ABSTRACT
Enzymes involved in de novo production of guanosine triphosphate (GTP) have recently been revealed as integral components of melanoma progression through modulation of the activity of small GTPases. Here, we discuss the biology and therapeutic implications of these findings.
KEYWORDS: GMPS, GMPR, GTP, invasion, melanoma, RAC1, RHO-GTPAses
Text
Guanosine triphosphate (GTP), through its effects on GTP-binding proteins, is arguably the most important small-molecule regulator of cellular processes in healthy cells and is also involved in the maintenance of transformed phenotypes. Importantly, the levels of enzymes that regulate nucleotide metabolism, including the metabolism of guanylates, are often changed in human cancers.1-5
G-proteins, including those that are commonly constitutively activated in cancers (e.g., RAS, Ras-related C3 botulinum toxin substrate 1 [RAC1], Ras homolog gene family, member A [RHOA], and CDC42), act as “molecular switches” that fluctuate between guanosine diphosphate (GDP)-bound (inactive) and GTP-bound (active) states. This cycling is controlled by 2 types of regulatory protein: GTPase-activating proteins (GAPs) promote the hydrolysis of G-protein-bound GTP molecules, thus converting a G-protein into its inactive GDP-bound state, whereas guanosine exchange factors (GEFs) release GDP from the G-protein, so that it can bind GTP and become active again (Fig. 1). However, very little is known about whether changes in intracellular GTP levels affect GTPase activity.
Figure 1.
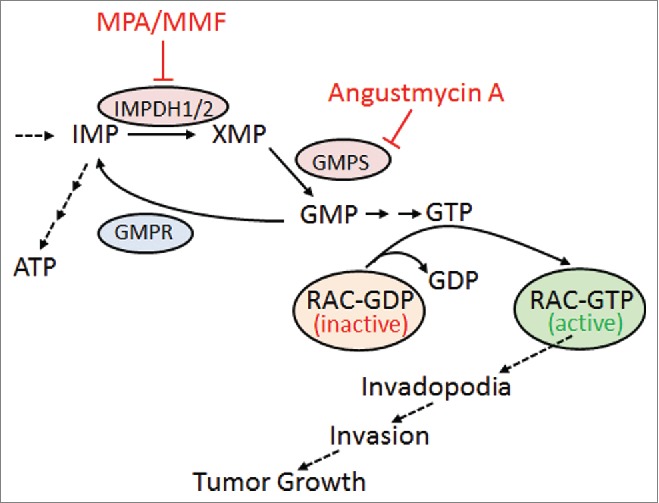
Inhibition of guanylate biosynthesis enzymes limits tumor invasion and growth. Simplified schematic of guanylate metabolic pathway and the proposed model. Enzymes are shown by ovals. Inhibitors of the pathway are indicated. ATP, adenosine triphosphate; GDP, guanosine diphosphate; GMP, guanosine monophosphate; GMPS, GMP synthase; GMPR, GMP reductase; GTP, guanosine triphosphate; IMP, inositol monophosphate; IMPDH, inositol monophosphate dehydrogenase; MMF, mycophenolate mofetil; MPA, mycophenolic acid; XMP, xanthosine monophosphate.
Notably, 2 recent studies from our group have highlighted a previously unrecognized role for guanylate metabolism enzymes in GTPase activation and tumor cell invasion. In our previous report, we established that guanosine monophosphate reductase (GMPR), a key enzyme in guanylate metabolism (Fig. 1), is a suppressor of melanoma metastasis,3 directly linking its activity to suppression of GTP levels and subsequent inhibition of RAC1 and formation of invadopodia. In our current work, we identify GMP synthase (GMPS), another key enzyme in guanylate metabolism and a functional antagonist of GMPR (Fig. 1), as a driver of melanoma invasion.1
Most intriguingly, although the variations in GTP levels detected upon manipulation of GMPR levels were relatively modest they translated into a disproportionate effect on the activation status of RAC1 (and to a lesser extent of RHOA and RHOC). Pharmacologic targeting of other key guanylate biosynthesis enzymes, including inosine monophosphate dehydrogenases (IMPDHs, Fig. 1) led to similar results.
These findings raise an obvious question: Why is GTPase activity affected if the intracellular concentration of GTP, albeit reduced, is still several fold higher than that required for saturation of these GTPases? One intriguing hypothesis is that, similar to ATP,6,7 GTP is not homogeneously distributed throughout the cell but rather fractionated in a gradient. Moreover, information about intracellular GTP levels is derived from methodologies that cannot detect local GTP changes in the cell, such as HPLC. Therefore, it is conceivable that spatiotemporal variation in GTP distribution throughout the cell may result in significant localized decreases in GTP concentration, ultimately affecting the activity of GTPases (as well as other GTP-binding proteins).
The importance of GTP pools for tumor progression is further supported by the finding that alteration of the levels of guanylate metabolism enzymes seems to be a widespread mechanism adopted by multiple types of cancer cell.3,8-10 Therefore, a more in-depth understanding of guanylate metabolism is likely to lead to the discovery of novel therapeutic targets and/or drugs. One such example is our current finding that Angustmycin A, a potent antibiotic produced by the fungus Streptomyces hygroscopius and a selective inhibitor of GMPS, reduces the invasion of metastatic melanoma cells in vitro and their growth as xenografts in vivo.1
Angustmycin A (also known as decoyinine) is a nucleoside that was first isolated in the mid-1950s. In one study, researchers evaluated the possible immunosuppressive properties of angustmycin A because of the convergence of its action with mycophenolic acid (MPA), an immunosuppressing agent that works via inhibition of IMPDH, the enzyme acting upstream of GMPS (Fig. 1). However, no significant activities were found. In a single follow-up study, analogs of angustmycin A showed minimal effects on cancer cell growth and, to the best of our knowledge, no further evaluations on its antitumor activities were performed until our current work. Interestingly, MPA (in the form of its salt mycophenolate mofetil, MMF) was reported to suppress growth of human tumor cell xenografts in mice.8,9 Our experiments demonstrated that angustmycin A possesses higher antimelanoma activity than MMF in in vivo settings. These findings underline the importance of guanylate metabolism enzymes for melanoma progression and identify a novel target for antimelanoma therapy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Bianchi-Smiraglia A, Wawrzyniak JA, Bagati A, Marvin EK, Ackroyd J, Moparthy S, Bshara W, Fink EE, Foley CE, Morozevich GE., et al. Pharmacological targeting of guanosine monophosphate synthase suppresses melanoma cell invasion and tumorigenicity. Cell Death Differ 2015; 22(11):1858-64; PMID:25909885; http://dx.doi.org/ 10.1038/cdd.2015.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mannava S, Grachtchouk V, Wheeler LJ, Im M, Zhuang D, Slavina EG, Mathews CK, Shewach DS, Nikiforov MA.. Direct role of nucleotide metabolism in C-MYC-dependent proliferation of melanoma cells. Cell Cycle 2008; 7:2392-400; PMID:18677108; http://dx.doi.org/ 10.4161/cc.6390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wawrzyniak JA, Bianchi-Smiraglia A, Bshara W, Mannava S, Ackroyd J, Bagati A, Omilian AR, Im M, Fedtsova N, Miecznikowski JC., et al. A purine nucloetide biosynthesis enzyme guanosine monophosphate reductase is a suppressor of melanoma invasion. Cell Rep 2013; 5:493-507; PMID:24139804; http://dx.doi.org/ 10.1016/j.celrep.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannava S, Moparthy KC, Wheeler LJ, Natarajan V, Zucker SN, Fink EE, Im M, Flanagan S, Burhans WC, Zeitouni NC., et al. Depletion of deoxyribonucleotide pools is an endogenous source of DNA damage in cells undergoing oncogene-induced senescence. Am J Pathol 2013; 182:142-51; PMID:23245831; http://dx.doi.org/ 10.1016/j.ajpath.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannava S, Moparthy KC, Wheeler LJ, Leonova KI, Wawrzyniak JA, Bianchi-Smiraglia A, Berman AE, Flanagan S, Shewach DS, Zeitouni NC., et al. Ribonucleotide reductase and thymidylate synthase or exogenous deoxyribonucleosides reduce DNA damage and senescence caused by C-MYC depletion. Aging 2012; 4: 917-22; PMID:23249808.19720993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imamura H., Nhat KP, Togawa H, Saito K, Iino R, Kato-Yamada Y, Nagai T, Noji H.. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci U S A 2009; 106:15651-6; PMID:19720993; http://dx.doi.org/ 10.1073/pnas.0904764106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Horssen R, Janssen E, Peters W, van de Pasch L, Lindert MM, van Dommelen MM, Linssen PC, Hagen TL, Fransen JA, Wieringa B.. Modulation of cell motility by spatial repositioning of enzymatic ATP/ADP exchange capacity. J Biol Chem 2009; 284:1620-7; PMID:19008233; http://dx.doi.org/ 10.1074/jbc.M806974200 [DOI] [PubMed] [Google Scholar]
- 8.Domhan S, Muschal S, Schwager C, Morath C, Wirkner U, Ansorge W, Maercker C, Zeier M, Huber PE, Abdollahi A.. Molecular mechanisms of the antiangiogenic and antitumor effects of mycophenolic acid. Mol Cancer Therap 2008; 7:1656-68; PMID:18566237; http://dx.doi.org/ 10.1158/1535-7163.MCT-08-0193 [DOI] [PubMed] [Google Scholar]
- 9.Dun B, Sharma A, Teng Y, Liu H, Purohit S, Xu H, Zeng L, She JX.. Mycophenolic acid inhibits migration and invasion of gastric cancer cells via multiple molecular pathways. PloS one 2013; 8:e81702; PMID:24260584; http://dx.doi.org/ 10.1371/journal.pone.0081702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arozarena I, Sanchez-Laorden B, Packer L, Hidalgo-Carcedo C, Hayward R, Viros A, Sahai E, Marais R.. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell 2011; 19:45-57; PMID:21215707; http://dx.doi.org/ 10.1016/j.ccr.2010.10.029 [DOI] [PubMed] [Google Scholar]


