) | 27 (28%) |
| P-value | .005 | < .001 | .007 | |
Note. Chi-square test was used to compare proportions between the groups.
Patients who are blind are compared with patients who are not blind.
Medication and ocular history were not known in all patients.
Monocular patients are compared with patients who are not blind and not monocular.
Patients in the driving restricted group are compared with patients who are not blind.
Hypertension, Diabetes, and Blindness in Glaucoma Patients
The use of systemic medications for hypertension or diabetes was higher among visually impaired patients (see Table 4). A total of 64% of glaucoma patients who were not blind were taking systemic antihypertensive medications, compared with 79% of glaucoma patients who were blind (P = .003). With regards to the use of diabetes medications, 18% of glaucoma patients who were not blind were taking medication for diabetes, compared with 28% of glaucoma patients who were blind (P = .005).
Subgroup Analysis of POAG Patients
Black race and use of systemic medications for hypertension or diabetes were associated with blindness among patients with all types of glaucoma (see Table 3). However, the visually impaired groups had more patients with neovascular glaucoma (9% of patients who were blind versus 3% of those who were not blind), which is a diagnosis related to ocular sequelae of hypertension and diabetes. A subgroup analysis of patients with POAG was performed, which excluded patients with neovascular glaucoma and other secondary causes of glaucoma (see Table 5). Both black race (P < .001) and the use of antihypertensive medications (P = .05) remained significantly associated with blindness in this analysis. The relationship between use of systemic antihypertensive medications and blindness was not statistically different between black patients and white patients (interaction P = .27). However, there was no significant difference between the POAG patients who were blind and those who were not blind with regards to use of diabetes medications (P = .39). This finding suggests that the association between use of diabetes medications and blindness in glaucoma patients was related to vision loss from diabetic retinopathy.
TABLE 5.
Subgroup Analysis of Patients With Primary Open-Angle Glaucoma (POAG)
| Patients with POAG who are blind (n = 105)a,b | Patients with POAG who are not blind (n = 764)a,b | Chi-square P-value | |
|---|---|---|---|
| Race | |||
| Black (n = 363)a | 61 (17% of black patients with POAG) | 302 (83% of black patients with POAG) | < .001 |
| White (n = 485) | 44 (9% of white patients with POAG) | 441 (91% of white patients with POAG) | |
| OR = 1.8 (95% CI, 1.3-2.5) | |||
| Antihypertensive medication use | |||
| Yes (n = 609)b | 79 (13% of patients with POAG who are using HTN medications) | 530 (87% of patients with POAG who are using HTN medications) | .05 |
| No (n = 255) | 21 (8% of patients with POAG who are not using HTN medications) | 234 (92% of patients with POAG who are not using HTN medications) | |
| OR = 1.6 (95% CI, 1.1-2.3) | |||
| Diabetes medication use | |||
| Yes (n = 163) | 22 (13% of patients with POAG who are using DM medications) | 141 (87% of patients with POAG who are using DM medications) | .39 |
| No (n = 701) | 78 (11% of patients with POAG who are not using DM medications) | 623 (89% of patients with POAG who are not using DM medications) | |
Note. DM, diabetes mellitus; HTN, systemic hypertension; OR, odds ratio; POAG, primary open-angle glaucoma.
Patients with POAG and race data were included.
Patients with POAG and medication history were included.
Discussion
An estimated 87,000 North Carolinians over the age of 40 years have some degree of visual impairment, and almost 28,000 are blind [1]. In this study, practical criteria for loss of vision were chosen based on definitions that are meaningful to patients: legal blindness (bilateral blindness), need for additional safety precautions due to having only 1 functional eye, and loss of driving privileges. Despite recent advances in both medical and surgical therapies for glaucoma, the results presented here show that 13% of glaucoma patients at a tertiary care eye center met criteria for legal blindness. Internationally, the prevalence of glaucoma blindness ranges from very low among patients in Sweden to greater than 20% among glaucoma patients in South Africa [2]. In the United States, an estimate of glaucoma blindness based on cross-sectional data for blindness in only 1 eye is around 15% for patients with open-angle glaucoma [10]; based on longitudinal data, the probability is 4.3% for bilateral glaucoma-related blindness [11]. In addition, our results showed that, at this tertiary eye care center, glaucoma patients who were blind were treated with more glaucoma medications on average, and they received more glaucoma surgeries, suggesting that their disease was progressing faster and required more aggressive treatment. There were statistically significant relationships between use of systemic antihypertensive medications and glaucoma blindness, and between black race and glaucoma blindness.
Treated Hypertension and Glaucoma Blindness
Glaucoma patients who were taking antihypertensive medications were more likely to have visual impairment than were patients who were not taking systemic antihypertensive medications. This association is supported by a number of studies that have assessed the connection between hypertension and the incidence of glaucoma [12]. Newman-Casey and colleagues found that having hypertension increased the risk of developing open-angle glaucoma, and the effect was amplified for patients who were being treated for both hypertension and diabetes mellitus [13]. However, some studies report no association between hypertension and glaucoma [14, 15]. With rare exceptions [16], population-based studies have documented the detrimental effect of systemic hypertension on unilateral and bilateral vision loss [17, 18].
There are several potential theories on how treated systemic hypertension could be associated with blindness in glaucoma patients. One theory posits that progression to blindness could be due to episodic hypotension resulting from oral antihypertensive treatment, which can lower blood perfusion pressures through the capillaries surrounding the optic nerve and may lead to nerve degeneration over time [16]. Another theory relates to deregulation of systemic and intraocular pressure due to suboptimal adherence to antihypertensive medication, which could undermine efforts to manage glaucoma with topical medications [19]. A third theory is that blindness could be due to increased perfusion pressure in the ciliary arteries due to systemic hypertension, with accelerated aqueous humor production [15, 20]. All are related to the theories that have been posited for the association between hypertension and diagnosis of glaucoma.
Race and Glaucoma Blindness
The results of this study show that a disproportionate number of black glaucoma patients were blind compared with white patients. This finding is depressingly familiar and was discussed at length in a 1982 landmark study by Grant and Burke [4]. Although more recent studies have found similar associations [8], other studies have not found an association between race and glaucoma blindness [21], even in ethnically diverse populations [22]. In the diverse patient population in this study, which is similar to the population of Durham County in terms of racial demography, the disparity in blindness between black patients and white patients was striking. The explanations for the continued disparity remain uncertain at present [23], but include a range of possible factors including genetic predisposition to disparities in medical intervention and access to care. Of the estimated 66,000 North Carolinians with POAG, one-third are black (almost 22,000 people), although blacks make up only 22% of the population [1]. Thus, continued research is needed to better understand this disparity and to close this gap.
Limitations
The goal of this study was to provide perspective on current glaucoma patients and vision loss; however, there are limitations to retrospective chart reviews, particularly with respect to the heterogeneity of the population and the potential for multiple confounding factors, especially vision loss due to ocular comorbidities such as ARMD and diabetic retinopathy. In addition, there are weaknesses to using the medical record, which reflects a single visit snapshot of a patient's treatment and thus lacks information about the longitudinal progression of vision loss. The study is limited to the data collected at the point of care and does not account for errors such as underreporting or overreporting of systemic medications. Additionally, patients’ adherence to medications for hypertension or glaucoma was not recorded. Unlike insurance claims data, which would encompass the patients’ care more comprehensively, this review is restricted to only those services rendered at a single medical center and included in the patient's history. Also, the chart reviewer/abstractor who recorded visual acuity measurements and assessed visual fields (which were used to determine the patient's degree of blindness) was not masked to explanatory variables such as systemic antihypertensive medications. Socioeconomic data were not recorded nor were specific details of systemic treatments. Finally, an academic medical center may see more complex cases than other glaucoma practices, thus increasing the proportion of patients with comorbidities and/or the number of patients who are blind compared with the rest of the population. For this reason, this study may not reflect the levels of glaucoma blindness in other glaucoma practices.
The main strength of this study is the large number of records reviewed (1,454 patients) representing approximately one-third of current glaucoma patients in a busy tertiary care eye center. The determination of blindness was made by recording visual acuity measurements and reviewing the patient's most recent visual field results; the glaucoma diagnosis code was confirmed using the attending physician's assessment as well as the ophthalmic history. This level of detail is not possible with large studies of de-identified patient populations, but it is essential for understanding the causes of blindness.
Conclusions
One in 9 glaucoma patients treated at an academic medical center is blind from glaucoma. Treated systemic hypertension may be associated with glaucoma blindness. Finally, there continues to be a disparity between black and white patients with regards to glaucoma blindness, so continued research in this area is warranted. NCMJ
Acknowledgments
Financial support. This study was supported by Research to Prevent Blindness, an unrestricted grant to the Duke Eye Center. J.A.R is funded by NEI grant 5K12EY016333. K.W.M receives salary support from a Veterans Affairs Health Services Research and Development Career Development Award. The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Potential conflicts of interest. All authors have no relevant conflicts of interest.
Contributor Information
Jordan S. Stone, University of California, San Diego, San Diego, California..
Kelly W. Muir, Duke University; director of clinical research, Durham Veterans Affairs Medical Center, Durham, North Carolina..
Sandra S. Stinnett, Duke University, Durham, North Carolina..
Jullia A. Rosdahl, Duke University, Durham, North Carolina..
References
- 1.Prevent Blindness America [January 7, 2014];Vision problems in the U.S. Prevent Blindness America website. www.visionproblemsus.org.
- 2.Cook C, Foster P. Epidemiology of glaucoma: what's new? Can J Ophthalmol. 2012;47(3):223–226. doi: 10.1016/j.jcjo.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Chen PP. Risk and risk factors for blindness from glaucoma. Curr Opin Ophthalmol. 2004;15(2):107–111. doi: 10.1097/00055735-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Grant WM, Burke JF., Jr Why do some people go blind from glaucoma? Ophthalmology. 1982;89(9):991–998. doi: 10.1016/s0161-6420(82)34675-8. [DOI] [PubMed] [Google Scholar]
- 5.United States Census Bureau [December 4, 2013];Durham County, North Carolina. United States Census Bureau website. http://quickfacts.census.gov/qfd/states/37/37063.html. Revised June 27, 2013.
- 6.Foster A, Resnikoff S. The impact of Vision 2020 on global blindness. Eye (Lond) 2005;19(10):1133–1135. doi: 10.1038/sj.eye.6701973. [DOI] [PubMed] [Google Scholar]
- 7.Taylor HR, Keeffe JE. World blindness: a 21st century perspective. Br J Ophthalmol. 2001;85(3):261–266. doi: 10.1136/bjo.85.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen PP. Blindness in patients with treated open-angle glaucoma. Ophthalmology. 2003;110(4):726–733. doi: 10.1016/S0161-6420(02)01974-7. [DOI] [PubMed] [Google Scholar]
- 9.Horvath MM, Winfield S, Evans S, Slopek S, Shang H, Ferranti J. The DEDUCE Guided Query tool: providing simplified access to clinical data for research and quality improvement. J Biomed Inform. 2011;44(2):266–276. doi: 10.1016/j.jbi.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broman AT, Quigley HA, West SK, et al. Estimating the rate of progressive visual field damage in those with open-angle glaucoma, from cross-sectional data. Invest Ophthalmol Vis Sci. 2008;49(1):66–76. doi: 10.1167/iovs.07-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malihi M, Moura Filho ER, Hodge DO, Sit AJ. Long-term trends in glaucoma-related blindness in Olmsted County, Minnesota. Ophthalmology. 2014;121(1):134–141. doi: 10.1016/j.ophtha.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanagi M, Kawasa R, Wang JJ, Wong TY, Crowston J, Kiuchi Y. Vascular risk factors in glaucoma: a review. Clin Experiment Ophthalmol. 2011;39(3):252–258. doi: 10.1111/j.1442-9071.2010.02455.x. [DOI] [PubMed] [Google Scholar]
- 13.Newman-Casey PA, Talwar N, Nan B, Musch DC, Stein JD. The relationship between components of metabolic syndrome and open-angle glaucoma. Ophthalmology. 2011;118(7):1318–1326. doi: 10.1016/j.ophtha.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B, BESs Study Group Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008;115(1):85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Kahn HA, Leibowitz HM, Ganley JP, et al. The Framingham Eye Study. II. Association of ophthalmic pathology with single variables previously measured in the Framingham Heart Study. Am J Epidemiol. 1977;106(1):33–41. doi: 10.1093/oxfordjournals.aje.a112429. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell P, Lee AJ, Rochtchina E, Wang JJ. Open-angle glaucoma and systemic hypertension: the Blue Mountains Eye Study. J Glaucoma. 2004;13(4):319–326. doi: 10.1097/00061198-200408000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Dielemans I, Vingerling JR, Algra D, Hofman A, Grobbee DE, de Jong PT. Primary open angle glaucoma, intraocular pressure, and systemic blood pressure in the general elderly population. The Rotterdam Study. Ophthalmology. 1995;102(1):54–60. doi: 10.1016/s0161-6420(95)31054-8. [DOI] [PubMed] [Google Scholar]
- 18.Zafra-Pérez JJ, Villegas-Pérez MP, Canteras Jordana M, Miralles De Imperial J. Intraocular pressure and prevalence of occult glaucoma in a village of Murcia. Arch Soc Esp Oftalmol. 2000;75(3):171–178. [PubMed] [Google Scholar]
- 19.Gherghel D, Orgül S, Gugleta K, Gekkieva M, Flammer J. Relationship between ocular perfusion pressure and retrobulbar blood flow in patients with glaucoma with progressive damage. Am J Ophthalmol. 2000;130(5):597–605. doi: 10.1016/s0002-9394(00)00766-2. [DOI] [PubMed] [Google Scholar]
- 20.McLeod SD, West SK, Quigley HA, Fozard JL. A longitudinal study of the relationship between intraocular and blood pressures. Invest Ophthalmol Vis Sci. 1990;31(11):2361–2366. [PubMed] [Google Scholar]
- 21.Paula JS, Furtado JM, Santos AS, Coelho Rde M, Rocha EM, Rodrigues Mde L. Risk factors for blindness in patients with open-angle glaucoma followed-up for at least 15 years. Arq Bras Oftalmol. 2012;75(4):243–246. doi: 10.1590/s0004-27492012000400004. [DOI] [PubMed] [Google Scholar]
- 22.Kooner KS, AlBdoor M, Cho BJ, Adams-Huet B. Risk factors for progression to blindness in high tension primary open angle glaucoma: comparison of blind and nonblind subjects. Clin Ophthalmol. 2008;2(4):757–762. doi: 10.2147/opth.s3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosoko-Lasaki O, Gong G, Haynatzki G, Wilson MR. Race, ethnicity and prevalence of primary open-angle glaucoma. J Nat Med Assoc. 2006;98(10):1626–1629. [PMC free article] [PubMed] [Google Scholar]