Abstract
Purpose of review
We will review the symptom burden in cancer patients in the last days of life, its impact on nutrition and hydration, and the role of artificial nutrition and hydration in patients with days of life expectancy.
Recent findings
In the last days of life, cancer patients often experience progressive functional decline and worsening symptom burden. Many symptoms such as anorexia-cachexia, dysphagia and delirium could impair oral intake. These, coupled with refractory cachexia, contribute to persistent weight loss and decreased quality of life. Furthermore, the inability to eat/drink and body image changes can result in emotional distress for patients and caregivers. Clinicians caring for these individuals need to ensure longitudinal communication about goals of care, education about the natural process of dying, optimization of symptom management, and provide appropriate emotional support for patients and caregivers. There is a lack of evidence to support that artificial nutrition and hydration can improve outcomes in the last days of life. Artificial nutrition is not recommended because of its invasive nature, while artificial hydration may be considered on a case-by-case basis.
Summary
This review highlights the need to conduct further research on symptom burden, nutrition and hydration in the last days of life.
Keywords: death, symptom assessment, fluid therapy, neoplasms, nutritional support, palliative care
Introduction
Patients with advanced cancer often experience symptom distress throughout the disease trajectory starting from the time of diagnosis.[1] In the final weeks to months, many patients develop progressive functional decline and nutritional compromise associated with decreased ability and desire to eat/drink and weight loss.[2] In the last days of life, patients develop characteristic changes in their neurocognitive, neuromuscular, cardiovascular and respiratory function.[3,4]
There has been a paucity of studies on symptom prevalence in the last days of life.[5] This gap in knowledge is related to several unique challenges (Box 1). A better understanding of the symptom prevalence in the final days can help clinicians to educate patients and families what to expect, guide treatment recommendations, and identify opportunities for improvement in symptom management. In this review, we will provide a comprehensive literature review on symptom burden in cancer patients in the last days of life, its impact on nutrition and hydration, and the role of artificial nutrition and hydration for these patients.
Box 1. Methodological Issues related to Symptom Research in the Last Days of Life.
Patients often develop delirium in the last days of life, losing their ability to communicate and to report their symptom experience.[16,41] Few studies have examined patient-reported outcomes in the last days of life.[7,16] Surrogate assessment from healthcare professionals or caregivers was often used. However, clinicians often under-estimate symptom burden, while caregivers often overestimate symptom intensity.[42]
The timing of death is challenging to predict, making it difficult to know when prospective data collection should be initiated. A majority of studies have either retrospectively reviewed data after patient’s death, or started documenting when the patients were recognized as dying, which may introduce ascertainment bias and selection bias.[8–10,13,15]
- Significant variations in study populations, methods to assess symptoms, and reporting of symptom burden make it challenging to compare study findings or combine the data.
- Symptom prevalence vary widely by patient population (e.g. cancer vs. non-cancer) and settings (e.g. hospice vs. acute hospital vs. nursing homes). For instance, patients with chronic obstructive pulmonary disease (COPD) were much more likely to experience dyspnea at the end-of-life compared to patients with colon cancer.[10]
- Some studies examined symptom prevalence (absent/present), a few only reported the frequency of moderate to severe symptoms, and others only provide symptom intensity.
The lack of investigators, research infrastructure and funding likely further contributed to the limited research on the last days of life.[43]
Symptom Burden in the Last Days of Life
In a prospective study of 120 cancer patients in the Italian home care setting, Ventafridda et al. reported that uncontrollable symptoms requiring sedation appeared in 63 (53%) patients an average of 49 hours before death, and included dyspnea (28%), pain (26%), delirium (9%) and vomiting (4%).[6]
Fainsinger et al. conducted a retrospective study of 100 consecutive patients admitted to a palliative care unit for at least 6 days, and reported the symptom intensity of pain, nausea and drowsiness in the last 7 days of life. The symptoms were assessed using a 0–100 visual analogue scale, and was completed by patients whenever possible (otherwise relatives or nurses). Drowsiness increased progressively, while pain and nauseas improved close to death.[7]
Lichter et al. examined the symptom burden among 200 consecutive patients who died in a hospice program in New Zealand.[8] In this study, research nurses started documenting symptoms when patients were recognized as likely to die or after death if the death was unexpected. The most common symptoms in the last 48 hours of life were noisy and moist breathing (56%), pain (51%), restlessness and agitation (42%), incontinence of urine (32%), difficulty in swallowing (29%) and dyspnea (22%). Patients enrolled in hospice care generally had good symptom control, which may partly explain the relatively low symptom prevalence.
In a prospective study, Conill et al. reported the symptom prevalence in the last 7 days of life in 176 consecutive patients with advanced cancer who died at home, in the hospital or a hospice in Spain.[9] Eighteen symptoms were assessed by clinicians as absent or present on first consultation and then during the last week of life (72% of the assessments were within the last 3 days of life). The mean time interval between study visits was 6.5 weeks. A majority of symptoms increased in frequency in the second visit, with anorexia (80%), dry mouth (70%), confusion (68%), constipation (55%), dyspnea (47%), dysphagia (46%) and anxiety (46%) being particularly common in the last 7 days.
In the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment (SUPPORT) Study, 9105 patients with life-limiting illnesses (e.g. cirrhosis, COPD, acute respiratory failure, multiple organ system failure with sepsis, colon and non-small cell lung cancer, heart failure) in the United States were observed from study entry at initial hospital admission until death or for up to 6 months.[10] Symptom burden in the last 3 days of life was assessed based on after-death interview with the patient’s surrogate decision makers. Moderate to severe pain was reported in 34–45% of patients and dyspnea was reported in 28–83%. In this study, 80% had severe fatigue, 24–34% had confusion, 12% had nausea approximately 25% had moderate to severe anxiety or dysphoria. McCarthy et al. further reported the symptom trajectory among 316 patients with metastatic colon cancer and 747 patients with stage III/IV lung cancer in this study.[11] Pain (45%), confusion (28%), depression and anxiety increased steadily over the last 6 months of life, reaching a peak in the last 3 days of life.
Hall et al. examined the symptom prevalence in the last 48 hours of life among a convenience sample of 185 residents of long term care facilities in Canada.[12] Sudden unexpected deaths were excluded. 14 (8%) patients had cancer. The investigators reported that dyspnea, pain, noisy breathing, delirium, dysphagia, fever, myoclonus were documented in 62%, 44%, 39%, 29%, 28%, 24% and 16% of patients in the last 48 hours of life, respectively. Importantly, many symptoms may be under-estimated in this retrospective chart review. The symptoms were documented by nursing staff alone 45–81% of the time, by physician alone 2–9% of the time, and by both 2–17% of the time.
In an Italian study, Toscani et al. interviewed nurses within 72 hours of death to document the symptom in 370 patients who died in general hospital wards (25% had cancer).[13] 80 (22%) death were considered unexpected. The most common symptoms in the last day of life were reported to be fatigue (98%), drowsiness (78%), pain (75%), anorexia (73%), dyspnea (70%), incontinence (57%) and confusion (55%).
Veerback et al. asked nurses to provide their assessment of patients’ symptoms in the last 3 days of life within 1 week after death among patients who died in hospitals (n=11), nursing homes (n=102) and under home care (n=27).[14] The questions were based on the European Organization for Research and Treatment of Cancer Quality of Life Core Questionnaire (EORTC QLQ-C30). The investigators reported the average symptom intensity but not prevalence, with fatigue (61–73/100), lack of appetite (42–77/100), dyspnea (41–92/100), pain (38–58/100) and difficulty concentrating (49–58/100) being the most significant concerns.
In a retrospective study conducted in Korea, Hwang et al. reported the occurrence of 17 signs and symptoms as documented by the clinical team in 181 patients with advanced cancer who died in a palliative care unit and had medical records available for the final week.[15] The symptoms confusion/delirium, pain, dyspnea at rest, increasing sleep, general weakness, anxiety/depression, abdominal discomfort, nausea/vomiting and poor appetite (finishing less than 1/3 of routine meal) were reported in 33%, 24%, 21%, 12%, 6%, 6%, 6%, and 5% of patients in the last 48 h of life, respectively. The low frequency of symptoms relative to other studies may be related to the patient population, how the symptoms were defined, and the documentation process.
The Investigating the Process of Dying study is a prospective, longitudinal observational study that examined the symptom expression in the last week of life.[16] It included 357 consecutive patients with advanced cancer from two acute palliative care units, in which those who were able to communicate rated the average intensity of 10 symptoms over the past 24 hours daily from admission until discharge or death using the Edmonton Symptom Assessment Scale. The symptom intensity was shown from death backwards, revealing that anorexia, drowsiness, well being, fatigue, shortness of breath and insomnia increased significantly in the final days of life (Table 1). In the same study, the presence or absence of 15 other symptoms that could be observed were assessed by bedside nurses every 12 hours from admission to discharge (regardless of communication capacity). Dysphagia to solids, dysphagia to liquids, urinary incontinence and constipation had the highest frequency and continued to worsen in the last days of life in the last days (Table 2).
Table 1.
Edmonton Symptom Assessment Scale Intensity >=4/10 in the Last 7 Days of Life among Cancer Patients who Died in Acute Palliative Care Units[16]
| Symptoms | Days before death (%) | ||||||
|---|---|---|---|---|---|---|---|
| −7 | −6 | −5 | −4 | −3 | −2 | −1 | |
| Appetite | 70 | 74 | 80 | 76 | 72 | 76 | 80 |
| Drowsiness | 65 | 69 | 68 | 73 | 61 | 75 | 74 |
| Well being | 70 | 77 | 68 | 76 | 73 | 81 | 77 |
| Fatigue | 59 | 56 | 60 | 68 | 74 | 79 | 75 |
| Shortness of Breath | 42 | 51 | 45 | 46 | 49 | 55 | 65 |
| Sleep | 61 | 44 | 49 | 57 | 63 | 65 | 62 |
| Anxiety | 54 | 56 | 58 | 61 | 64 | 63 | 66 |
| Depression | 54 | 59 | 44 | 46 | 41 | 47 | 47 |
| Pain | 45 | 49 | 50 | 43 | 44 | 45 | 44 |
| Nausea | 27 | 21 | 26 | 20 | 16 | 23 | 11 |
Table 2.
Symptom Prevalence in the Last 7 Days of Life According to Nursing Assessment among Cancer Patients who Died in Acute Palliative Care Units [16]
| Symptoms | Days before death (%) | ||||||
|---|---|---|---|---|---|---|---|
| −7 | −6 | −5 | −4 | −3 | −2 | −1 | |
| Urinary incontinence | 31 | 45 | 39 | 41 | 48 | 39 | 54 |
| Dysphagia to solids | 31 | 34 | 42 | 32 | 36 | 38 | 49 |
| Dysphagia to liquids | 23 | 30 | 33 | 25 | 24 | 35 | 41 |
| Constipation | 32 | 29 | 45 | 45 | 42 | 36 | 31 |
| Fecal incontinence | 11 | 22 | 17 | 14 | 21 | 18 | 22 |
| Cough | 18 | 22 | 19 | 18 | 22 | 16 | 27 |
| Sweating | 14 | 12 | 8 | 7 | 15 | 15 | 18 |
| Myoclonus | 4 | 6 | 8 | 8 | 8 | 11 | 11 |
| Urinary incontinence | 31 | 45 | 39 | 41 | 48 | 39 | 54 |
| Dysphagia to solids | 31 | 34 | 42 | 32 | 36 | 38 | 49 |
Impact of Symptoms on Nutrition and Hydration in the Last Days of Life
There are two major drivers of weight loss in the last days of life: starvation and refractory cachexia. As shown in Table 3, patients experience significant symptom burden in the last days of life. Symptoms such as anorexia, dysphagia, weakness and confusion often worsen as death approaches. These symptoms can have a major impact on nutrition and hydration (Figure 1), contributing either directly or indirectly to reduced oral intake, dehydration, weight loss and decreased quality of life.[17,18] Moreover, patients in the last days of life often have refractory cachexia with inflammatory response, hypermetabolism and an overall catabolic state, resulting in persistent weight loss and functional decline even if the starvation component can be addressed.[19]
Table 3.
Symptom Prevalence in the Final Days of Life
| Study | Lichter et al. 1990[8] | Conill et al. 1997[9] | Freeborne et al. 2000[10] | Hall et al. 2002[12] | Toscani et al. 2005[13] | Hwang et al. 2012[15] | Hui et al. 2015[16] |
|---|---|---|---|---|---|---|---|
| Design | Prospective/retrospective | Prospective | Retrospective | Retrospective | Retrospective | Retrospective | Prospective |
| Population | 200 deaths | 176 consecutive patients with advanced cancer | 2598 enrolled patients (41% cancer) | 185 convenience sample (14% cancer) | 370 patients (25% cancer) | 181 patients with advanced cancer | 357 consecutive patients with advanced cancer |
| Setting (place of death) | Hospice (home or inpatient) | Home, hospitals, hospices | Home, hospitals | Long term care | Hospitals | Palliative care unit | Palliative care units |
| Symptom assessment | Research nurses’ impression when patients were likely to die or after death | Clinicians’ impression in the last week of life | Caregiver recall after death | Chart documentation | Nurse recall after death | Chart documentation | Edmonton symptom assessment scale among patients who were able to communicate, nursing impression |
| Time frame | Last 2 d | Last 7 d | Last 3 d | Last 2 d | Last 3 d | Last 2 d | Last 3 d serially |
| Fatigue | NR | 82% | 80% | NR | 93% | NR | 60–78%† |
| Pain | 51% | 30% | 34–45%* | 44% | 75% | 24% | 38–50%† |
| Anorexia | NR | 80% | NR | NR | 73% | 5% | 74–83%† |
| Dyspnea | 22% | 47% | 28–83%* | 62% | 70% | 21% | 48–73%† |
| Confusion | 9% | 68% | 24–34%* | 29% | 55% | 33% | NR |
| Dysphagia | 29% | 46% | NR | 28% | 49% | NR | 24–53%‡ |
| Death rattle | 56% | NR | NR | 39% | 45% | NR | 11–39%‡ |
| Myoclonus | 12% | NR | NR | 16% | NR | NR | 8–11%‡ |
| Incontinence | 32% | NR | NR | NR | 57% | NR | 39–65%‡ |
| Anxiety | NR | 46% | 25% | NR | NR | 6% | 49–57%† |
Abbreviations: NR, not reported
Patients with advanced lung and colon cancer
Frequency of symptoms with intensity of >=4/10 as reported by patients in the last 3 days of life
Frequency of symptoms with as observed by nurses in the last 3 days of life
Figure 1. Contributors to Decreased Oral Intake in the Last Days of Life.
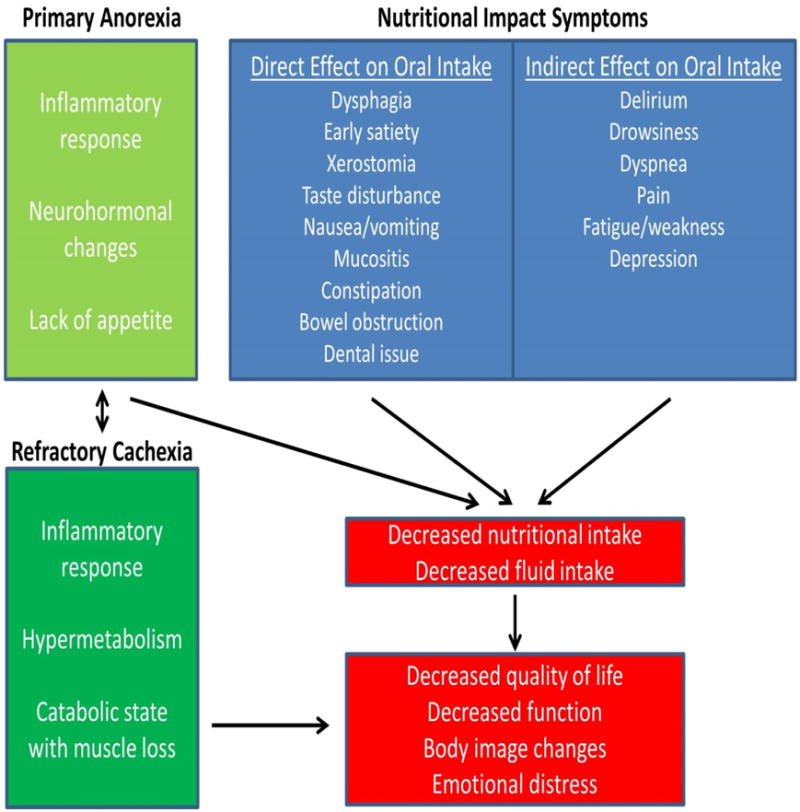
In the final days, cancer patients often have limited desire to eat/drink (anorexia). This, coupled with refractory cachexia and an array of nutritional impact symptoms, contribute to persistent weight loss and functional decline.
It is important for clinicians to recognize that a patient has reached the final phase of life, that many symptoms and/or acute complications may not be reversible even with aggressive interventions, and to communicate realistic expectations with patients and families.[16,20] It often takes weeks for weight to improve even when patients present at earlier stages of cancer cachexia.[21] Thus, patients with refractory cancer and only days of survival are unlikely to benefit from nutritional/anti-cachexia interventions. Anorexia-cachexia is associated with a shorter life expectancy;[22] however, it is unlikely that treatment of this syndrome, even if successful, would have any meaningful impact on survival because of the progressive cancer. In the following sections, we shall discuss the role of artificial nutrition and hydration in the last days of life, and a practical approach to patient care taking into account the individual’s prognosis.
The Role of Artificial Nutrition in the Last Days of Life
Is there evidence to support artificial nutrition in patients with days of life expectancy? A Cochrane systematic review on artificial nutrition for palliative care patients identified no randomized controlled trials on this topic and only 5 prospective non-controlled studies.[23] Importantly, the survival of patients in all 5 studies was in terms of months instead of days. They concluded that there was insufficient evidence to make recommendations regarding artificial nutrition at the end-of-life. Another systematic review also highlighted the paucity of evidence to support that artificial nutrition can improve survival or quality of life.[24]
While there is a lack of research to support benefits of artificial nutrition, it is important to note that enteral and parenteral nutrition are invasive medical interventions that can cause significant complications. Adverse events associated with enteral feeding include pain and bleeding at the insertion site, tube blockage, diarrhea, constipation, aspiration, electrolyte deficiencies, hyperglycemia, refeeding syndrome and tube feeding syndrome. Parenteral nutrition is also associated with many complications such as sepsis, hypoglycemia, hyperglycemia, hepatic dysfunction, electrolyte abnormalities, volume overload, and cholecystitis. Other concerns related to artificial nutrition include the requirement for frequent monitoring and investigations and cost.
The 2006 European Society for Clinical Nutrition and Metabolism (ESPEN) guideline recommended against provision of enteral nutrition to patients with incurable cancer who were dying.[25] A separate ESPEN Guideline suggested that parenteral nutrition should only be considered in patients with advanced cancer if life expectancy is at least 2–3 months, enteral nutrition is insufficient, parenteral nutrition is expected to stabilize or improve performance status and quality of life and patient desires this mode of nutritional support.[26] The prognostic criterion is based on the observation that healthy individual may live for 2–3 months without macronutrients.[27] Because cancer patients with shorter survival have competing risk factors for mortality, their survival is unlikely going to be improved even if reversal of starvation was successful.
The Role of Artificial Hydration in the Last Days of Life
Artificial hydration may be given enterally, subcutaneously, intravenously or rectally. Unlike artificial nutrition that is generally not recommended in the last days of life, the decision to hydrate dying patients or not has been a topic of heated debate.[28] Proponents of artificial hydration stated that it is a basic human right (i.e. food),[29] that hydration may alleviate thirst and other symptoms, reduce complications without significantly prolonging the dying process and build rapport with family caregivers who often demand interventions. Opponents of artificial hydration believe that hydration may increase the risk of complications (e.g. edema, ascites and pleural effusion), may introduce burden associated with need to urinate, does not improve quality of life, is not required in dying patients who do not complain of thirst, and may even interfere with the natural process of dying. Some healthcare professionals view terminal dehydration could even lessen the burden of suffering.[30] Indeed, there is wide variation in the prescription of artificial hydration among clinicians, which is driven by their beliefs toward the clinical/emotional benefits of hydration.[31]
The literature on artificial hydration in the last days of life is mixed, although no study has provided definitive evidence to support its use. In a before-and-after comparison, Bruera et al. suggested that hydration coupled with opioid rotation may reduce the incidence of agitated delirium.[32] Morita et al. was not able to confirm this benefit in a study utilizing a similar design.[33] Krishna et al. conducted a retrospective cohort study consisting of 238 patients and reported that artificial hydration in the last 48 hours of life did not improve symptoms or survival.[34] In a double-blind, randomized controlled trial, Bruera et al. randomized 51 cancer patients to receive either 1 L of normal saline per day or 100 mL per day by subcutaneous infusion. By day 2, patients experienced a significant improvement in dehydration symptoms (sum of hallucinations, myoclonus, fatigue and sedation).[35] However, a larger study that included 129 patients with longer duration of hydration revealed no difference in the same primary outcome by day 7 nor any secondary outcomes (symptoms, delirium, quality of life, creatinine, urea or survival).[36] Because both studies excluded patients with severe dehydration and only provided hydration with 1 L of normal saline, future studies should examine if hydration could be beneficial for selected patients (e.g. severe hydration symptoms) or if given in a different manner (e.g. fluid type, fluid volume).
Approach to Hydration and Nutrition in the Last Days of Life
Patients and caregivers often perceive artificial nutrition and hydration to be beneficial, while health professionals are concerned about their side effects.[37,38] As Koretz nicely stated, patients and families often worry about “starving to death” and frequently get confused “between dying in a malnourished state and dying as a direct consequence of nutrient deprivation; cancer patients fit into the former category.”[39] An interdisciplinary team consisting of physicians, nurses, psychologists, speech pathologists, physical/occupational therapists and dieticians can provide appropriate education, gentle reassurance, emotional support and nutritional guidance for patients in the last days and their families:
Clinicians should have honest discussions with patients and families about prognosis and goals of care.
In the last days of life, patients often have symptoms that prevent them to eat/drink properly (e.g. dysphagia) and do not desire eating. Patients should not feel guilty about not being able to eat/drink or be force fed.
If desired, patients may try eating and/or drinking small amounts orally as tolerated, with the goal of maximizing comfort while balancing the risk of complications (e.g. aspiration). A small randomized controlled trial found that some patients could ingest nutritional supplements even in the final days of life.[40]
Active measures may be undertaken to treat any potential nutritional impact symptoms and/or complications (Figure 1), providing that they are consistent with the patients’ goals of care. For example, pain control should be optimized and oral care should be provided regularly.
The inability to eat/drink and body image issues can be associated with significant emotional distress among patients and caregivers. It is important to normalize their reaction, and provide longitudinal education and counseling.
For patients in the last days of life, artificial nutrition is not recommended because it has no benefit and may cause harm (Table 4). There is no ethical distinction between withholding and withdrawing artificial nutrition.
Although there is also no definitive evidence to support that artificial hydration offer any benefits in this patient population, the adverse effects associated with hydration are generally limited. Thus, artificial hydration may be considered in selected patients after careful discussion of the risk, benefits and goals of care.
Table 4.
Prognosis-Based Decision Making Regarding Artificial Nutrition
| Nutritional State | Life expectancy: months or longer (active cancer treatments considered; pre-cachexia/cachexia state) | Life expectancy: days to weeks (progressive cancer with no standard treatment options; refractory cachexia) |
|---|---|---|
| Reduced oral intake and normal absorption | Continue with oral intake, consider nutritional supplements | Continue with oral intake, consider nutritional supplements |
| Significantly compromised oral intake (e.g. dysphagia, severe mucositis) and normal absorption | Consider enteral nutrition | Conservative measures Consider parenteral hydration Artificial nutrition not recommended |
| Significantly compromised absorption (e.g. bowel obstruction) or failure of enteral nutrition | Consider parenteral nutrition | Conservative measures Consider parenteral hydration Artificial nutrition not recommended |
Conclusion
In the last days of life, cancer patients often experience progressive functional decline and worsening symptom burden. Many symptoms such as anorexia, dysphagia and delirium could impair oral intake. These, coupled with refractory cachexia, contribute to persistent weight loss and decreased quality of life. Furthermore, the inability to eat and drink and body image changes may contribute to significant emotional distress among patients and caregivers. Clinicians caring for patients in the last days of life need to ensure impeccable communication about goals of care, education about the natural process of dying, optimization of symptom management balancing the risks, benefits and limited prognosis and provision of emotional support. There is currently a lack of evidence to support that artificial nutrition and hydration improve outcomes in patients in the last days of life; artificial nutrition is generally not recommended because of its invasive nature, while artificial hydration may be considered on a case-by-case basis.
Key points.
In the last days of life, cancer patients often experience progressive functional decline and worsening symptom burden. They often have decreased ability and desire to eat/drink.
If desired, patients may try eating and/or drinking small amounts orally as tolerated, with the goal of maximizing comfort while balancing the risk of complications (e.g. aspiration).
The use of artificial nutrition in the last days of life is not recommended because of the lack of confirmed benefit and likelihood of adverse effects.
A recent large randomized controlled trial of parenteral hydration found no benefit compared to control when given to patients with only weeks of life expectancy; however, parenteral hydration is generally well tolerated, and may be considered on a case-by-case basis.
The inability to eat and drink and body image changes at the end-of-life can be associated with significant distress. Education and counseling for patients and families are essential.
Acknowledgments
None
Financial support and sponsorship
David Hui is supported in part by a National Institutes of Health grant (R21CA186000-01A1) and an American Cancer Society Mentored Research Scholar Grant in Applied and Clinical Research (MRSG-14-1418-01-CCE).
Footnotes
Conflicts of interest
The author reports no relevant conflict of interest.
References
- 1.Seow H, Barbera L, Sutradhar R, et al. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J Clin Oncol. 2011;29:1151–1158. doi: 10.1200/JCO.2010.30.7173. [DOI] [PubMed] [Google Scholar]
- 2.Van der Riet P, Brooks D, Ashby M. Nutrition and hydration at the end of life: Pilot study of a palliative care experience. J Law Med. 2006;14:182–198. [PubMed] [Google Scholar]
- 3.Hui D, Dos Santos R, Chisholm G, et al. Bedside clinical signs associated with impending death in patients with advanced cancer: Preliminary findings. Cancer. 2015;121:960–967. doi: 10.1002/cncr.29048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui D, Dos Santos R, Chisholm G, et al. Clinical signs of impending death in cancer patients. Oncologist. 2014;19:681–687. doi: 10.1634/theoncologist.2013-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui D, Parsons HA, Damani S, et al. Quantity, design, and scope of the palliative oncology literature. The Oncologist. 2011;16:694–703. doi: 10.1634/theoncologist.2010-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ventafridda V, Ripamonti C, De Conno F, et al. Symptom prevalence and control during cancer patients’ last days of life. J Palliat Care. 1990;6:7–11. [PubMed] [Google Scholar]
- 7.Fainsinger R, Miller MJ, Bruera E, et al. Symptom control during the last week of life on a palliative care unit. J Palliat Care. 1991;7:5–11. [PubMed] [Google Scholar]
- 8.Lichter I, Hunt E. The last 48 hours of life. J Palliat Care. 1990;6:7–15. [PubMed] [Google Scholar]
- 9.Conill C, Verger E, Henriquez I, et al. Symptom prevalence in the last week of life. J Pain Symptom Manage. 1997;14:328–331. doi: 10.1016/s0885-3924(97)00263-7. [DOI] [PubMed] [Google Scholar]
- 10.Freeborne N, Lynn J, Desbiens NA. Insights about dying from the support project. The study to understand prognoses and preferences for outcomes and risks of treatments. J Am Geriatr Soc. 2000;48:S199–205. [PubMed] [Google Scholar]
- 11.McCarthy EP, Phillips RS, Zhong Z, et al. Dying with cancer: Patients’ function, symptoms, and care preferences as death approaches. J Am Geriatr Soc. 2000;48:S110–121. doi: 10.1111/j.1532-5415.2000.tb03120.x. [DOI] [PubMed] [Google Scholar]
- 12.Hall P, Schroder C, Weaver L. The last 48 hours of life in long-term care: A focused chart audit. J Am Geriatr Soc. 2002;50:501–506. doi: 10.1046/j.1532-5415.2002.50117.x. [DOI] [PubMed] [Google Scholar]
- 13.Toscani F, Di Giulio P, Brunelli C, et al. How people die in hospital general wards: A descriptive study. J Pain Symptom Manage. 2005;30:33–40. doi: 10.1016/j.jpainsymman.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Veerbeek L, van Zuylen L, Swart SJ, et al. The last 3 days of life in three different care settings in the netherlands. Support Care Cancer. 2007;15:1117–1123. doi: 10.1007/s00520-006-0211-x. [DOI] [PubMed] [Google Scholar]
- 15.Hwang IC, Ahn HY, Park SM, et al. Clinical changes in terminally ill cancer patients and death within 48 h: When should we refer patients to a separate room? Support Care Cancer. 2012;21:835–840. doi: 10.1007/s00520-012-1587-4. [DOI] [PubMed] [Google Scholar]
- 16**.Hui D, Dos Santos R, Chisholm G, et al. Symptom expression in the last 7 days of life among cancer patients admitted to acute palliative care units. J Pain Symptom Manage. 2015 doi: 10.1016/j.jpainsymman.2014.09.003. (in press). This study serially documents the frequency of multiple symptoms in patients with advanced cancer in the last 7 days of life This is only of the first studies that uses patient-reported outcomes instead of surrogate reporting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Del Fabbro E, Hui D, Dalal S, et al. Clinical outcomes and contributors to weight loss in a cancer cachexia clinic. J Palliat Med. 2011;14:1004–1008. doi: 10.1089/jpm.2011.0098. This research outlines many nutritional impact symptoms that could contribute to weight loss in the advanced cancer setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laviano A, Meguid MM, Rossi-Fanelli F. Cancer anorexia: Clinical implications, pathogenesis, and therapeutic strategies. Lancet Oncol. 2003;4:686–694. doi: 10.1016/s1470-2045(03)01247-6. [DOI] [PubMed] [Google Scholar]
- 19*.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. This Delphi consensus outlines the different stages of cachexia: pre-cachexia, cachexia and refactory cachexia. [DOI] [PubMed] [Google Scholar]
- 20.Hui D, dos Santos R, Reddy S, et al. Palliat Med. 2015 Acute symptomatic complications among patients with advanced cancer admitted to acute palliative care units: A prospective observational study. (in press) [Google Scholar]
- 21.Garcia JM, Boccia RV, Graham CD, et al. Anamorelin for patients with cancer cachexia: An integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncol. 2015;16:108–116. doi: 10.1016/S1470-2045(14)71154-4. [DOI] [PubMed] [Google Scholar]
- 22.Mercadante S, Valle A, Porzio G, et al. Prognostic factors of survival in patients with advanced cancer admitted to home care. J Pain Symptom Manage. 2013;45:56–62. doi: 10.1016/j.jpainsymman.2011.12.288. [DOI] [PubMed] [Google Scholar]
- 23*.Good P, Richard R, Syrmis W, et al. Medically assisted nutrition for adult palliative care patients. Cochrane Database Syst Rev. 2014;4:CD006274. doi: 10.1002/14651858.CD006274.pub3. This systematic review highlighted the lack of evidence to support artificial nutriton for patients in the palliative care setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Rio MI, Shand B, Bonati P, et al. Hydration and nutrition at the end of life: A systematic review of emotional impact, perceptions, and decision-making among patients, family, and health care staff. Psychooncology. 2012;21:913–921. doi: 10.1002/pon.2099. [DOI] [PubMed] [Google Scholar]
- 25.Arends J, Bodoky G, Bozzetti F, et al. Espen guidelines on enteral nutrition: Non-surgical oncology. Clin Nutr. 2006;25:245–259. doi: 10.1016/j.clnu.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Bozzetti F, Arends J, Lundholm K, et al. Espen guidelines on parenteral nutrition: Non-surgical oncology. Clin Nutr. 2009;28:445–454. doi: 10.1016/j.clnu.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Chalela JA, Lopez JI. Medical management of hunger strikers. Nutr Clin Pract. 2013;28:128–135. doi: 10.1177/0884533612462896. [DOI] [PubMed] [Google Scholar]
- 28.Dev R, Dalal S, Bruera E. Is there a role for parenteral nutrition or hydration at the end of life? Curr Opin Support Palliat Care. 2012;6:365–370. doi: 10.1097/SPC.0b013e328356ab4a. [DOI] [PubMed] [Google Scholar]
- 29.Torres-Vigil I, Cohen MZ, de la Rosa A, et al. Food or medicine: Ethnic variations in perceptions of advanced cancer patients and their caregivers regarding artificial hydration during the last weeks of life. BMJ supportive & palliative care. 2012;2:276–279. doi: 10.1136/bmjspcare-2012-000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Riet P, Good P, Higgins I, et al. Palliative care professionals’ perceptions of nutrition and hydration at the end of life. Int J Palliat Nurs. 2008;14:145–151. doi: 10.12968/ijpn.2008.14.3.28895. [DOI] [PubMed] [Google Scholar]
- 31.Torres-Vigil I, Mendoza TR, Alonso-Babarro A, et al. Practice patterns and perceptions about parenteral hydration in the last weeks of life: A survey of palliative care physicians in latin america. J Pain Symptom Manage. 2012;43:47–58. doi: 10.1016/j.jpainsymman.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruera E, Franco JJ, Maltoni M, et al. Changing pattern of agitated impaired mental status in patients with advanced cancer: Association with cognitive monitoring, hydration, and opioid rotation. J Pain Symptom Manage. 1995;10:287–291. doi: 10.1016/0885-3924(95)00005-J. [DOI] [PubMed] [Google Scholar]
- 33.Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003;6:557–563. doi: 10.1089/109662103768253669. [DOI] [PubMed] [Google Scholar]
- 34.Krishna LK, Poulose JV, Goh C. Artificial hydration at the end of life in an oncology ward in singapore. Indian journal of palliative care. 2010;16:168–173. doi: 10.4103/0973-1075.73668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruera E, Sala R, Rico MA, et al. Effects of parenteral hydration in terminally ill cancer patients: A preliminary study. J Clin Oncol. 2005;23:2366–2371. doi: 10.1200/JCO.2005.04.069. [DOI] [PubMed] [Google Scholar]
- 36**.Bruera E, Hui D, Dalal S, et al. Parenteral hydration in patients with advanced cancer: A multicenter, double-blind, placebo-controlled randomized trial. J Clin Oncol. 2013;31:111–118. doi: 10.1200/JCO.2012.44.6518. This well-desgined large randomized controlled trial revealed that parenteral hydration offers no clinical benefit for patients under hospice care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malia C, Bennett MI. What influences patients’ decisions on artificial hydration at the end of life? A q-methodology study J Pain Symptom Manage. 2011;42:192–201. doi: 10.1016/j.jpainsymman.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 38.Raijmakers NJ, Fradsham S, van Zuylen L, et al. Variation in attitudes towards artificial hydration at the end of life: A systematic literature review. Curr Opin Support Palliat Care. 2011;5:265–272. doi: 10.1097/SPC.0b013e3283492ae0. [DOI] [PubMed] [Google Scholar]
- 39.Koretz RL. Should patients with cancer be offered nutritional support: Does the benefit outweigh the burden? Eur J Gastroenterol Hepatol. 2007;19:379–382. doi: 10.1097/MEG.0b013e3280bdc093. [DOI] [PubMed] [Google Scholar]
- 40.Ishiki H, Iwase S, Gyoda Y, et al. Oral nutritional support can shorten the duration of parenteral hydration in end-of-life cancer patients: A randomized controlled trial. Nutr Cancer. 2015;67:105–111. doi: 10.1080/01635581.2015.976312. [DOI] [PubMed] [Google Scholar]
- 41.Morita T, Tei Y, Inoue S. Impaired communication capacity and agitated delirium in the final week of terminally ill cancer patients: Prevalence and identification of research focus. J Pain Symptom Manage. 2003;26:827–834. doi: 10.1016/s0885-3924(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 42.Hui D, Morgado M, Vidal M, et al. Dyspnea in hospitalized advanced cancer patients: Subjective and physiologic correlates. J Palliat Med. 2013;16:274–280. doi: 10.1089/jpm.2012.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui D, Reddy A, Parsons HA, et al. Reporting of funding sources and conflict of interest in the supportive and palliative oncology literature. J Pain Symptom Manage. 2012;44:421–430. doi: 10.1016/j.jpainsymman.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


