Abstract
Alzheimer’s disease (AD) is a progressive neurological disorder that impairs memory and other cognitive functions in the elderly. The social and financial impacts of AD are overwhelming and are escalating exponentially as a result of population aging. Therefore, identifying AD-related risk factors and the development of more efficacious therapeutic approaches are critical to cure this neurological disorder. Current epidemiological evidence indicates that life experiences, including chronic stress, are a risk for AD. However, it is unknown if short-term stress, lasting for hours, influences the onset or progression of AD. Here, we determined the effect of short-term, multi-modal ‘modern life-like’ stress on AD pathogenesis and synaptic plasticity in mice bearing three AD mutations (the 3xTg-AD mouse model). We found that combined emotional and physical stress lasting 5 h severely impaired memory in wild-type mice and tended to impact it in already low-performing 3xTg-AD mice. This stress reduced the number of synapse-bearing dendritic spines in 3xTg-AD mice and increased Aβ levels by augmenting AβPP processing. Thus, short-term stress simulating modern-life conditions may exacerbate cognitive deficits in preclinical AD by accelerating amyloid pathology and reducing synapse numbers.
Keywords: Alzheimer’s disease, amyloid, dementia, dendritic spines, hippocampus, stress
Alzheimer’s disease (AD) is the most significant cause of dementia among elderly populations, accounting for 60–80% of cases. The afflicted brain contains several pathological hallmarks including extracellular Aβ-plaques, intraneuronal neurofibrillary tau tangles and extensive neuronal and synaptic loss (Galimberti et al. 2006). AD is categorized into early onset or familial AD and late onset or sporadic AD (sAD). Familial AD represents a small proportion of AD cases (~2%), and is inheritable in an autosomal dominant manner due to mutations in one of three genes [amyloid-β protein precursor (AβPP); presenilin-1, PS1; and presenilin-2, PS2]. However, the etiology underlying sporadic AD (sAD), which accounts for more than 98% of AD cases, is complex and multi-factorial (Galimberti et al. 2006). Despite current intensive research, the mechanisms modulating the pathogenesis of sAD are poorly defined and effective treatments have yet to be identified. Notably, epidemiological studies reveal that adverse lifestyle factors including stress play a key role in modulating AD progression (Pardon 2011; Zverova et al. 2013). In fact, hypothalamic-pituitary-adrenal axis dysfunction as well as elevated levels of cortisol in plasma and cerebrospinal fluid (CSF) are found in AD patients (Umegaki et al. 2000; Csernansky et al. 2006; Hoogendijk et al. 2006; Huang et al. 2009; Brureau et al. 2013). Furthermore, recent studies in animal models have found that stress and stress hormones, including glucocorticoids (GCs; cortisol in humans and corticosterone in rodents) and corticotrophin-releasing hormone (CRH) play a crucial role in AD pathogenesis by modulating Aβ production and degradation and stimulating tau pathology (Kulstad et al. 2005; Green et al. 2006; Jeong et al. 2006; Kang et al. 2007; Rissman et al. 2007, 2012; Dong et al. 2008, 2012; Sotiropoulos et al. 2008, 2011; Catania et al. 2009; Li et al. 2010; Cuadrado-Tejedor et al. 2012; Filipcik et al. 2012; Rothman et al. 2012). In addition, using a glucocorticoid receptor antagonist strategy, we have reduced Aβ and tau levels and restored cognitive performance in 3xTg-AD mice (Baglietto-Vargas et al. 2013). Together, these findings suggest that stress and several stress mediators, play key roles in modulating AD pathogenesis.
The effects of stress on cognitive function have been extensively studied (McEwen 2007; Arnsten 2009; Maras and Baram 2012; Schwabe et al. 2012). Indeed, several lines of evidence support the importance of stress duration on cognitive function (McEwen 2007; Arnsten 2009; Maras and Baram 2012; Schwabe et al. 2012). Acute stress, lasting seconds to minutes, enhances learning and memory; however, chronic stress, lasting weeks, generally impairs these processes (Bullitt 1990; Chan et al. 1993; Cullinan et al. 1995; Emmert and Herman 1999; Chowdhury et al. 2000; Jankord and Herman 2008; Schwabe et al. 2012). In addition, specific modalities of stress (physical or psychological) have distinct effects on cognitive function (Cullinan et al. 1995; Emmert and Herman 1999; Dayas et al. 2001; Watts and Sanchez-Watts 2002; Day et al. 2004). This matter is extremely important, because modern-life stress often involves multiple concurrent psychological, social, and physical stresses (Maras et al. 2014). Given that modern-life stressful experiences are not unitary or discrete, it is fundamental to elucidate the effect of multiple concurrent stresses on the onset and progress of AD pathogenesis.
Here, we investigate the impact of short-term, multi-modal modern-life like stress on AD progression and its implication in synaptic plasticity and cognitive function. We found that short-term multimodal stress lasting for 5 h significantly reduced the number of the spines in 3xTg-AD mice compared to non-transgenic (Ntg) mice. In addition, short-term, multimodal stress increased Aβ-oligomers by modulation of amyloid-β protein precursor (AβPP) processing via upregulation of beta-site amyloid precursor protein-cleaving enzyme 1 (BACE-1) steady state levels without altering Aβ degradation. Overall, our data suggest that short-term stress recapitulating salient features of modern-life conditions promotes synaptic and memory loss and accelerates AD pathogenesis.
Material and methods
Transgenic mice
Here, 5–6 months old homozygous 3xTg-AD and Non-transgenic (Ntg), 10–12 mice per group (males) were used. All mice (Ntg and 3xTg-AD) had the same genetic background (hybrid 129/C57BL6 background). The characterization of 3xTg-AD mice has been described previously (Oddo et al. 2003). Briefly, two independent transgenes encoding human APPSwe and the human tauP301L (both under control of the mouse Thy1.2 regulatory element) were co-microinjected into single-cell embryos harvested from homozygous mutant PS1M146V knockin (PS1-KI) mice (Oddo et al. 2003). All animal procedures were performed in accordance with National Institutes of Health and University of California guidelines and Use Committee at the University of California, Irvine.
Stress paradigm
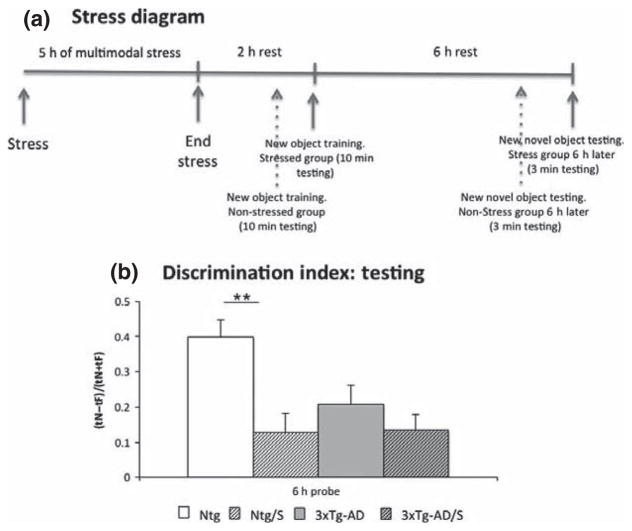
A multimodal short-term stress paradigm was employed in the current study (Chen et al. 2008, 2010; Maras et al. 2014). Briefly, Ntg and 3xTg-AD mice were restrained singly in 50-mL tubes (Corning Incorporated, Corning, NY, USA) that were ventilated and allowed urine exit. Mice were placed 5 per cage on a rapid laboratory shaker in a brightly lit room accompanied by a loud noise generated by a random noise-generator (dB level < 80; frequency 80–300 Hz) for 5 h (Chen et al. 2008, 2010; Maras et al. 2014). Following the stress, mice were returned to their home cages. Control animals from both genotypes were kept in the housing room without manipulation. Afterwards, all four groups of mice (Ntg, Ntg-stress, 3xTg-AD and 3xTg-AD-stress) were subjected to a novel object recognition task, and subsequently killed. (Fig. 1a).
Fig. 1.
Multimodal acute stress impairs cognition. (a) Significant impairment in memory evaluated by novel object recognition (a) and novel/familiar Ratio was observed in Ntg stressed compared to Ntg non-stressed mice. No differences were observed between stressed and non-stressed 3xTg-Alzheimer’s disease (AD) mice. The values represent the mean ± SEM. (N = 10–12 per group). **p < 0.01.
Novel object
Before testing, each mouse was habituated to an empty Plexiglas arena (45 × 25 × 20 cm) for 6 consecutive days. The lighting intensity in each behavioral task was measured at 44 lux. The arena and the stimulus objects were cleaned thoroughly between trials and ensure the absence of olfactory cues. If an animal did not explore both objects during the training phase for any behavioral task, the test was not scored. During the scoring procedure, because some mice exhibited freezing or fearful behavior on introduction to the chamber, scoring did not start until the mice physically moved from their initial starting position, which was always in the corner closest to the familiar object. Animal exploration was considered if the mouse’s head was within 2.54 cm of the object, with its neck extended and vibrissae moving. Simple proximity, chewing, or standing on the object did not count as exploratory. All exploratory segments and tests were videotaped for scoring purposes.
For the novel object recognition testing, mice were exposed to two identical objects placed at opposite ends of the arena for 10 min. 6 h later, mice were presented for 3 min with one of the familiar and a novel object of similar dimensions. The discrimination index represents the percentage of the time that mice spend exploring the novel object (Baglietto-Vargas et al. 2013).
Tissue preparation
After deep anesthesia with sodium pentobarbital (60 mg/Kg), Ntg and 3xTg-AD control and stressed mice were perfused transcardially with 0.1 M phosphate-buffered saline (pH7.4). Protein extracts were prepared by homogenizing the hippocampus samples in T-per (Thermo Fisher Scientific, Rockford, IL, USA) extraction buffer (150 mg/mL), complemented with proteases inhibitor (Complete Mini Protease Inhibitor Tablets, Roche Diagnostics GmbH, Germany) and phosphatases inhibitor (5 mmol/L, Sigma-Aldrich, St. Louis, MO, USA), followed by centrifugation at 100 000 g for 1 h. After that, the pellet was resuspended with 70% formic acid followed by centrifugation at 100 000 g for another hour. Protein concentration in the supernatant was determined using the Bradford assay.
For Golgi staining, Ntg and 3xTg-AD mice were perfused transcardially with 0.1 M phosphate-buffered saline (pH 7.4) and brains were processed using superGolgi Kit (Bioenno Tech LLC, Santa Ana, CA, USA). Briefly, brains were incubated for 11 days in impregnation solutions, followed by 2 days incubation in a post-impregnation solution. Once the impregnation of neurons was complete, thick (150 μm) free-floating sections were obtained using a HA752 vibratome (Campden Instruments Ltd, Lafayette, IN, USA) and serially collected in a mounting buffer. Sections mounted on coated slides were stained and post-stained, respectively for 20 min, dehydrated in graded ethanol, cleared with xylene, and coverslipped with dibutylphthalate polystyrene xylene (DPX) mounting medium.
Dendritic spine analysis
Two independent methods/approaches were employed in this study to analyse the number and density of dendritic spines. We determined the number of the spines in the CA3 hippocampal region using the stereological fractionator method. We augmented the analysis by examining the density of dendritic spines on pyramidal cells in CA3 hippocampal region using the NeuronStudio software (NeuronStudio Documentation © CNIC, Mount Sinai School of Medicine, New York, NY). Thus, stereological quantifications were performed using Stereo-Investigator software from Microbrightfield Bioscience (MBF Bioscience, Williston, VT, USA) to determine the number of spines in the stratum radiatum (SR) and stratum lacunosum-moleculare of the hippocampal CA3 region. Briefly, every 2nd section was used through the entire antero-posterior extent of the hippocampus (between −1.46 mm anterior and −3.40 mm posterior to Bregma according to the atlas of Franklin and Paxinos, Third Edition, 2007). SR and stratum lacunosum-moleculare in the CA3 region were defined using a 5× objective and spines were counted using a 100x/1.4 objective. The numbers of the spines were obtained following the optical fractionator methods (West 1999; Baglietto-Vargas et al. 2010). In this study, we used a counting frame of 25 × 25 μm, a sampling grid of 200 × 200 μm, a guard zone of 10 μm and disector height of 50 μm. The precision of the study was estimated by calculating the coefficient of error (CE) (Gundersen et al. 1999). The CEs value for each individual animal ranged between 0.03 and 0.08.
NeuronStudio software was utilized to determine the number of spines per dendritic length (Rodriguez et al. 2006, 2008). Briefly, 10–13 images stacks were collected in Ntg and 3xTg-AD from the SR and SLM layer, respectively in the CA3 hippocampal area. The images were collected, using 100x/1.4 oil objective from a Zeiss AxioImager M2 microscope (Zeiss, Thornwood, NY, USA). Next, images were modified to greyscale with 8 bit-depth using Image J (NIH, Bethesda, MD) 1.36b software. Then, NeuronStudio software was used to perform unbiased and automatic spines quantification per dendritic length. An investigator who was blind to the experimental condition (treatment and genotype) performed the spines quantification and analysis.
Immunoblotting
Equal amounts of protein (20 μg) were separated on 4–12% Bis-Tris gel (Invitrogen, Carlsbad, CA, USA), and transferred to nitrocellulose membranes. Membranes were blocked for 1 h in 5% (w/v) suspension of nonfat milk in 0.2% Tween 20 Tris-buffered saline (TBS) (pH 7.5). After blocking, the membranes were incubated overnight at 4°C, with one of the following primary antibodies: anti-APP-CT20 (1 : 1000) for C99 and C83 (Calbiochem, San Diego, CA, USA), desintegrin and metalloproteinase domain-containing protein (ADAM)10 (1 : 1000), ADAM17 (1 : 1000), BACE-1 (1 : 1000), HT7 (1 : 5000) and AT8 (1 : 1000) (Thermo Scientific), PHF1 (Dr Peter Davies, Albert Einstein College of Medicine, Manhasset, NY, USA), anti-CRH (1 : 500) (Sigma-Aldrich), anti-CRHR1 (1 : 1000) (Everest Biotech, Ramona, CA, USA), anti-insulin-degrading enzyme (IDE) (1 : 1000), anti-neprilysin (CD10) (1 : 1000), anti-apolipoprotein E (1 : 1000) and anti-Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1 : 5000, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The membranes were washed in Tween-TBS for 20 min and incubated at 20°C with the specific secondary antibody at a dilution of 1 : 10 000 (Pierce Biotechnology, Grand Island, NY, USA) for 60 min. The blots were developed using Super Signal (Thermo Scientific).
Aβ-Elisa
Aβ levels were quantified using the MSD96-well multi-spot 6E10 Aβ triple ultra-sensitive assay kit, according to the manufacture’s instruction (Meso Scale Discovery, Rockville, MD, USA). T-Per soluble fractions were loaded directly onto the ELISA plate, and the formic acid supernatants (insoluble fractions) were diluted 1 : 2 in neutralization buffer (1 M Tris-base and 0.5 M NaH2PO4) before loading. Standards (including Aβ1-38, Aβ1-40, and Aβ1-42), and samples, were added to the 96-well plate and incubated overnight, washed, and read in a Sector Imager plate reader Meso Scale Discovery (MSD), immediately after addition of the MSD read buffer. Aβ concentration was calculated with reference to the standard curves and expressed as picograms per micrograms of proteins.
Dot-blot
Equal amounts of protein (3 μg) were transferred into the nitrocellulose membranes. Membranes were blocked for 1 h in 5% (w/v) suspension of non-fat milk in 0.2% Tween 20 TBS (pH 7.5). After blocking, the membranes were incubated overnight at 4°C, with one of the following primary antibodies: anti-A11 (1 : 1000, Life technologies, Grand Island, NY, USA) and anti-OC (1 : 3000, EMD Millipore, Billerica, MA, USA). The membranes were washed in Tween-TBS for 20 min and incubated at 20°C with the specific secondary antibody at a dilution of 1 : 10 000 (Pierce Biotechnology) for 60 min. The blots were developed using Super Signal (Thermo Scientific).
Plasma corticosterone
A corticosterone competitive ELISA kit (Assay Systems, Ann Arbor, MI, USA) was used to measure corticosterone levels as per the instructions of the manufacturer. Plasma samples were diluted 1 : 50 in the bugger provided.
Cell culture
N2A cells were maintained in Dulbecco’s modified Eagle’s medium (invitrogen) supplemented with 10% fetal bovine serum (FBS). For experiments, equal numbers of cells (100 000 cells per well) were plated down in 6-well plates. Wells were treated 24 h later by removal of media and replacing with 2 mL of fresh media containing CRH (Bachem, Torrance, CA, USA) at a final concentration of 10 and 50 nM. Control wells contained fresh media only. After a treatment period of 5 h, cell lysates were collected using M-per (Thermo Fisher Scientific) extraction buffer (150 mg/mL), complemented with proteases inhibitor (Complete Mini Protease Inhibitor Tablets) and phosphatases inhibitor (5 mmol/L, Sigma-Aldrich), followed by centrifugation at 100 000 g for 1 h. Protein concentration in the supernatant was determined using the Bradford assay.
Quantitative and statistical analyses
All data were quantitatively analyzed using Image J 1.36b software. The data were subsequently analyzed by Student’s t-test comparisons and one-way or two-way analysis of variance (ANOVA), followed by Bonferroni’s comparisons using Graphpad Prism software (Graphpad Prism Inc., San Diego, CA, USA). The significance was set at 95% of confidence. All values are presented as mean ± SEM.
Results
Short-term modern life-like stress impairs memory
Single traumatic and emotional experiences are experienced by humans, and may contribute to age-related cognitive decline as well as to the development of neurodegenerative disorders such as AD. To model this, we subjected non-transgenic and 3xTg-AD mice to a single short multimodal stress paradigm, and tested their hippocampal-dependent memory 8-h later using novel object recognition, which involves hippocampal and parahippocampal structures, although cortical areas are also involved in this behavioral test (Broadbent et al. 2010). Memory of wild-type mice subjected to stress was impaired compared to non-stress controls, consistent with previous studies (Chen et al. 2008, 2010, 2013). The 3xTg-AD mice were already impaired and no further deficits were found (Fig. 1b).
Short-term modern life-like stress severely affects dendritic spines in 3xTg-AD mice
Dendritic spines are dynamic structures whose plasticity is thought to underlie learning and memory process (Rochefort and Konnerth 2012; Koleske 2013). Importantly, several lines of evidence revealed that stress and/or stress mediator are important factors that impair the function and stability of dendritic spines (Chen et al. 2008, 2010, 2013; Andres et al. 2013; Maras et al. 2014). Given that, we analyzed the impact of short modern-life like stress on dendritic spines in the 3xTg-AD mice in comparison to Ntg mice. In our current study, we focused on the CA3 hippocampal area because many of the structural and functional consequences of stress on hippocampus occur in this area.
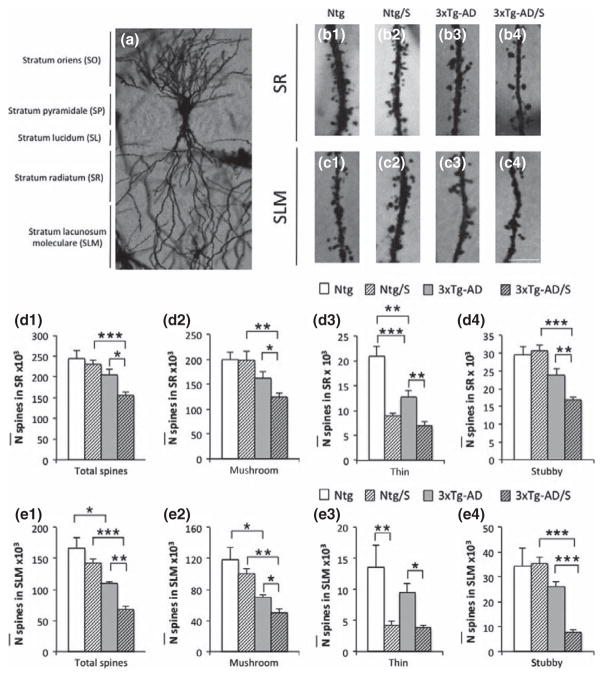
The total number of spines in CA3 area (Fig. 2) and the density of spines in CA3 pyramidal cells (Fig. 3) were investigated in the current study. Stereological quantifications in the stratum radiatum (sr) and stratum lacunosum-moleculare (SLM) of CA3 hippocampus shows a significant reduction in the total number of dendritic spines in 6-month-old stressed 3xTg-AD mice compared to non-stressed-matched mice (Fig. 2d1 and e1), 8 h after the short multimodal stress paradigm. Interestingly, all types of spines were affected in 3xTg-AD mice (including thin, mushroom, and stubby) in SR and SLM of hippocampal CA3 (Fig. 2 b3–b4, c3–c4, d2–d4, and e2–e4). This was in contrast to Ntg stressed mice, where only thin spines were significantly affected in both sr and slm layer (Fig. 2 b1–b2, c1–c2, d3 and e3), while mushroom and stubby were not affected. These data are in accordance to previous studies that have shown that short-term stress affects mainly thin spines (Chen et al. 2008, 2010, 2013).
Fig. 2.
Multimodal acute stress severely impairs the number of spine in CA3 area in 3xTg-Alzheimer’s disease (AD) mice. (a–c) Light microscopy images of dendritic spines in CA3 subfield of sr (b1–b4) and slm (c1–c4) in Ntg (b1 and c1), Ntg-stressed (b2 and c2), 3xTg-AD (B3 and C3) and 3xTg-AD-stressed (b4 and c4) mice at 5–6 months of age. Stereological quantifications showed significant decreases in the total number of spines observed in 3xTg-AD-stressed compared to non-stressed 3xTg-AD mice in both sr and slm layers (d1 and e1). In addition, not differences were observed in Ntg-stressed versus non-stressed Ntg mice (d1 and e1). Furthermore, stereological quantifications based on spines types (including, mushroom, thin and stubby) demonstrate that only thin spines are affected in Ntg-stressed mice compared to non-stressed Ntg (d2–d4 and e2–e4). (The definition/standard of thin, mushroom spines should be cited or described briefly.) Notably, all types of spines are affected after multimodal acute stress in 3xTg-AD mice (d2–d4 and e2–e4). The values represent the mean ± SEM (N = 5 per group). *p < 0.05, **p < 0.01 and ***p < 0.001. SO: stratum oriens; SP: straum pyramidale; SL: stratum lucidum; SR: stratum radiatum; SLM; stratum lacunosum-moleculare. Scale bars: 5 μm (B1–C4).
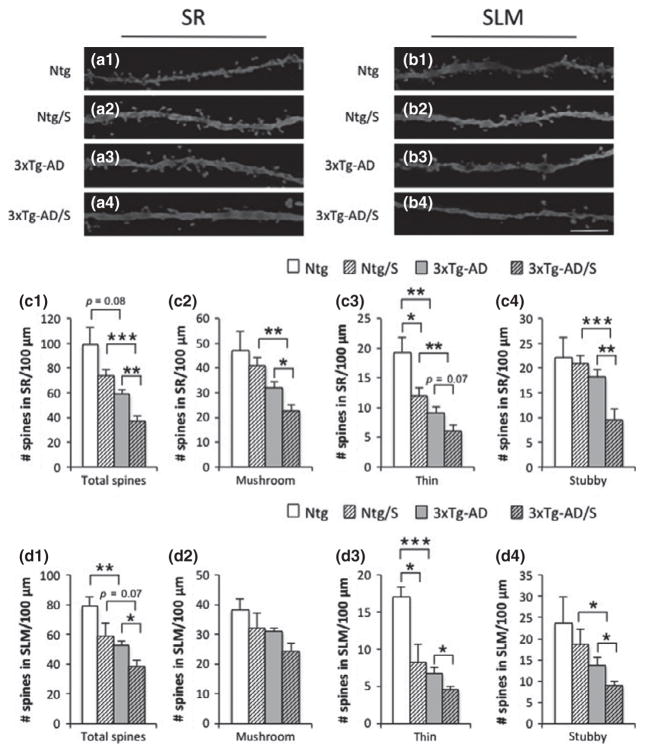
Fig. 3.
Multimodal acute stress severely impairs spine densities on CA3 pyramidal cells in 3xTg-Alzheimer’s disease (AD) mice. (a and b) Light microscopy images of dendritic spines on pyramidal cells in CA3 subfield of sr (a1–a4) and slm (b1–b4) in Ntg (a1 and b1), Ntg-stressed (a2 and b2), 3xTg-AD (a3 and b3) and 3xTg-AD-stressed (a4 and b4) mice at 5–6 months of age. Spines quantifications per dendritic length showed significant decreases in spine density of total spines observed in 3xTg-AD stressed compared to non-stressed 3xTg-AD mice in both sr and slm layers (c1 and d1). Furthermore, not differences were observed in Ntg-stressed versus non-stressed Ntg mice (c1 and d1). Quantifications based on types of spines (including mushroom, thin, and stubby) demonstrate that only thin spines are affected in Ntg-stressed mice compared to non-stressed Ntg (c2–c4 and d2–d4), meanwhile all types of spines are affected in 3xTg-AD-stressed versus non-stressed 3xTg-AD mice (c2–c4 and d2–d4). The values represent the mean ± SEM (N = 5 per group). *p < 0.05, **p < 0.01 and ***p < 0.001. SR: stratum radiatum; SLM; stratum lacunosum-moleculare. Scale bars: 5 μm (a1–b4).
In CA3 pyramidal cells area (Fig. 3a1–a4 and b1–b4), a significant decrease in the density of the total spines in stressed 3xTg-AD mice was observed in both sr and slm layers (Fig. 3 c1 and d1). In addition, only thin spines were significantly affected in Ntg-stressed mice compared to non-stressed matched mice (Fig. 3 c3 and d3) while in 3xTg-AD mice significant decreases in the densities of all types of spines occurred (Fig. 3 c2–c4 and d2–d4). In sum, short modern-life like stress significantly reduces the number of dendritic spines in 3xTg-AD mice, and this effect is global compared to the selective effect on thin spines found in Ntg mice.
Short-term modern life-like stress increases Aβ pathology
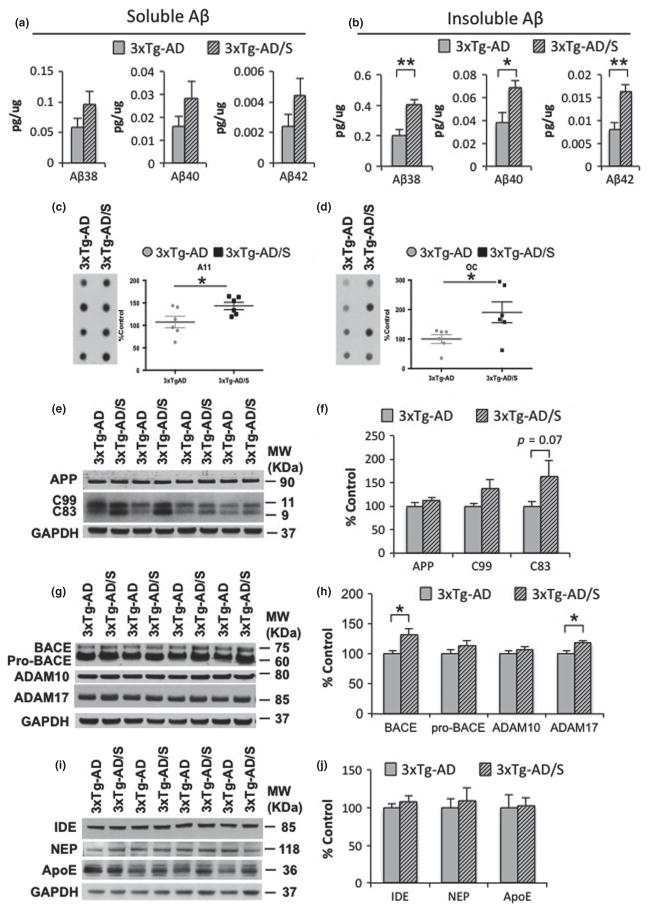
To determine why short-term stress has more severe effects on spine densities in 3xTg-AD mice than in Ntg mice, we evaluated if short multimodal stress paradigm had any effects on Aβ or tau pathology. Soluble and insoluble Aβ levels, measured by ELISA assay, show an increase in the levels of Aβ1-38, Aβ1-40, and Aβ1-42 (Fig. 4a and b). In addition, dot-blot analysis showed elevated levels of A11- and OC-positive Aβ oligomers, in 3xTg-AD mice exposed to short-term multimodal stress, compared to non-stressed 3xTg-AD mice (Fig. 4c and d). We further examined whether the increase on Aβ induced by short-term multimodal stress was caused by any change in AβPP processing. Steady-state levels of full-length AβPP holoprotein were unaffected between stressed and non-stressed 3xTg-AD mice (Fig. 4e and f). Notably, the C-terminal fragments (CTFs), which are membrane stubs produced by APP cleavage, were significantly increased in 3xTg-AD stressed versus non-stressed mice (Fig. 4g and 4h). Steady-state levels of constitutive proteases, including α-secretases (ADAM 10 and 17) and β-secretase (BACE1), involved on AβPP processing using western blot analysis shows significant increases in BACE1 and ADAM 17 in stressed 3xTg-AD mice compared to non-stressed 3xTg-AD mice. Additionally, we evaluated the effect of stress on the major putative Aβ clearance pathways, including IDE, neprilysin and apolipoprotein E, and find no differences (Fig. 4i and j). Our data indicate that the increase on Aβ levels induced by short modern-life like stress is likely caused by triggering AβPP processing.
Fig. 4.
Multimodal acute stress increases Aβ levels. (a and b) Aβ measurements by sandwich ELISA of both the soluble and insoluble (Aβ1-38: 98.18 ± 15.80%, **p < 0.01, t-test; Aβ1-40: 78.40 ± 16.93%, *p < 0.05, t-test; Aβ1-42: 99.96 ± 20.20%, **p < 0.01, t-test) fractions shows an increase in Aβ levels in stressed 3xTg-Alzheimer’s disease (AD) mice compared to non-stressed mice. (c and d) Dot blot analysis shows a significant increase in the level of Aβ–oligomers recognized by the antibody A11 (c) (43.27 ± 8.12%, *p < 0.05, t-test) and OC (d) (90.60 ± 35.40%, *p < 0.05, t-test) in stressed 3xTg-AD mice compared to non-stressed mice. (e) Immunoblot analysis of AβPP holoprotein and C-terminal amyloid precursor protein (APP) fragment (CTFs) C99 and C83 from hippocampal-brain homogenates of non-stressed and stressed 3xTg-AD mice at 5–6 month-old shown as alternating lanes. (f) Quantification of E normalized to GAPDH and expressed, as a % of control shows no differences in AβPP and C99 in non-stressed versus stressed 3xTgAD mice. In addition, significant differences were observed in C83 levels (82.12 ± 37.91%, p = 0.07, t-test). g) Immunoblot analysis of constitutive proteases, including beta-site amyloid precursor protein-cleaving enzyme 1 (BACE-1), ADAM-10 and -17 from hippocampal-brain homogenates of non-stressed and stressed 3xTg-AD mice at 5–6 month-old shown as alternating lanes. (h) Quantification of G normalized to GAPDH and expressed, as a % of control shows significant increases on BACE1 (31.79 ± 9.10%, *p < 0.05, t-test) and ADAM17 (18.84 ± 8.06%, *p < 0.05, t-test) in stressed 3xTg-AD mice compared to non-stressed 3xTg-AD mice. (i–j) Immunoblot analysis of insulin-degrading enzyme (IDE), neprilysin (NEP) and anti-apolipoprotein E (ApoE) from hippocampal-brain homogenates of non-stressed and stressed 3 × Tg-AD mice at 5–6 month-old shown as alternating lanes. The values represent the mean ± SEM (N = 6). *p < 0.05 and **p < 0.01.
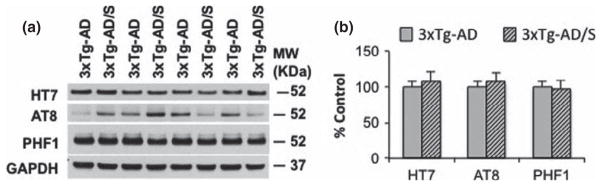
We next investigated the effect of short multimodal stress on tau in the 3xTg-AD mice. Western blot analysis revealed that neither steady-state tau nor phospho-tau species recognized by antibodies AT8 (Ser199/202) and PH1 (Ser396/404) were altered by multimodal stress 8 h after its termination (Fig. 5a and b). These data are consistent with a previous study in that phospho-tau levels returned to baseline level after 90 min of acute stress (Rissman et al. 2007).
Fig. 5.
Multimodal acute stress does not alter tau levels. (a) Immunoblot analysis of human tau (HT7), phosphotau Ser199/202 (AT8) and phosphotau Ser396/404 (PHF1) from hippocampal-brain homogenates of non-stressed and stressed 3xTg-Alzheimer’s disease (AD) mice at 6 months old shown as alternating lanes. (b) Quantification of A normalized to GAPDH and expressed, as a % of control shows no differences in any of the markers.
Levels of CRH prepropeptide are increased in 3xTg-AD mice after short-term modern life-like stress
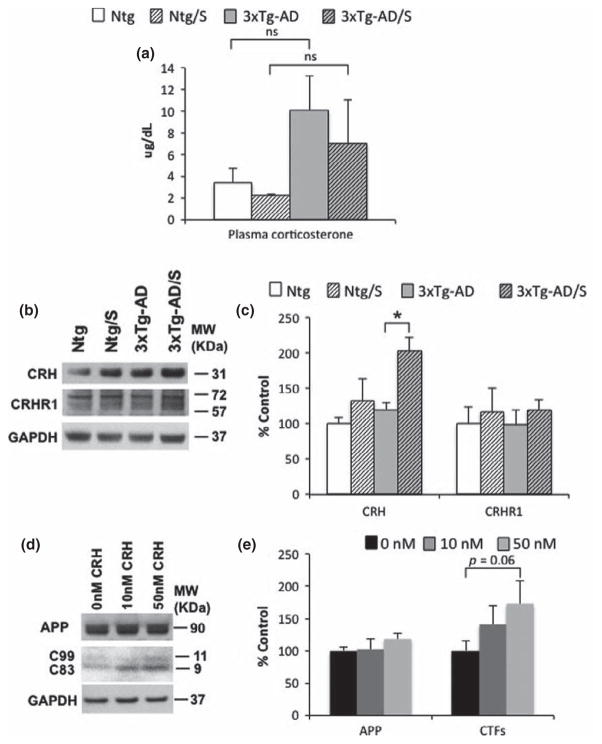
We next sought to determine the potential mechanism by which short multimodal stress could increase Aβ levels in 3xTg-AD mice. Previous studies in animal models showed that several stress mediators such as GC and CRH influence Aβ pathology via modulating AβPP production or Aβ degradation (Kulstad et al. 2005; Green et al. 2006; Jeong et al. 2006; Kang et al. 2007; Dong et al. 2008, 2012; Catania et al. 2009; Li et al. 2010; Cuadrado-Tejedor et al. 2012; Rothman et al. 2012). Therefore, we measured the levels of both GC and CRH to determine whether these mediators could be the underlying cause of the increase in Aβ levels observed in stressed 3xTg-AD mice. We found that basal plasma corticosterone levels were elevated in 3xTg-AD mice compared to Ntg mice at 5–6 month of age (Fig. 6a). However, no differences were observed between stressed and non-stressed mice in both Ntg and 3xTg-AD mice 8 h after of the end of multimodal short-term stress (Fig. 6a). Western-blot analysis reveals significantly elevated steady state levels of likely CRH prepropeptide in 3xTg-AD stressed mice compared to non-stressed mice. In Ntg-mice, the differences in CRH levels between stressed mice versus non-stressed mice were not significant (Fig. 6b and c). Corticotropin-releasing hormone receptor 1 (CRH1) levels did not vary with genotype or treatment (Fig. 6b and c).
Fig. 6.
Multimodal acute stress elevates corticotrophin-releasing hormone (CRH) prepropeptide levels in 3xTg-Alzheimer’s disease (AD) mice. (a) No significant differences were observed in plasma corticosterone levels from stressed and non-stressed Ntg and 3xtg-AD mice at 5–6 months of age measured 6 h after the end of the stress. (b) Immunoblot analysis of CRH and CRHR1 from hippocampal-brain homogenates of non-stressed and stressed 3 × Tg-AD mice at 6 month-old shown as alternating lanes. (c) Quantification of B normalized to GAPDH and expressed, as a % of control shows significant differences in CRH in non-stressed versus stressed 3 × TgAD mice (69.41 ± 15.29% two-way ANOVA, Turkey post hoc ***p < 0.05). In addition, no differences were detected in CRHR1 levels. (d) Immunoblot analysis of AbPP holoprotein and C-terminal amyloid precursor protein (APP) fragment (CTFs) from hippocampalbrain homogenates of non-stressed and stressed 3 × Tg-AD mice at 5–6 month-old shown as alternating lanes. (e) Quantification of A normalized to GAPDH and expressed, as a % of control shows no differences in AbPP in N2A cells. In addition, significant differences were observed in N2A cells treated with 50 nM of CRH versus control (46.23 ± 28.44%, one way ANOVA, Bonferroni post hoc p = 0.06). The values represent the mean ± SEM (N = 6–9). *p < 0.05.
To determine whether CRH may regulate/increase Aβ levels, we turned to an in vitro system. We treated N2A cells with CRH and found that increased levels of CRH stimulate AβPP processing by increasing CTFs (Fig. 6d and e). Together, our data suggest that elevated levels of CRH might contribute to increase Aβ in 3xTg-AD mice by stimulating AβPP processing.
Discussion
Epidemiological and animal studies suggest that adverse lifestyle factors such as chronic stress play a key role in modulating AD pathogenesis and further impair cognitive function (Umegaki et al. 2000; Csernansky et al. 2006; Hoogendijk et al. 2006; Huang et al. 2009; Pardon 2011; Brureau et al. 2013; Zverova et al. 2013). In fact, several studies suggest that stress and/or stress mediators (including GC and CRH) regulate Aβ pathogenesis via modulating key molecular factors involved in AβPP processing and Aβ degradation (Kulstad et al. 2005; Green et al. 2006; Jeong et al. 2006; Kang et al. 2007; Dong et al. 2008, 2012; Catania et al. 2009; Li et al. 2010; Cuadrado-Tejedor et al. 2012; Rothman et al. 2012) and impairs tau pathology by modulating key kinases involved in tau phosphorylation or by mislocalizing tau protein to the somatodendritic compartment (Green et al. 2006; Rissman et al. 2007, 2012; Sotiropoulos et al. 2011; Filipcik et al. 2012).
Although there has been an intense investigation in the last decade to elucidate the impact of stress on AD pathogenesis, the majority of these studies have been focused on the effect of acute (minutes) or chronic stress (days/weeks). However, these types of stress do not fully represent the diverse spectrum of modern-life stress that we experience nowadays. Modern-life stressful experiences are not unitary or discrete and involve multiple concurrent psychological, social, and physical stresses (Maras et al. 2014). In order to mimic a short-term modern-life stressful experience, such as in car accidents and shooting events, which often last for hours rather than minutes or days/weeks, mice were exposed to a multimodal type of stress consisting of concurrent psychological and physical stresses (Chen et al. 2010). In addition, recent studies provide evidence that this short-term multimodal stress, lasting hours, may impact memory significantly and adversely (Maras et al. 2014). The utilization of this technical approach has allowed us to determine the impact of a short-term, modern life-like stress event, on AD pathogenesis and its implication on synaptic plasticity.
Multiple key studies indicate that stress is an important modulator that affects synaptic plasticity and memory processes (Kim and Diamond 2002; Joels and Baram 2009; Lupien et al. 2009). One of the important regions in memory formation that is highly sensitive to stress is the hippocampus (Kim and Diamond 2002). Interestingly, novel studies reveal that different types and durations of stress significantly influence synaptic plasticity and long-term potentiation (LTP) in complex manners that are governed by the duration, severity and complexity of the stress (Pavlides et al. 2002; Brunson et al. 2005; Wilson et al. 2007; Chen et al. 2008, 2010; Cazakoff and Howland 2010; Ivy et al. 2010). However, it is not known how short-term, multimodal stress affects synaptic plasticity in AD (Zhou et al. 2004; Segal 2005; Chen et al. 2007). Our study demonstrates that a short-term multimodal stress paradigm severely decreases the number of dendritic spines in the hippocampus of 3xTg-AD mice. In addition, mushroom, thin and stubby spines are all significantly affected in 3xTg-AD mice following short-term multimodal stress. In contrast, only thin spines are affected in Ntg mice, as previous studies have shown (Chen et al. 2008, 2010, 2013). Hence, our findings indicate that modern lifelike stress severely diminishes dendritic spines in 3xTg-AD mice.
The fact that dendritic spines were severely diminished in 3xTg-AD mice compared to Ntg mice after exposure to short-term multimodal stress is an interesting phenomenon. To understand the molecular mechanism underlying this phenomenon, we hypothesized if short-term multimodal stress might severely affect synaptic plasticity in 3xTg-AD mice by modulating AD pathology. In this regard, multiple studies have revealed that stress or stress mediators (i.e., GC and CRH) trigger both Aβ and tau pathogenesis (Kulstad et al. 2005; Green et al. 2006; Jeong et al. 2006; Kang et al. 2007; Rissman et al. 2007, 2012; Dong et al. 2008, 2012; Sotiropoulos et al. 2008, 2011; Catania et al. 2009; Li et al. 2010; Cuadrado-Tejedor et al. 2012; Filipcik et al. 2012; Rothman et al. 2012). In agreement with theses studies, we found that a short-term multimodal stress event increases Aβ levels, and the formation of Aβ-oligomers. This augmentation in Aβ levels occurs in part by modulating AβPP processing and increasing CTFs levels. Thus, it is plausible that short-term multimodal stress might significantly diminish the dendritic spines by increasing Aβ levels. In this regard, accumulating evidence indicates that soluble oligomers and intermediate aggregates of amyloids are the most neurotoxic forms of Aβ leading to impairment of long-term potentiation, causing synaptic dysfunction, neuronal loss, and memory deficits (Galimberti et al. 2006; Walsh and Selkoe 2007). In addition, we looked at tau levels, which appeared to be unaffected by exposure to multimodal acute stress. This finding is supported by a previous report performed by Rissman et al. (2007), which showed that phospho-tau levels returned to baseline level after 90 min of acute restrain stress. Overall, our study suggests that increasing of Aβ-oligomers levels stimulated by modern life-like stress might impact the synaptic plasticity and induce a robust synaptic loss in the 3xTg-AD mice.
Previous studies revealed that several stress mediators, including GC and CRH, are able to trigger Aβ pathology (Green et al. 2006; Kang et al. 2007; Dong et al. 2008, 2012; Li et al. 2010). Several mechanisms are proposed by which Aβ pathology is triggered by these stress mediators, like AβPP misprocessing and by reducing Aβ clearance via decreasing the activity of IDE (Green et al. 2006; Kang et al. 2007; Dong et al. 2008, 2012; Li et al. 2010). Therefore, to determine how multimodal acute stress increases Aβ levels in 3xTg-AD mice, we analyzed the levels of both GC and CRH. Our study demonstrated that plasma GCs levels are elevated in 3xTg-AD mice at 6 months of age compared to Ntg mice; however, these differences are not significant, as a previous study has shown (Green et al. 2006). In addition, no difference in plasma GCs was observed between 3xTg-AD-stressed and non-stressed 3xTg-AD mice. These findings are in agreement with a previous report by Trojanowski and group who found that after an induction of acute restrain stress, plasma GCs levels return to baseline level in 90 min (Carroll et al. 2011). In our study, we measured plasma GCs level 8 h after the induction of multimodal acute stress; thus, as expected, no differences in GC levels between non-stress and stressed mice were found. In addition, we measured the levels of CRH and found significantly elevated steady-state levels of CRH in the hippocampus of 3xTg-AD stressed mice compared to non-stressed 3xTg-AD mice. CRH has been proposed to increase Aβ pathology, although its mechanism remains unclear (Kang et al. 2007; Dong et al. 2012). We found that CRH significantly increases both Aβ and CTFs fragments. Therefore, our data suggest that CRH might trigger Aβ pathology via modulation of AβPP processing. However, we cannot discard the idea that GCs might also modulate Aβ levels via the induction of secondary messenger.
In conclusion, short-term, complex (multimodal) stress is a key factor that triggers AD pathogenesis and severely affects synaptic plasticity in 3xTg-AD mice. These findings support the concept that short-term, modern-life stress comprised of several modalities might augment AD pathology in preclinical AD patients. However, further studies will be necessary to determine the long-lasting effect of this short-term multimodal stress event in AD pathogenesis, and whether repetitive multimodal stress episodes may accelerate the pathological cascade of events, perpetuating the development of AD later in life. As a result, therapies aimed to reduce this type of stress might be a promising approach to mitigate AD pathology and alleviate cognitive impairments.
Acknowledgments
D.B.V, Y.C, and D.S, were responsible for data acquisition, analysis, and interpretation. R.R.A was responsible for the in vitro study. K. M was responsible for the behavior test. C. J. R.O performed statistical analysis. D.B.V, Y.C, D.S, R.R.A, C. J. R.O, R.M, K.M, K.N.G, T.Z.B and F.M.L performed critical revision of the manuscript. D.B.V, Y.C, T.Z.B and F.M.L provide study concept and design, drafting the manuscript and funding support. This study was supported by grants from The Larry Hillblom Foundation #2013-A-016-FEL (DBV) and the National Institute of Health (NIH): NIH/NIA AG027544, OD010420 (FML), NS28912 (TZB) and NS45260 (YC, TZB; Gall PI).
All experiments were conducted in compliance with the ARRIVE guidelines.
Abbreviations used
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- AβPP
amyloid-β protein precursor
- BACE-1
beta-site amyloid precursor protein-cleaving enzyme 1
- CRH
corticotrophin-releasing hormone
- CTFs
C-terminal fragments
- IDE
insulin-degrading enzyme
- TBS
Tris-buffered saline
Footnotes
Conflict of interest disclosure
No potential conflicts of interest relevant to this article were reported.
References
- Andres AL, Regev L, Phi L, Seese RR, Chen Y, Gall CM, Baram TZ. NMDA receptor activation and calpain contribute to disruption of dendritic spines by the stress neuropeptide CRH. J Neurosci. 2013;33:16945–16960. doi: 10.1523/JNEUROSCI.1445-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglietto-Vargas D, Moreno-Gonzalez I, Sanchez-Varo R, et al. Calretinin interneurons are early targets of extracellular amyloid-beta pathology in PS1/AbetaPP Alzheimer mice hippocampus. J Alzheimers Dis. 2010;21:119–132. doi: 10.3233/JAD-2010-100066. [DOI] [PubMed] [Google Scholar]
- Baglietto-Vargas D, Medeiros R, Martinez-Coria H, LaFerla FM, Green KN. Mifepristone alters amyloid precursor protein processing to preclude amyloid beta and also reduces tau pathology. Biol Psychiatry. 2013;74:357–366. doi: 10.1016/j.biopsych.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2010;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brureau A, Zussy C, Delair B, Ogier C, Ixart G, Maurice T, Givalois L. Deregulation of hypothalamic-pituitary-adrenal axis functions in an Alzheimer’s disease rat model. Neurobiol Aging. 2013;34:1426–1439. doi: 10.1016/j.neurobiolaging.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Iba M, Bangasser DA, Valentino RJ, James MJ, Brunden KR, Lee VM, Trojanowski JQ. Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J Neurosci. 2011;31:14436–14449. doi: 10.1523/JNEUROSCI.3836-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania C, Sotiropoulos I, Silva R, Onofri C, Breen KC, Sousa N, Almeida OF. The amyloidogenic potential and behavioral correlates of stress. Mol Psychiatry. 2009;14:95–105. doi: 10.1038/sj.mp.4002101. [DOI] [PubMed] [Google Scholar]
- Cazakoff BN, Howland JG. Acute stress disrupts paired pulse facilitation and long-term potentiation in rat dorsal hippocampus through activation of glucocorticoid receptors. Hippocampus. 2010;20:1327–1331. doi: 10.1002/hipo.20738. [DOI] [PubMed] [Google Scholar]
- Chan RK, Brown ER, Ericsson A, Kovacs KJ, Sawchenko PE. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. J Neurosci. 1993;13:5126–5138. doi: 10.1523/JNEUROSCI.13-12-05126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in synaptic morphology accompany actin signaling during LTP. J Neurosci. 2007;27:5363–5372. doi: 10.1523/JNEUROSCI.0164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dube CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Rex CS, Rice CJ, Dube CM, Gall CM, Lynch G, Baram TZ. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc Natl Acad Sci USA. 2010;107:13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Kramar EA, Chen LY, Babayan AH, Andres AL, Gall CM, Lynch G, Baram TZ. Impairment of synaptic plasticity by the stress mediator CRH involves selective destruction of thin dendritic spines via RhoA signaling. Mol Psychiatry. 2013;18:485–496. doi: 10.1038/mp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GM, Fujioka T, Nakamura S. Induction and adaptation of Fos expression in the rat brain by two types of acute restraint stress. Brain Res Bull. 2000;52:171–182. doi: 10.1016/s0361-9230(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Neurobiol Aging. 2006;163:2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado-Tejedor M, Ricobaraza A, Frechilla D, Franco R, Perez-Mediavilla A, Garcia-Osta A. Chronic mild stress accelerates the onset and progression of the Alzheimer’s disease phenotype in Tg2576 mice. J Alzheimers Dis. 2012;28:567–578. doi: 10.3233/JAD-2011-110572. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neuorsci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Dong H, Yuede CM, Yoo HS, Martin MV, Deal C, Mace AG, Csernansky JG. Corticosterone and related receptor expression are associated with increased beta-amyloid plaques in isolated Tg2576 mice. Neuroscience. 2008;155:154–163. doi: 10.1016/j.neuroscience.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Murphy KM, Meng L, Montalvo-Ortiz J, Zeng Z, Kolber BJ, Zhang S, Muglia LJ, Csernansky JG. Corticotrophin releasing factor accelerates neuropathology and cognitive decline in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2012;28:579–592. doi: 10.3233/JAD-2011-111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert MH, Herman JP. Differential forebrain c-fos mRNA induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Res. 1999;845:60–67. doi: 10.1016/s0006-8993(99)01931-9. [DOI] [PubMed] [Google Scholar]
- Filipcik P, Novak P, Mravec B, Ondicova K, Krajciova G, Novak M, Kvetnansky R. Tau protein phosphorylation in diverse brain areas of normal and CRH deficient mice: up-regulation by stress. Cell Mol Neurobiol. 2012;32:837–845. doi: 10.1007/s10571-011-9788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti D, Fenoglio C, Lovati C, et al. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiol Aging. 2006;27:1763–1768. doi: 10.1016/j.neurobiolaging.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology–reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Hoogendijk WJ, Meynen G, Endert E, Hofman MA, Swaab DF. Increased cerebrospinal fluid cortisol level in Alzheimer’s disease is not related to depression. Neurobiol Aging. 2006;27:780 e781–780 e782. doi: 10.1016/j.neurobiolaging.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Huang CW, Lui CC, Chang WN, Lu CH, Wang YL, Chang CC. Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer’s disease. J Clin Neurosci. 2009;16:1283–1286. doi: 10.1016/j.jocn.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Rex CS, Chen Y, Dube C, Maras PM, Grigoriadis DE, Gall CM, Lynch G, Baram TZ. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci. 2010;30:13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong YH, Park CH, Yoo J, Shin KY, Ahn SM, Kim HS, Lee SH, Emson PC, Suh YH. Chronic stress accelerates learning and memory impairments and increases amyloid deposition in APPV717I-CT100 transgenic mice, an Alzheimer’s disease model. FASEB J. 2006;20:729–731. doi: 10.1096/fj.05-4265fje. [DOI] [PubMed] [Google Scholar]
- Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Cirrito JR, Dong H, Csernansky JG, Holtzman DM. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proc Natl Acad Sci USA. 2007;104:10673–10678. doi: 10.1073/pnas.0700148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Koleske AJ. Molecular mechanisms of dendrite stability. Nat Rev Neurosci. 2013;14:536–550. doi: 10.1038/nrn3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulstad JJ, McMillan PJ, Leverenz JB, et al. Effects of chronic glucocorticoid administration on insulin-degrading enzyme and amyloid-beta peptide in the aged macaque. J Neuropathol Exp Neurol. 2005;64:139–146. doi: 10.1093/jnen/64.2.139. [DOI] [PubMed] [Google Scholar]
- Li WZ, Li WP, Yao YY, Zhang W, Yin YY, Wu GC, Gong HL. Glucocorticoids increase impairments in learning and memory due to elevated amyloid precursor protein expression and neuronal apoptosis in 12-month old mice. Eur J Pharmacol. 2010;628:108–115. doi: 10.1016/j.ejphar.2009.11.045. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maras PM, Baram TZ. Sculpting the hippocampus from within: stress, spines, and CRH. Trends Neurosci. 2012;35:315–324. doi: 10.1016/j.tins.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras PM, Molet J, Chen Y, Rice C, Ji SG, Solodkin A, Baram TZ. Preferential loss of dorsal-hippocampus synapses underlies memory impairments provoked by short, multimodal stress. Mol Psychiatry. 2014;19:811–822. doi: 10.1038/mp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Pardon MC. Therapeutic potential of some stress mediators in early Alzheimer’s disease. Exp Gerontol. 2011;46:170–173. doi: 10.1016/j.exger.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–257. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- Rissman RA, Lee KF, Vale W, Sawchenko PE. Corticotropin-releasing factor receptors differentially regulate stress-induced tau phosphorylation. J Neurosci. 2007;27:6552–6562. doi: 10.1523/JNEUROSCI.5173-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman RA, Staup MA, Lee AR, Justice NJ, Rice KC, Vale W, Sawchenko PE. Corticotropin-releasing factor receptor-dependent effects of repeated stress on tau phosphorylation, solubility, and aggregation. Proc Natl Acad Sci USA. 2012;109:6277–6282. doi: 10.1073/pnas.1203140109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort NL, Konnerth A. Dendritic spines: from structure to in vivo function. EMBO Rep. 2012;13:699–708. doi: 10.1038/embor.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Ehlenberger DB, Hof PR, Wearne SL. Rayburst sampling, an algorithm for automated three-dimensional shape analysis from laser scanning microscopy images. Nat Protoc. 2006;1:2152–2161. doi: 10.1038/nprot.2006.313. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS ONE. 2008;3:e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Herdener N, Camandola S, Texel SJ, Mughal MR, Cong WN, Martin B, Mattson MP. 3xTgAD mice exhibit altered behavior and elevated Abeta after chronic mild social stress. Neurobiol Aging. 2012;33:830 e831–812. doi: 10.1016/j.neurobiolaging.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Joels M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: an update and integration. Neurosci Biobehav Rev. 2012;36:1740–1749. doi: 10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6:277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos I, Catania C, Riedemann T, Fry JP, Breen KC, Michaelidis TM, Almeida OF. Glucocorticoids trigger Alzheimer disease-like pathobiochemistry in rat neuronal cells expressing human tau. J Neurochem. 2008;107:385–397. doi: 10.1111/j.1471-4159.2008.05613.x. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos I, Catania C, Pinto LG, Silva R, Pollerberg GE, Takashima A, Sousa N, Almeida OF. Stress acts cumulatively to precipitate Alzheimer’s disease-like tau pathology and cognitive deficits. J Neurosci. 2011;31:7840–7847. doi: 10.1523/JNEUROSCI.0730-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umegaki H, Ikari H, Nakahata H, Endo H, Suzuki Y, Ogawa O, Nakamura A, Yamamoto T, Iguchi A. Plasma cortisol levels in elderly female subjects with Alzheimer’s disease: a cross-sectional and longitudinal study. Brain Res. 2000;881:241–243. doi: 10.1016/s0006-8993(00)02847-x. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G. Interactions between heterotypic stressors and corticosterone reveal integrative mechanisms for controlling corticotropin-releasing hormone gene expression in the rat paraventricular nucleus. J Neurosci. 2002;22:6282–6289. doi: 10.1523/JNEUROSCI.22-14-06282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68:2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Zverova M, Fisar Z, Jirak R, Kitzlerova E, Hroudova J, Raboch J. Plasma cortisol in Alzheimer’s disease with or without depressive symptoms. Med Sci Monit. 2013;19:681–689. doi: 10.12659/MSM.889110. [DOI] [PMC free article] [PubMed] [Google Scholar]