Abstract
Chromogranin A (CgA) is a member of the granin family of molecules found in secretory granules of endocrine and neuro-endocrine cells. Here, we have identified a new 23-mer CgA-derived peptide secreted from pituitary AtT-20 cells, which we named pyroGlu-serpinin (pGlu-serpinin). LC–MS studies of peptides in conditioned medium of AtT-20 cells indicate that pGlu-serpinin is derived from initial processing of mouse CgA at paired basic residues, Arg461–Arg462 and Arg433–Arg434, to yield a previously described 26 amino acid peptide, serpinin. Three amino acids are then cleaved from the N terminus of serpinin, yielding a peptide with an N-terminal glutamine, which is then subsequently pyroglutaminated. Immunocytochemistry showed co-localization of pGlu-serpinin with adrenocorticotropic hormone in secretory granules of AtT-20 cells, and it was released in an activity-dependent manner. Functional studies demonstrated that pGlu-serpinin was able to prevent radical oxygen species (hydrogen peroxide)-induced cell death of AtT-20 cells and cultured rat cerebral cortical neurons at a concentration of 1 and 10 nM, respectively. These data indicate that pGlu-serpinin has anti-apoptotic effects that may be important in neuroprotection of central nervous system neurons and pituitary cells. Furthermore, pGlu-serpinin added to the media of AtT-20 cells up-regulated the transcription of the serine protease inhibitor, protease nexin-1 (PN-1) mRNA. pGlu-serpinin’s ability to increase levels of PN-1, a potent inhibitor of plasmin released during inflammatory processes causing cell death, may play a role in protecting cells under adverse pathophysiological conditions.
Keywords: Serpinin, Chromogranin A, Neuroprotection, Protease nexin-1, Pyroglutamination
Introduction
Chromogranin A (CgA) is a member of the granin family found in neurons, endocrine cells, and chromaffin cells. It is proteolytically processed to yield several peptide fragments that have various physiological functions including ones that regulate cardiac function and blood pressure and others that have anti-microbial or anti-angiogenesis activity (for reviews see Special issue of Regulatory Peptides, 2010). At the cell biological level, we showed that CgA is a granulogenic protein and plays a critical role in the formation of dense core secretory vesicles in (neuro) endocrine cells (Kim and Loh 2006; Kim et al. 2001). We also demonstrated that a CgA fragment, but not intact CgA, secreted in an activity-dependent manner, was able to augment granule biogenesis in AtT-20 cells, a pituitary cell line, by up-regulating a serine protease inhibitor protein, protease nexin-1 (PN-1), which then stabilizes granule proteins to increase their levels in the Golgi complex (Kim and Loh 2006). We subsequently found that a synthetic 26 amino acid residue peptide (mouse CgA435–460), which we named serpinin (Koshimizu et al. 2010) was able to induce PN-1 mRNA up-regulation. The serpinin region of CgA is highly conserved in mammals, suggesting that peptide(s) derived from this domain are likely to have important physiological functions.
In this and a previous study (Koshimizu et al. 2011), we searched for endogenous serpinin-related peptides in AtT-20 pituitary cells. In addition to endogenous serpinin, we found a 23-mer serpinin-like molecule that was subsequently identified as pyroglutaminated serpinin (pGlu-serpinin), in AtT-20 cell-conditioned medium (CM). pGlu-serpinin was present in the highest amount compared to the other serpinin-related peptides in AtT-20 cell CM (Koshimizu et al. 2011); therefore, we focused on studying the function of this peptide. Since CgA is a potential Alzheimer’s disease (AD) biomarker in cerebrospinal fluid (CSF) (Hu et al. 2010) and has been implicated in neurotoxicity by activating microglia causing neuronal cell death (Kingham and Pocock 2001), we investigated the possibility that the pGlu-serpinin fragment of CgA might play a regulatory function in neuronal cell survival. Often, different parts of the same molecule can have different or even opposite biological effects, for example, while the mature form of brain-derived neuro-trophic factor (matBDNF) inhibits the low potassium (LK)-induced apoptosis of cultured rat cerebellar granule neurons, the precursor form of BDNF (proBDNF) enhances the LK-induced apoptosis (Koshimizu et al. 2009). We also tested the effect of pGlu-serpinin on PN-1 mRNA transcription in AtT-20 cells, since expression of PN-1 is up-regulated by CgA in AtT-20 cells (Kim and Loh 2006) and PN-1 is a potent inhibitor of plasmin which causes apoptosis of neurons if chronically exposed during vascular injury or inflammatory processes (Ho-Tin-Noé et al. 2009). We found that pGlu-serpinin had an anti-apoptotic effect on AtT-20 cells and cultured CNS neurons that were challenged to oxidative stress. Additionally, we show that treatment of AtT-20 cells with pGlu-serpinin up-regulated PN-1 mRNA expression in these cells potentially leading to effective inhibition of plasmin.
Materials and Methods
Cell Culture
AtT-20 cells were grown in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS) and primocin (Invitrogen, San Diego, CA). For steady state analysis of conditioned media from AtT-20 and 6T3 cells, 3–4 10 cm dishes of cells were cultured, and their media collected and concentrated by reverse phase chromatography through C18 sep-pak cartridges (Peninsula, Belmont, CA) described previously (Koshimizu et al. 2011). For the stimulated secretion assay on AtT-20 cells, the cells were rinsed with basal medium (DMEM supplemented with 0.01% BSA) and incubated in 1 ml of fresh basal medium for 2 h at 37°C. This medium was collected, and after rinsing with basal medium, the cells were incubated again in basal medium for 10 min and then in stimulation medium (50 mM K+ DMEM supplemented with 0.01% BSA) for an additional 10 min at 37°C. The basal and stimulated samples were concentrated with the C18 sep-pak cartridges and lyophilized. The samples were reconstituted with Block and Sample Buffer (BSB, Promega, Madison, WI) for enzyme immunoassays. Primary rat cerebral cortical neurons, prepared from E18 rat embryos, were purchased from Genlantis (San Diego, CA) and plated at a density of 2.5×105 cells/cm2 in an 8-well chambered and polyethyleneimine-coated slide in complete DMEM containing 10% heat-inactivated FBS. After 4–5 days, the media was changed to serum free neurobasal medium (Invitrogen, Carlsbad, CA) containing 2% B27 supplement (Invitrogen) and 10 μM cytosine-arabinoside (AraC) to suppress glial growth. The culture was then further incubated for 5–7 days. All animal work was performed with the approval of the animal care and use committee (ACUC) of NICHD. Cell death induction of the cultured cells was performed as previously described (Woronowicz et al. 2008). In brief, AtT-20 cells and cerebral cortical cultures were treated with 50 μM hydrogen peroxide in the presence or absence of serpinin or pGlu-serpinin for 1 day.
Generation of Anti-serpinin and Anti-pGlu-Serpinin Antisera
A rabbit polyclonal antiserum against serpinin was generated under contract with Covance (Princeton, NJ). The serpinin peptide, CKK-AEDQELESLSAIEAELEKVAHQLQAL (serpinin sequence underlined), was synthesized by Princeton Biomolecules (Longhorne, PA) and contained a di-lysine linker and N-terminal cysteine residue to which keyhole limpet hemocyanin (KLH) was coupled as a carrier for immunization. Immune serum was collected and affinity purified through a serpinin peptide column coupled at the N-terminal cysteine. This anti-serpinin antiserum detected full-length CgA on Western blots (data not shown), indicating it could detect any CgA fragment with an intact C terminus containing serpinin. It was also successfully used in immunocytochemistry to detect CgA-immunoreactivity throughout the cell (Fig. 2) and in enzyme-linked immunosorbent assays (ELISA) for serpinin peptide (Koshimizu et al. 2011). A rabbit polyclonal antiserum against a short sequence within pGlu-serpinin was generated under contract with Affinity BioReagents (ABR) (Golden, CO). This company synthesized the pGlu-serpinin peptide fragment (pGlu-ELESLSA-KKC; pGlu-serpinin fragment underlined) with the di-lysine linker and cysteine residue at its C terminus for subsequent cross-linking to KLH. The immune serum was affinity purified by a pGlu-serpinin peptide column. This antibody failed to detect full-length CgA on Western blot but was used successfully in direct and sandwich directed ELISA protocols for the detection of pGlu-serpinin standard (see below). In ELISA, this antibody cross reacted with serpinin peptide by <10% (data not shown), and it did not detect pGlu-TRH.
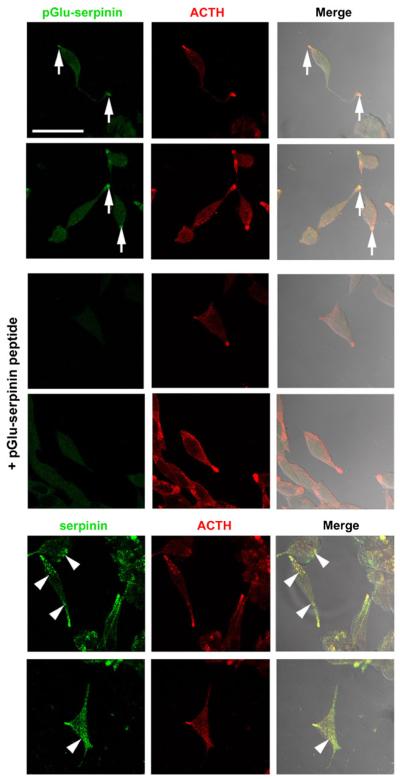
Fig. 2.
Intracellular distribution of pGlu-serpinin in AtT-20 cells. The images show that immuno-reactive pGlu-serpinin (Alexa 488, green) was detected mainly in the tips of AtT-20 cells (arrows), and co-localized (merged, yellow) with a secretory granule marker, ACTH (Cy3, red) (upper panels), whereas no immunosignal was detected in the absorption control group (+pGlu-serpinin peptide, middle panels), which was incubated with anti-pGlu-serpinin antibody in the presence of synthetic pGlu-serpinin peptide. In contrast (lower panels), immunore-active serpinin/CgA detected by anti-serpinin antibody (Alexa 488, green) was distributed not only in the tips, but also in the processes and soma (arrow heads) of AtT-20 cells. Scale bar=50 μm
Enzyme Immunoassay for pGlu-serpinin
A direct ELISA for serpinin has previously been described (Koshimizu et al. 2011) but for the specific detection of pGlu-serpinin, a sandwich ELISA was developed (Fig. 3a). As mentioned, the anti-serpinin and anti-pGlu-serpinin rabbit polyclonal antibodies were generated against a serpinin peptide or pGlu-serpinin peptide fragment, respectively. IgGs were purified and the anti-pGlu-serpinin IgGs were conjugated with biotin using Biotin Protein Labeling Kit (Roche Applied Science, Indianapolis, IN). Ninety six-well immunoassay plates (Nunc, Roskilde, Denmark) were incubated overnight at 4°C with the anti-serpinin rabbit polyclonal antibody (HL6013, 1:3,000 dilution) in a carbonate/bicarbonate buffer (pH 10.0). The plate was rinsed with tris-buffered saline containing 0.05% Tween 20 (T-TBS) and then blocked with Block and Sample Buffer (BSB, Promega, Madison, WI) at RT for 1 h. After washing, pGlu-serpinin standard or experimental sample diluted in BSB was added to the plate which was then incubated overnight at 4°C. After washing, the plate was incubated with biotin-conjugated anti-pGlu-serpinin IgG in BSB (1:3,000) for 3 h at RT. The plate was then rinsed with T-TBS five times and incubated with poly-horse radish peroxidase (pHRP)-streptavidin (1:3,000, Thermo Fisher Scientific, Waltham, MA) for 1.5 h at RT. After that, the plate was rinsed with T-TBS 5 times and the chromogenic reaction was induced with TMB-One solution (Promega) at RT for 15~30 min and terminated with 1 N hydrochloric acid. The absorbance at 450 nm was detected by Synergy HT spectrofluorometer (BIO-TEK, Winooski, VT). The anti-pGlu-serpinin IgG used is highly specific for pGlu-serpinin and cross-reacts poorly with serpinin. It also does not cross-react with thyrotropin releasing hormone (TRH), a tripeptide with a pyroglutamyl modification at the N terminus (data not shown), indicating that the antibody recognizes the pyroglutamyl moiety only in the context of the serpinin sequence.
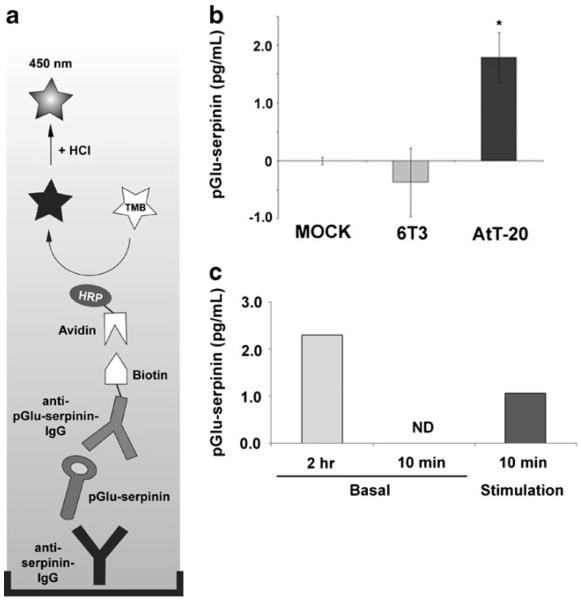
Fig. 3.
Detection of secreted pGlu-serpinin. a Schematic of the pGlu-serpinin-specific ELISA assay (see “Materials and Methods”). HRP=horseradish peroxidase; TMB=3,3′,5,5′-tetramethylbenzidine. b pGlu-serpinin-specific ELISA detected immunoreactive pGlu-serpinin in AtT-20 cell CM but none was detected in mock (medium alone without cells) or conditioned medium from 6T3 cells that do not express CgA (n=3). c Bar graphs showing pGlu-serpinin in basal (2 h and 10 min) and high K+ stimulation (10 min) incubation media from AtT-20 cells (n=2)
High Performance Liquid Chromatography and Mass Spectrometric Analysis of pGlu-Serpinin
Conditioned media from AtT-20 and 6T3 cells were previously analyzed by high performance liquid chromatography (HPLC) and ELISA, as described in detail for the initial discovery of serpinin (Koshimizu et al. 2011). Briefly, samples were concentrated through C18 reverse phase Sep-pak cartridges (Peninsula, Belmont, CA) and after reconstitution were separated by HPLC (Gilson, Middleton, WI). Fractions were assayed by ELISA for serpinin-like immunoreactivity using the anti-serpinin ELISA. Under these conditions, serpinin standard eluted into fractions #35 and #36 (see (Koshimizu et al. 2011) for HPLC profile). Mass spectrometric analysis was carried out on a parallel aliquot that was prepared from fractions #35 and #36 from which authentic serpinin was identified (Koshimizu et al. 2011). At that time, our studies continued to focus on serpinin; however, we subsequently identified the other peaks in the mass spectrum and report them here, particularly that of pGlu-serpinin (Fig. 1b).
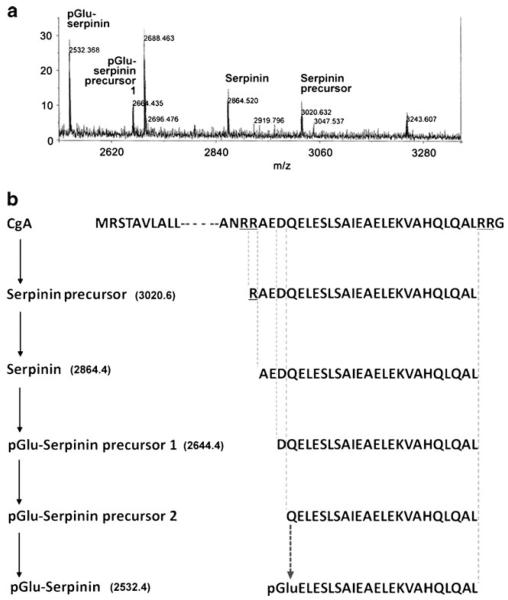
Fig. 1.
Analysis of serpinin-related peptides in AtT-20 cells. a Detection and identification of endogenous serpinin-related peptides secreted from AtT-20 cells by MALDI-TOF analysis. HPLC-fractionated AtT-20 cell-conditioned medium were analyzed by MS. The reflector spectrum shows the identification of four serpinin-related peptides. b Schematic diagram showing a predicted pGlu-serpinin biosynthetic pathway from mouse CgA based on the endogenous serpinin-related peptides identified. Ion mass of pGlu-serpinin (m/z 2532.5), pGlu-serpinin precursor 1 (m/z 2664.4), serpinin (m/z 2864.5), and serpinin precursor (m/z 3020.6) identified by MALDI-TOF analysis. pGlu-serpinin precursor 2 is a predicted intermediate of pGlu-serpinin biosynthesis
Immunocytochemistry
AtT-20 cells were grown in 8-well chamber slide (Nunc, Roskilde, Denmark) until 80% confluent. The cells were fixed in 4% paraformaldehyde (PFA) and permeabilized with 0.1% Triton X-100 in PBS. Rabbit polyclonal anti-pGlu-serpinin antibody (1:1,000), rabbit polyclonal anti-serpinin antibody (1:1,000), mouse monoclonal anti-ACTH antibody (1:1,000, Abcam, Cambridge, MA), chicken polyclonal anti-MAP-2 antibody (1:1,000, Abcam), and diamidino-2-phenyl-indole (Dapi) (1 μM; Sigma-Aldrich) were used at indicated dilutions/concentration in 1% BSA/PBS. Secondary antibody conjugated with either FITC (Molecular Probes, Eugene, OR), Alexa Fluor 488 (Invitrogen, San Diego, CA) or Cy3 (Molecular Probes) was used at 1:3,000 in 1% BSA/PBS. Images were taken using confocal microscopes (LSM 510, Carl Zeiss, Thornwood, NY) at the Microscopy and Imaging Core (MIC) facility in NICHD and processed using software (Zeiss LSM Image Browser, Carl Zeiss), Photoshop 6.0 software (Adobe, San Jose, CA) and ImageJ 1.37 (NIH, Bethesda, MD).
Cell Viability Assay
Cell viability was quantified by counting the number of dead cells by measuring lactate dehydrogenase (LDH) released into the medium using the kit (Promega) or using MAP-2 staining according to previous reports (Koshimizu et al. 2002; Nishiyama et al. 2005) with minor modifications. For the LDH assay, culture media were centrifuged at 10,000×g for 10 min and 50 μl of dye buffer were added to 10–20 μl of each supernatant. The mixtures were incubated at 37°C for 15 min and 50 μl of terminating buffer was added to each sample. The absorbance was photometrically determined at a wavelength of 560 nm. For immunostaining with anti-MAP2 polyclonal antibody, the cultured neurons were fixed using 4% paraformaldehyde (PFA) for 15 min at room temperature. The neurons were then permeabilized with 0.1% Triton X-100-containing PBS for 1 min on ice and blocked with 5% FBS-containing PBS for 1 h at RT. Immunocytochemistry was performed using chicken anti-MAP-2 polyclonal antibody and FITC-conjugated anti-chicken IgY antibody as described above. Immunosignal was quantified using ImageJ 1.37.
Real-Time RT-PCR
Real-time RT-PCR was carried out for amplification and quantitative comparison of mouse PN-1 mRNAs as previously described (Kim and Loh 2006). Briefly, total RNAs were purified using RNeasy mini kit (Qiagen, Germantown, MD) and amplified with a primer set for PN-1 (PN1-B5; 5′-GAAGGAACCATGAATTGGCAT-3′ and PN1-C3; 5′-TGCAGCATGCCCAAGATGGAC-3′) using the LightCycler and RNA master SYBR Green I (Roche Diagnostics, Indianapolis, IN). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was also amplified using a primer set (Clontech, Mountain View, CA) and used for normalization of PN-1 mRNA levels in the samples. Analyses of PCR amplification were performed using the LightCycler3 software version 5.3 (Roche Diagnostics).
Statistical Analysis
Data are presented as the means±SEM. For multiple group comparison, one-way ANOVA followed by Tukey’s post hoc test was used. For comparison between two group of means, Student’s t test was used. P<0.05 was considered statistically significant.
Results
Analysis of Serpinin-Related Peptides Secreted from Pituitary AtT-20 Cells
To search for endogenous serpinin-related peptides in pituitary cells, MALDI-TOF analysis was carried out on conditioned medium from AtT-20 cells. Four serpinin-related peptides with peptide ions in the reflector spectrum with m/z (mass-to-charge ratio) of 3,020.6, 2,864.5, 2,664.4, and 2,532.4 were detected (Fig. 1a). These peptide ions were identified as serpinin precursor (RAEDQELESLSAIEAELEKVAHQLQAL), serpinin (AEDQELESLSAIEAELEKVAHQLQAL), and its derivatives (DQELESLSAIEAELEKVAHQLQAL and pGlu-ELESLSAIEAELEKVAHQLQAL), respectively. We named the pyroglutaminated serpinin-derived peptide, which appears to be a major peptide secreted from AtT-20 cells, pGlu-serpinin (Fig. 1a). In Fig. 1b, a putative biosynthetic pathway for generation of pGlu-serpinin from mouse CgA, based on the identified serpinin-related peptides, is shown. An additional precursor peptide of pGlu-serpinin (pGlu-serpinin precursor 2, QELESLSAIEAELEKVAHQLQAL) is predicted (Fig. 1b).
Subcellular Localization of pGlu-serpinin in AtT-20 Cells
Using the anti-pGlu-serpinin specific antibody, subcellular localization of pGlu-serpinin was analyzed by immunocytochemistry in AtT-20 cells. The immunosignal of pGlu-serpinin was mainly detected in the tips of the processes and co-localized with the secretory granule marker, ACTH (Fig. 2, upper panels). To confirm that the immunosignal was derived from authentic pGlu-serpinin, an absorption control was carried out. In the presence of a synthetic pGlu-serpinin peptide, no immunostaining was observed showing that the immunosignal at the tips was specific (Fig. 2, middle panels). In addition, the intracellular distribution of pGlu-serpinin precursor molecule(s) was analyzed using anti-serpinin antibody. The immunosignal detected by the anti-serpinin antibody was distributed not only in the tips but also in the processes and soma (Fig. 2, lower panels). The differential distribution of the immunostaining pattern between the serpinin and the pGlu-serpinin-specific antibodies supports the conclusion that maturation of pGlu-serpinin precursors to form pGlu-serpinin occurs in secretory granules, and this peptide is stored in these organelles at the tips of processes together with ACTH in AtT-20 cells.
Activity-Dependent Secretion of pGlu-serpinin from AtT-20 Cells
To detect and quantify secreted pGlu-serpinin, a pGlu-serpinin ELISA was developed (Fig. 3a). To test whether pGlu-serpinin ELISA can specifically detect pGlu-serpinin secreted from CgA-expressing cells, conditioned media from AtT-20 cells and a mutant of AtT-20 cell line, 6T3 cells, which do not express CgA (Kim et al. 2001), were assayed. A significant immunosignal was detected in AtT-20 cell CM when normalized to the mock group, while no significant difference was seen in 6T3 cell CM, indicating that pGlu-serpinin secreted from CgA-expressing cells could be detected directly in a specific manner in the media using this assay (Fig. 3b). Since pGlu-serpinin is co-localized with ACTH, we assayed basal and stimulated secretion media from AtT-20 cells (Fig. 3c) to confirm its localization in secretory granules of the regulated secretory pathway. Secretion of pGlu-serpinin was detected in the medium after 2-h incubation of the cells in basal medium. After 10 min incubation in basal medium, pGlu-serpinin was below the detection limit in the secretion medium. However, after 10 min incubation in high K+ medium, pGlu-serpinin was detected, indicating this peptide is secreted in an activity-dependent manner. By calculating the proportionate amount of pGlu-serpinin in 10 min basal secretion medium based on the 2-h basal secretion medium, the fold stimulation was 5.5-fold.
Anti-Apoptotic Effect of pGlu-serpinin on AtT-20 Cells and CNS Neurons
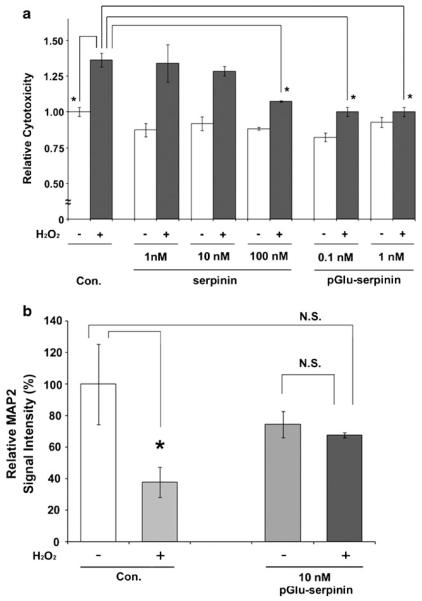
Hydrogen peroxide-induced cell death is a model system for reactive oxygen species (ROS)-induced apoptosis in neuronal cells (Woronowicz et al. 2008). In this study, AtT-20 cells were challenged with 50 μM hydrogen peroxide in the presence or absence of 1, 10, 100 nM serpinin or 0.1, 1 nM pGlu-serpinin for 1 day. Cell viability was quantified by LDH-cytotoxicity assay. pGlu-serpinin (0.1 and 1 nM) and 100 nM serpinin significantly inhibited hydrogen peroxide-induced cell death (*P<0.05), whereas 1 and 10 nM serpinin failed to inhibit the cell death (Fig. 4a). Next, cultured rat cerebral cortical neurons were challenged with 50 μM hydrogen peroxide in the presence or absence of 10 nM pGlu-serpinin for 1 day. MAP-2 immunosignal intensity was quantified. In the absence of pGlu-serpinin, treatment of hydrogen peroxide significantly induced cell death. However, in the presence of pGlu-serpinin, the hydrogen peroxide-induced neuronal cell death was inhibited (*P<0.05; N.S., no significant difference) (Fig. 4b).
Fig. 4.
Anti-apoptotic effects of pGlu-serpinin and serpinin. a AtT-20 cells were challenged with 50 μM hydrogen peroxide in the presence or absence of 1, 10, 100 nM serpinin or 0.1, 1 nM pGlu-serpinin for 1 day. Cell viability was quantified by the LDH assay. Serpinin and pGlu-serpinin significantly inhibited hydrogen peroxide-induced cell death (n=3, *P<0.05). b Cultured rat cortical neurons were challenged with 50 μM hydrogen peroxide in the presence or absence of 10 nM pGlu-serpinin for 1 day. MAP-2 immunosignal intensity was quantified. pGlu-serpinin significantly inhibited hydrogen peroxide-induced neuronal cell death (n=3 view fields, P<0.05; N.S., no significant difference)
pGlu-serpinin Up-Regulated PN-1 mRNA Expression in AtT-20 Cells
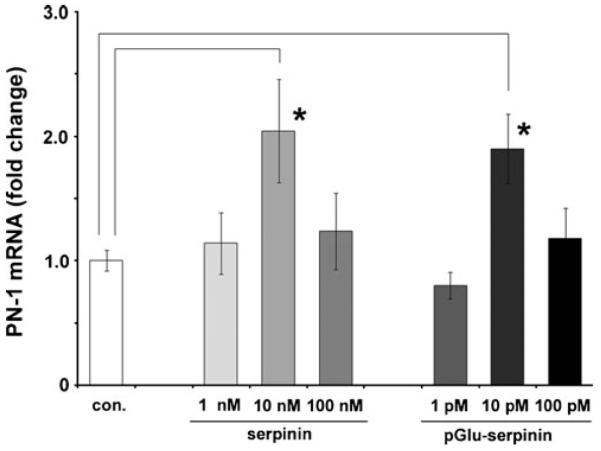
Our previous study demonstrated that serpinin induces expression of the protease inhibitor, protease nexin-1 (PN-1) in AtT-20 cells (Koshimizu et al. 2011). PN1 is a known inhibitor of plasmin, which is elevated under conditions of inflammation and leads to cell death (Ho-Tin-Noé et al. 2009). To test if pGlu-serpinin has a similar effect, AtT-20 cells were treated with pGlu-serpinin or serpinin peptide at different concentrations. Twenty hours after the treatment, real-time RT-PCR was performed to quantify the amount of PN-1 mRNA in the cells. pGlu-serpinin and serpinin significantly up-regulated PN-1 mRNA at 10 pM and 10 nM concentrations, respectively (*P<0.05), relative to untreated cells, indicating that pGlu-serpinin is a much more potent inducer of PN-1 expression than serpinin (Fig. 5). Similar to that reported previously for serpinin (Koshimizu et al. 2011), a bell-shaped curve of bioactivity was seen for serpinin as expected, but also for pGlu-serpinin. This kind of bioactivity profile is not uncommon as it has been seen for several bioactive peptides such as ghrelin, leptin, and epidermal growth factor (EGF) (Depoortere et al. 2005; Ojaniemi and Vuori 1997; Tezuka et al. 2002).
Fig. 5.
pGlu-serpinin up-regulated PN-1 mRNA expression. Bar graphs showing the effect of treatment of AtT-20 cells with synthetic serpinin (n=4) or pGlu-serpinin (n=3) for 20 h on PN-1 mRNA expression. Serpinin and pGlu-serpinin significantly up-regulated the PN-1 mRNA at 10 nM (*P<0.05) and 10 pM (*P<0.05) concentrations, respectively, relative to untreated cells (Con., n=3)
Discussion
In this study, we examined the processing of the C-terminal domain of CgA in mouse AtT-20 cells. Analysis of conditioned medium (CM) of AtT-20 cells revealed the presence of endogenous serpinin (Koshimizu et al. 2011) which indicated cleavage at the Arg433–Arg434 and Arg461–Arg462 pairs of basic residues of the C terminus domain of mouse CgA, presumably by one of the proprotein convertases (PC1 or PC2) which cleaves at paired basic residues (Fig. 1) (Steiner 1998). Carboxypeptidase E (CPE) then removes the C terminus basic residues, Arg–Arg, to yield serpinin (Fricker 1988). Two other peptides found in the CM indicated aminopeptidase activity to remove N-terminal residues from serpinin to form a peptide with a glutamine (Q) as the first amino acid at the N terminus. Interestingly, peptidomic studies of chromaffin granules from human pheochromocytomas have revealed the presence of this same serpinin-like peptide beginning with the Q residue (Hook et al. 2010), indicating that such a processing pattern of CgA is not unique to the mouse. In addition we found that the N terminus Q underwent pyroglutamination to form pGlu-serpinin which is the major product in the secretion medium and therefore likely to represent the final processed product. Nevertheless, some serpinin was found in the CM. Immunocytochemical studies using a pGlu-serpinin specific antibody revealed that the peptide is predominantly localized in the tips of processes of AtT-20 cells and co-localized with ACTH, a peptide hormone in the secretory granules of these cells (Moore et al. 1983). To confirm that pGlu-serpinin resides in secretory granules of the regulated secretory pathway, we showed that this peptide was released in an activity-dependent manner upon high K+ stimulation. Processing of CgA occurs in secretory granules and indeed proprotein convertases (Seidah et al. 2008), aminopeptidases (Gainer et al. 1984; Yasothornsrikul et al. 1998), carboxypeptidase E (CPE) (Hook and Loh 1984; Laslop et al. 1986), and glutamyl cyclase (QC) (Busby et al. 1987; Fischer and Spiess 1987) enzymes have been reported to be present in these organelles. Although QC found in several tissues such as pituitary and adrenal medulla have been shown to have a pH optimum of 7.0–7.5, unlike the other prohormone processing enzymes that have an acidic pH optimum consistent with the pH of secretory granules, the activity only drops off at pH below 5.0 and above pH 8.0 (Busby et al. 1987) and therefore can be functional within the granules. Hence, pyroglutamination and formation of pGlu-serpinin can and does occur in secretory granules of AtT-20 cells, although this process may continue extracellularly.
Our studies showed that pGlu-serpinin has anti-apoptotic effects on AtT-20 cells subjected to ROS-induced stress. pGlu-serpinin was 1,000 times more effective than serpinin at inhibiting cell death (Fig. 4a). This anti-apoptotic effect was also found for cerebral cortical neurons treated with hydrogen peroxide to induce ROS-induced stress (Fig. 4b). Our preliminary results suggest that the neuroprotective effects of serpinin may not be limited to the inhibition of hydrogen peroxide-induced cell death of AtT-20 cells and cerebral cortical neurons, since we found that low K+ (5 mM) induced apoptosis of mouse cerebellar granule neuron (CGN) culture, which is a well-established model system for neuronal apoptosis (D’Mello et al. 1993), was also significantly inhibited by addition of 10 nM serpinin to the culture medium (our unpublished data).
In cognitively impaired patients, CgA expression is elevated (Hu et al. 2010). CgA has been shown to stimulate microglia reactivity thereby promoting neurotoxicity and neurodegeneration (Kingham and Pocock 2001). It would be interesting to determine if pGlu-serpinin might also be elevated in the Alzheimer’s disease (AD) brain to counteract this damaging effect, since compensatory mechanisms often exist in many pathological conditions. Thus, pGlu-serpinin could be a useful therapeutic agent for treating AD patients. During brain injury, accompanying vascular damage and inflammatory processes occur, resulting in release of plasminogen which is rapidly converted to plasmin by tissue plasminogen activator (Kaur et al. 2004). Plasmin has been shown to degrade extracellular matrix proteins causing neuronal detachment from the matrix. Prolonged treatment (>72 h) of neurons with plasminogen leads to increase rates of apoptosis (Ho-Tin-Noé et al. 2009). Hence, increase in expression of PN-1, a potent inhibitor of plasmin (Farrell et al. 1988), which is secreted constitutively from neurons could potentially inhibit the destructive effects of plasmin under pathophysiological conditions. pGlu-serpinin’s ability to increase PN-1 synthesis (Fig. 5) may play a role in protecting cells from apoptosis by inhibiting the action of plasmin in tissues undergoing a inflammatory response. Indeed, circulating CgA has been shown to be elevated in various diseases in which inflammatory process are involved such as AD (Skaper 2007), rheumatoid arthritis (Kingsley and Panayi 1997), and irritable bowel syndrome (Ohman and Simrén 2010).
In conclusion, pGlu-serpinin is a new CgA-derived peptide that is secreted in an activity-dependent manner from secretory granules of endocrine cells and presumably other cells such as neurons that synthesize CgA. pGlu-serpinin’s anti-apoptotic effect on endocrine cells and CNS neurons and its ability to increase production of PN-1 to inhibit the destructive effect of plasmin on cells, renders it a potentially important molecule in protecting cells from dying under various pathological insults.
Acknowledgements
We would like to thank Dr. Hong Lou for technical assistance, and Chip Dye and Dr. Vincent Schram (Microscopy and Imaging Core, NICHD, NIH) for assistance with microscopic imaging. This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health intramural research program.
Abbreviations
- ACTH
Adrenocorticotropic hormone
- CgA
Chromogranin A
- HPLC
High performance liquid chromatography
- LC/MS
Liquid chromatography–mass spectrometry
- MALDI-TOF
Matrix assisted laser desorption/ionization time of flight
- PN-1
Protease nexin-1
- QC
Glutamyl cyclase
- ROS
Radical oxygen species
Contributor Information
Hisatsugu Koshimizu, Section on Cellular Neurobiology, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Bethesda, MD 20892, USA.
Niamh X. Cawley, Section on Cellular Neurobiology, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Bethesda, MD 20892, USA
Alfred L. Yergy, Section on Metabolic Analysis and Mass Spectrometry, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Bethesda, MD 20892, USA
Y. Peng Loh, Section on Cellular Neurobiology, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Bethesda, MD 20892, USA.
References
- Busby WH, Jr, Quackenbush GE, Humm J, Youngblood WW, Kizer JS. An enzyme(s) that converts glutaminyl-peptides into pyroglutamyl-peptides. Presence in pituitary, brain, adrenal medulla, and lymphocytes. J Biol Chem. 1987;262:8532–8536. [PubMed] [Google Scholar]
- Depoortere I, De Winter B, Thijs T, De Man J, Pelckmans P, Peeters T. Comparison of the gastroprokinetic effects of ghrelin, GHRP-6 and motilin in rats in vivo and in vitro. Eur J Pharmacol. 2005;515:160–168. doi: 10.1016/j.ejphar.2005.04.008. [DOI] [PubMed] [Google Scholar]
- D’Mello SR, Galli C, Ciotti T, Calissano P. Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc Natl Acad Sci USA. 1993;90:10989–10993. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell DH, Wagner SL, Yuan RH, Cunningham DD. Localization of protease nexin-1 on the fibroblast extracellular matrix. J Cell Physiol. 1988;134:179–188. doi: 10.1002/jcp.1041340203. [DOI] [PubMed] [Google Scholar]
- Fischer WH, Spiess J. Identification of a mammalian glutaminyl cyclase converting glutaminyl into pyroglutamyl peptides. Proc Natl Acad Sci USA. 1987;84:3628–3632. doi: 10.1073/pnas.84.11.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker LD. Carboxypeptidase E. Annu Rev Physiol. 1988;50:309–321. doi: 10.1146/annurev.ph.50.030188.001521. [DOI] [PubMed] [Google Scholar]
- Gainer H, Russell JT, Loh YP. An aminopeptidase activity in bovine pituitary secretory vesicles that cleaves the N-terminal arginine from beta-lipotropin 60–65. FEBS Lett. 1984;175:135–139. doi: 10.1016/0014-5793(84)80586-4. [DOI] [PubMed] [Google Scholar]
- Hook VY, Loh YP. Carboxypeptidase B-like converting enzyme activity in secretory granules of rat pituitary. Proc Natl Acad Sci USA. 1984;81:2776–2780. doi: 10.1073/pnas.81.9.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook V, Bark S, Gupta N, Lortie M, Lu WD, Bandeira N, Funkelstein L, Wegrzyn J, O’Connor DT, Pevzner P. Neuropeptidomic components generated by proteomic functions in secretory vesicles for cell–cell communication. AAPS J. 2010;12:635–645. doi: 10.1208/s12248-010-9223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho-Tin-Noé B, Enslen H, Doeuvre L, Corsi JM, Lijnen HR, Anglés-Cano E. Role of plasminogen activation in neuronal organization and survival. Mol Cell Neurosci. 2009;42:288–295. doi: 10.1016/j.mcn.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Hu WT, Chen-Plotkin A, Arnold SE, Grossman M, Clark CM, Shaw LM, Pickering E, Kuhn M, Chen Y, McCluskey L, et al. Novel CSF biomarkers for Alzheimer’s disease and mild cognitive impairment. Acta Neuropathol. 2010;119:669–678. doi: 10.1007/s00401-010-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J, Zhao Z, Klein G, Lo E, Buchan A. The neurotoxicity of tissue plasminogen activator? J Cereb Blood Flow Metab. 2004;24:945–963. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
- Kim T, Loh YP. Protease nexin-1 promotes secretory granule biogenesis by preventing granule protein degradation. Mol Biol Cell. 2006;17:789–798. doi: 10.1091/mbc.E05-08-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Tao-Cheng JH, Eiden LE, Loh YP. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell. 2001;106:499–509. doi: 10.1016/s0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- Kingham PJ, Pocock JM. Microglial secreted cathepsin B induces neuronal apoptosis. J Neurochem. 2001;76:1475–1484. doi: 10.1046/j.1471-4159.2001.00146.x. [DOI] [PubMed] [Google Scholar]
- Kingsley G, Panayi GS. Joint destruction in rheumatoid arthritis: biological bases. Clin Exp Rheumatol. 1997;15(Suppl 17):S3–S14. [PubMed] [Google Scholar]
- Koshimizu H, Araki T, Takai S, Yokomaku D, Ishikawa Y, Kubota M, Sano S, Hatanaka H, Yamada M. Expression of CD47/integrin-associated protein induces death of cultured cerebral cortical neurons. J Neurochem. 2002;82:249–257. doi: 10.1046/j.1471-4159.2002.00965.x. [DOI] [PubMed] [Google Scholar]
- Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, Uegaki K, Nagappan G, Zaitsev E, Hirokawa T, Tatsu Y, et al. Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Mol Brain. 2009;2:27. doi: 10.1186/1756-6606-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu H, Kim T, Cawley NX, Loh YP. Chromogranin A: a new proposal for trafficking, processing and induction of granule biogenesis. Regul Pept. 2010;160:153–159. doi: 10.1016/j.regpep.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu H, Cawley NX, Kim T, Yergey AL, Loh YP. Serpinin: a novel chromogranin a-derived, secreted peptide up-regulates protease nexin-1 expression and granule biogenesis in endocrine cells. Mol Endocrinol. 2011 doi: 10.1210/me.2010-0124. doi: 10.1210/me.2010-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laslop A, Fischer-Colbrie R, Hook V, Obendorf D, Winkler H. Identification of two glycoproteins of chromaffin granules as the carboxypeptidase H. Neurosci Lett. 1986;72:300–304. doi: 10.1016/0304-3940(86)90530-6. [DOI] [PubMed] [Google Scholar]
- Moore HP, Gumbiner B, Kelly RB. Chloroquine diverts ACTH from a regulated to a constitutive secretory pathway in AtT-20 cells. Nature. 1983;302:434–436. doi: 10.1038/302434a0. [DOI] [PubMed] [Google Scholar]
- Nishiyama K, Konishi A, Nishio C, Araki-Yoshida K, Hatanaka H, Kojima M, Ohmiya Y, Yamada M, Koshimizu H. Expression of cystatin C prevents oxidative stress-induced death in PC12 cells. Brain Res Bull. 2005;67:94–99. doi: 10.1016/j.brainresbull.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163–173. doi: 10.1038/nrgastro.2010.4. [DOI] [PubMed] [Google Scholar]
- Ojaniemi M, Vuori K. Epidermal growth factor modulates tyrosine phosphorylation of p130Cas. Involvement of phosphatidylinositol 3′-kinase and actin cytoskeleton. J Biol Chem. 1997;272:25993–25998. doi: 10.1074/jbc.272.41.25993. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Mayer G, Zaid A, Rousselet E, Nassoury N, Poirier S, Essalmani R, Prat A. The activation and physiological functions of the proprotein convertases. Int J Biochem Cell Biol. 2008;40:1111–1125. doi: 10.1016/j.biocel.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Skaper SD. The brain as a target for inflammatory processes and neuroprotective strategies. Ann NY Acad Sci. 2007;1122:23–34. doi: 10.1196/annals.1403.002. [DOI] [PubMed] [Google Scholar]
- Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- Tezuka M, Irahara M, Ogura K, Kiyokawa M, Tamura T, Matsuzaki T, Yasui T, Aono T. Effects of leptin on gonadotropin secretion in juvenile female rat pituitary cells. Eur J Endocrinol. 2002;146:261–266. doi: 10.1530/eje.0.1460261. [DOI] [PubMed] [Google Scholar]
- Woronowicz A, Koshimizu H, Chang SY, Cawley NX, Hill JM, Rodriguiz RM, Abebe D, Dorfman C, Senatorov V, Zhou A, et al. Absence of carboxypeptidase E leads to adult hippocampal neuronal degeneration and memory deficits. Hippocampus. 2008;18:1051–1063. doi: 10.1002/hipo.20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasothornsrikul S, Toneff T, Hwang S, Hook V. Arginine and lysine aminopeptidase activities in chromaffin granules of bovine adrenal medulla: relevance to prohormone processing. J Neurochem. 1998;70:153–163. doi: 10.1046/j.1471-4159.1998.70010153.x. [DOI] [PubMed] [Google Scholar]