Abstract
Hepatocellular carcinoma, one of the most common solid tumors worldwide, is poorly responsive to available chemotherapeutic approaches. While systemic chemotherapy is of limited benefit, intra-arterial delivery of doxorubicin to the tumor frequently produces tumor shrinkage. Its utility is limited, in part, by the frequent emergence of doxorubicin resistance. The mechanisms of this resistance include increased expression of multidrug resistance efflux pumps, alterations of the drug target, topoisomerase, and modulation of programmed cell death pathways. Many of these effects result from changes in miRNA expression and are particularly prominent in tumor cells with a stem cell phenotype. This review will summarize the current knowledge on the mechanisms of doxorubicin resistance of hepatocellular carcinoma and the potential for approaches toward therapeutic chemosensitization.
Practice points.
Doxorubicin-based chemoembolization is a key therapy for hepatocellular carcinoma (HCC), but its utility is limited by pre-existing and acquired tumor resistance.
The doxorubicin anti-tumor effect primarily involves topoisomerase-mediated double-strand DNA breaks with the subsequent triggering of DNA damage associated cell cycle arrest and apoptosis pathways.
Clinical resistance of HCC to doxorubicin involves multiple mechanisms largely related to changes in drug accumulation, topoisomerase activity or apoptosis signaling.
Changes in DNA damage triggered apoptosis are responsible for the major fraction of clinical resistance.
Specific miRNA increases and decreases are a primary mechanism for effecting these changes.
Therapeutic approaches to overcome resistance are an important future goal but have not yet reached clinical practice.
Hepatocellular carcinoma (HCC) is one of the major causes of cancer deaths worldwide and its incidence is increasing [1]. The therapy for HCC remains suboptimal and treatment with traditional cytotoxic chemotherapeutic agents such as cisplatin, doxorubicin and 5-FU has been limited by systemic toxicity, poor efficacy, and acquired resistance of the tumors after exposure [2–4]. Sorafenib, a multi-kinase inhibitor, has been shown to produce modest increases in survival of selected patients [5,6], but its effects are relatively small and it is not tolerated by patients with more advanced liver disease. Improvements in patient outcome have thus largely resulted from the use of surgical resection, local ablative techniques and liver transplantation [7,8].
One of the more promising developments in HCC treatment has been in targeted delivery of cytotoxic chemotherapy agents directly to the tumor. Selective injection of embolizing agents in combination with doxorubicin into arteries feeding tumors, or trans-arterial chemoembolization (TACE), has been shown to provide a survival benefit in patients with unresectable HCC [9,10] and is now the standard of care for patients with intermediate stage HCC [7]. Embolization of the tumor alone causes ischemia and can produce tumor shrinkage. However, the combination of the embolic effect with the addition of a chemotherapy agent, typically doxorubicin, has been shown in large randomized studies to increase tumor response, decrease progression and improve overall survival [11,12]. The use of embolic drug-eluting microspheres that release doxorubicin (DEBDOX) in a controlled manner has thus improved the TACE technique allowing for higher doses with reduced systemic exposure [13]. Doxorubicin-based TACE now plays an important role in shrinking (downstaging) tumor size and number to allow eligibility for liver transplantation [9].
Resistance to doxorubicin has thus emerged as a central problem limiting treatment of patients with HCC. While TACE is highly effective in many patients, approximately 50% of tumors treated with DEBDOX show no response and only 27% show a complete response [12,13]. One of the important goals of therapy for HCC is to better understand the mechanism of doxorubicin resistance so that new or adjunct approaches can improve the effectiveness of treatment. This review will summarize our knowledge of the mechanisms of doxorubicin resistance in HCC with an eye toward possible development of chemosensitization approaches.
Mechanisms of doxorubicin antitumor effects
Doxorubicin is an anthracycline antibiotic that is widely used as a human antitumor therapeutic agent. Doxorubicin sensitivity is the result of diffusion of the drug to the nucleus and a series of signaling events that are initiated by doxorubicin's interaction with DNA. This ultimately leads to a series of programmed responses culminating in cell apoptosis. It appears to have multiple antitumor effects but the best understood of these involves its interaction with topoisomerase IIα (TOP2A) [14]. This enzyme is involved in separating entangled DNA strands and as part of its function it transiently generates and then repairs protein-bound double-strand DNA breaks (DSBs) [15]. Doxorubicin stabilizes the cleaved-strand intermediate, suppressing the completion of the process resulting in numerous protein-bound DSBs [14]. DSBs have numerous negative consequences for cells and notably trigger caspase-dependent apoptosis programs that involve the activation of master regulators p53 and FOXO3, and suppression of pro-growth signaling pathways, that lead to changes in the ratio of anti/pro-apoptotic Bcl-2 family proteins [16]. This DNA damage response is the primary factor accounting for the antitumor effect of doxorubicin and blocking just this downstream response to DNA damage is sufficient to attenuate doxorubicin toxicity [17]. Multiple other mechanisms have been observed to be involved in doxorubicin cytotoxicity as well and these include the formation of TOP2A-independent DNA adducts [18], inhibition of DNA and RNA synthesis and mitochondrial ROS production triggering apoptosis [19].
Molecular mechanisms of doxorubicin resistance
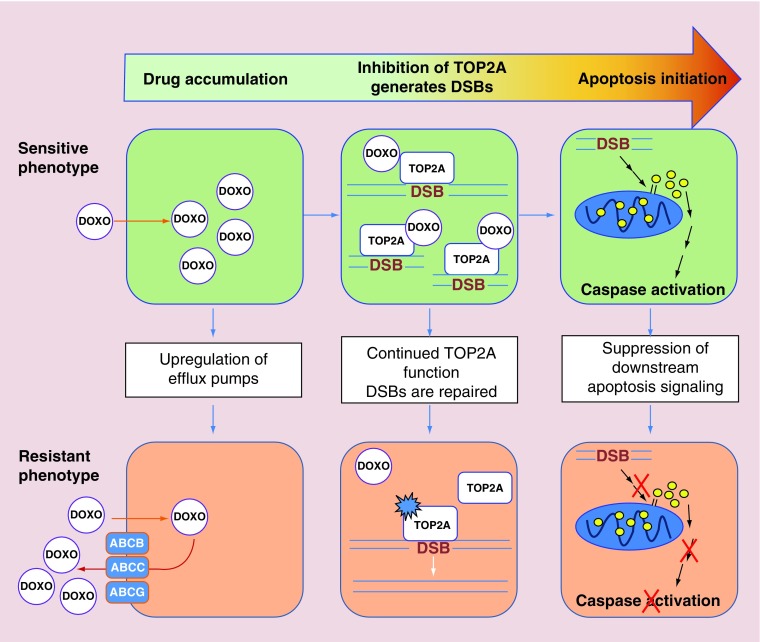
Doxorubicin resistance results from reduction in the ability of the drug to accumulate in the nucleus, decreased DNA damage and suppression of the downstream events that transduce the DNA damage signal into apoptosis. The general mechanisms involved are illustrated in Figure 1.
Figure 1. . General mechanisms of doxorubicin resistance in hepatocellular carcinoma.
Doxorubicin first must accumulate within the cell but this process is inhibited by the upregulation of ABC family efflux pumps in resistant cells. Doxorubicin then prevents the repair of TOP2A-generated DSBs in DNA, increasing TOP2A-bound DSBs. Overexpression and mutations in TOP2A allow continued TOP2A function in resistant cells. Finally, DNA damage induces apoptotic signaling pathways causing cytochorome C release from mitochondria which leads to caspase activation and cell death. Downregulation of the effectors of apoptosis and upregulation of anti-apoptotic proteins prevents the completion of apoptosis in resistant cells.
ABC: ATP-binding cassette; DSB: Double-strand DNA break.
• Multidrug resistance transporters
Doxorubicin is a hydrophobic molecule that passes through cellular membranes independently of specific transporters. However, cells can fail to accumulate the drug through active drug efflux via ATP-dependent efflux transporters. This phenomenon, first described in a number of cancers and labeled ‘multidrug resistance’, results from the expression of a group of multidrug resistance efflux pumps. These proteins, members of the ATP-binding cassette (ABC) transporter family, were initially identified for their pathological role in tumors before their normal physiological functions were understood. They are now known to be important components of transport in a number of tissues. Hepatocytes use multiple different ABC transporters for the transport of organic ions such as bile acids and conjugated bilirubin [20]. Since these pumps are highly abundant in hepatocytes, it is not surprising that they are expressed in HCC as well where increased expression results in chemotherapy resistance.
The basal expression of ABC proteins is controlled by multiple transcription factors including NF-Y and members of the Sp family [21]. Additionally, p53 has been shown to repress transcription of ABC family proteins [22] while several transcription factors including both AP-1 [23] and NF-κB [24] are capable of upregulating their expression. Activity of the enzyme COX–2 has also been implicated in the control of MDR1 expression as the COX-2 inhibitor, celecoxib, decreases MDR1 expression in multidrug-resistant HCC cells [25,26]. In HCC, three ABC subfamilies, ABCB (the MDR proteins), ABCC (MRP proteins) and ABCG (BCRP proteins) may contribute to doxorubicin resistance. Although they have different substrate specificities during function in normal physiologic conditions, they have all shown an ability to transport doxorubicin [27–29]. MDR1, MRP1, MRP2 and MRP3 are all expressed in HCC at the transcriptional level. MDR1 protein expression is found in 80–90% of HCC cases [27]. MDR family proteins have also been found to be expressed and functionally active on mitochondrial membranes, perhaps protecting mitochondrial DNA from drug-induced damage by keeping the drug out of mitochondria, or suppressing apoptosis by altering mitochondrial outer membrane permeability [30].
MDR1 expression was found to inversely correlate with response to systemic chemotherapy in one study [27], but the precise extent to which expression of ABC proteins accounts for clinical drug resistance is less clear. The situation is somewhat clearer in cell culture models of HCC. One method of generating doxorubicin-resistant cultured HCC cells for study is to select for resistant HCC cells in vitro after exposing them to incrementally increasing doses of doxorubicin. This method consistently induces the expression of MDR1 and other ABC family members [31,32], and the upregulation of these transporters can be shown to cause drug resistance since inhibitors of ABC proteins such as verapamil and cyclosporine A are able to restore doxorubicin sensitivity [33]. However, verapamil has not proven to be useful as a doxorubicin sensitizing agent in patients, perhaps due to the presence of other efflux transporters, pharmacological interactions between the drugs [34], the loss of important normal physiological functions [35], or the presence of unrelated resistance mechanisms. Therefore, while overexpression of MDR efflux pumps may be an important cause of drug resistance in deliberately selected HCC cell lines in vitro, other mechanisms appear likely to be important in human disease.
• Topoisomerase II α
The primary means by which doxorubicin causes cellular toxicity is by targeting the alpha isoform of topoisomerase II (TOP2A) [14], resulting in numerous protein-bound DSBs and the subsequent triggering of apoptosis [36]. It has been hypothesized that one mechanism of resistance to doxorubicin might be through the reduction in TOP2A expression and increased reliance on the beta isoform of topoisomerase II that is less sensitive to doxorubicin [36]. Supporting this hypothesis is the finding that breast cancers with co-amplified HER2 and TOP2A genes have increased sensitivity to doxorubicin, while tumors with a TOP2A deletion have increased resistance [37,38]. Additionally, this mechanism of resistance to doxorubicin has been seen in several cancer cell lines [39]. The situation in HCC, however, appears to be different where TOP2A is increased rather than decreased. In HCC, TOP2A protein level has been shown to be increased independently of gene amplification in 73% of human HCC tumors compared with adjacent non-tumor tissue [40]. It is also overexpressed in several HCC cell lines with acquired doxorubicin resistance [41]. Furthermore, TOP2A expression was found to be positively correlated with histological grade, vascular invasion and early age of onset in a tissue microarray of 172 HCC tumors, and it positively correlated with doxorubicin resistance and shorter survival in 148 patients in a prospective randomized study [42]. TOP2A overexpression has been found to be associated with several indices of tumor aggressiveness in many other types of cancer as well, presumably due to the role of TOP2A in facilitating DNA replication and transcription. While the association of increased TOP2A with tumor growth seems logical, it is not understood why it is also associated with doxorubicin resistance. One hypothesis is that the high expression levels are associated with the development of mutations in TOP2A that lead to its insensitivity to doxorubicin [43]. Another possibility is that in order for cells to survive the high levels of TOP2A they must simultaneously suppress the downstream apoptosis programs normally triggered by DNA strand breaks and it is the acquisition of this adaptive characteristic that confers doxorubicin resistance. At the present time, this issue remains unresolved.
• p53
The tumor suppressor p53 is a frequently altered target in doxorubicin-resistant HCC. It is one of the key DNA damage sensors and acts as a transcriptional activator of pro-apoptotic factors including Bax, Bak, CD95 and TRAIL receptors [44,45]. In addition, it transcriptionally represses anti-apoptotic factors including Bcl-2 and Survivin [46,47]. Doxorubicin upregulates p53 [48], which occurs through its phosphorylation by DDR kinases, which inhibit its binding to and phosphorylation by MDM2, part of the pathway of constitutive ubiquitination and proteosomal degradation that normally leads to low steady-state levels of p53 [49]. An inhibitor of MDM2–p53 binding, Nutlin-3 has been shown to enhance p53 stabilization and activation, and increases doxorubicin sensitivity in HCC cells with wild-type p53 [50]. Mutation or deletion of p53, or disruption of p53 activation pathways are frequent events in HCC tumorigenesis, providing a possible mechanism for intrinsic resistance to doxorubicin [51]. The specific role of p53 in doxorubicin resistance has been illustrated by experiments showing that restoring p53 expression in HCC cells promotes doxorubicin-induced apoptosis [52].
While it thus might seem attractive to target the regulation of p53, attempts to manipulate p53 by interfering with upstream regulators have produced some unanticipated and paradoxical results. For example, one recent study showed that inhibiting the deubiquitinase USP9x increased p53 ubiquitination and thus decreased p53 levels, yet it still increased doxorubicin sensitivity in HCC cells. This suggests that the effects of ubiquitination inhibitors are more complex than simply causing the degradation of p53 [53]. Furthermore, increasing p53 is clearly not the only way to enhance doxorubicin sensitivity as the three hepatoma cell lines, Huh7, Hep3B, and HepG2 illustrate. HepG2 cells, which have wild-type p53, are the most resistant, and Huh7 and Hep3B which are p53 defective are more doxorubicin sensitive. Clearly, p53, while important, does not account for the complete phenomenon of doxorubicin resistance [54].
• NF-κB
NF-κB is also a transcription factor that has multiple, sometimes opposing functions, such as tumor suppression or promotion depending on the cellular context. In HCC associated with inflammation, such as in HCV or HBV infection, NF-κB tends to have a tumor promoting effect, while in tumors induced by carcinogens such as DEN, NF-κB functions as a tumor suppressor [55]. NF-κB signaling is activated by DNA damage and can have varying effects on subsequent apoptosis primarily through regulation of its target genes, such as Bcl-XL and XIAP [56]. In general, NF-κB has an anti-apoptotic effect in response to drugs that induce DSBs in DNA such as doxorubicin although it may be partially dependent on the cancer cell type [57]. There are few studies investigating the role of NF-κB in resistance to doxorubicin in HCC although it has been shown to be activated in HCC cells in response to doxorubicin [58] and several studies have indicated that activation of NF-κB is a mechanism by which a diverse set of stimuli generate an anti-apoptotic effect. For example, the antiapoptotic gene BAG–1 was found to enhance doxorubicin resistance by potentiating the transcriptional activity of NF-κB [59]. Additionally, the HBV protein HBx has been shown to increase doxorubicin resistance through the activation of NF-κB in HCC cells [60], and reduced expression of miR-26b in HCC promotes doxorubicin resistance due to the loss of its suppression of NF-κB signaling [58].
• FOXO3
FOXO3 is a multifunctional transcription factor involved in the adaptation of cells to a nonproliferating state. It was initially identified as a longevity factor responsible for antioxidant responses, cell cycle arrest, and stem cell survival [61]. Under certain conditions, however, it also promotes apoptosis and some combination of its cell cycle arrest and apoptosis-inducing properties allows it to function as a tumor suppressor [62]. FOXO3 may function as a tumor suppressor in HCC and has been shown to promote apoptosis in HCC cells exposed to various toxic compounds [63–68]. Regulation of FOXO3 is primarily through post-translational modifications including phosphorylation by Akt at three sites that promotes its nuclear export and degradation [61].
FOXO3 mediates doxorubicin-induced apoptosis in a number of different tumor cell types. Doxorubicin increases nuclear accumulation of FOXO3 in breast cancer [69,70], lung cancer, neuroblastoma [71] and osteosarcoma cells [72], and pharmacological approaches that inhibit Akt or otherwise increase FOXO3 nuclear accumulation work synergistically with doxorubicin to enhance apoptosis [73,74]. The mechanisms by which FOXO3 mediates doxorubicin-induced apoptosis include its transcriptional repression of miR-21 which represses translation of Fas-L [71], transcriptional upregulation of Bim, a pro-apoptotic Bcl-2 homolog [75], and transcriptional repression of Bcl-2 [76] and Survivin, an anti-apoptotic Bcl-2 family member [77].
Understanding the role of FOXO3 in doxorubicin resistance is complicated by the fact that FOXO3 can be responsible either for enhanced cell survival or enhanced apoptosis [78]. This ability of FOXO3 to transition between a cell survival factor and a cell death factor may explain the seemingly paradoxical finding that this ‘tumor suppressor’ is frequently increased in poor prognosis tumors. Increased FOXO3 expression has been observed in breast cancer [69] and certain leukemias that have developed doxorubicin resistance [79], with one potential mechanism being the ability of FOXO3 to transcriptionally activate MDR1 [80].
Recent evidence has shown that the pro-death versus prosurvival balance of FOXO3 is controlled by specific post-translational modifications. Phosphorylation of FOXO3 at serine-7 by p38 causes it to translocate to the nucleus in response to doxorubicin [70] and phosphorylation at serine-574 causes it to selectively bind to pro-apoptotic promoters and induces cell death. In the absence of this phosphorylation, FOXO3 initiates an antioxidant and cell protective transcriptional program [76]. Thus, whether FOXO3 serves as a pro-apoptotic or pro-survival factor likely depends on the state of its modification by upstream enzymes. The situation in HCC has not been studied in as much detail but preliminary studies from our lab show that FOXO3 mediates doxorubicin-induced apoptosis in HCC cells and doxorubicin-resistant human HCCs have higher cytosolic FOXO3 than doxorubicin-sensitive tumors [81].
• PI3K/Akt
Another class of resistance mechanisms is signaling pathways that are drivers of tumor cell proliferation. These frequently inhibit apoptosis during tumorigenesis as well as after chemotherapy exposure. One such signaling pathway is the PI3K/Akt pathway. Akt is activated through phosphorylation by the second messenger PI3K following growth factor stimulation and in response to many cell stressors [82]. It is negatively regulated by the phosphatase, PTEN [83]. Akt then directly and indirectly regulates cell proliferation and apoptosis by phosphorylating and modulating target protein function including FOXO3, Bad, p53, and cyclin-dependent kinase inhibitors [82], as well as by activating parallel pro-growth pathways [83]. This pathway is frequently activated in HCC and is correlated with decreased overall survival [84]. Several studies have shown that inhibiting PI3K/Akt function using pharmacological inhibitors [85,86] or by exogenous overexpression of an upstream inhibitor [87,88] increases HCC cell sensitivity to doxorubicin, while activating PI3K/Akt has the opposite effect [87,89].
• MAP kinases
The MEK/ERK signaling pathway is another important pathway that translates growth signals from the cell surface to transcription factors and other regulatory proteins to promote cell proliferation and inhibit apoptosis [83]. It promotes HCC tumor cell growth and it is frequently activated in HCC. It has also been shown to be activated by doxorubicin [90], serving as a tumor cell response that counters doxorubicin-induced toxicity. Direct inhibition of ERK activity increases doxorubicin sensitivity in HCC cells by inhibiting cell proliferation and promoting apoptosis [90]. Inhibition of EGFR, an upstream activator of the MEK/ERK pathway, also increases doxorubicin sensitivity in HCC cells [91]. In addition, the mechanism of action of the tyrosine kinase inhibitor, Sorafenib, which has been used as a systemic chemotherapeutic treatment for advanced HCC, also involves inhibition of the MEK/ERK pathway [92]. In a randomized controlled trial of patients with advanced stage HCC, sorafenib plus systemic doxorubicin was shown to increase patient overall survival compared with doxorubicin treatment alone [93].
The p38 MAPK pathway is also activated by doxorubicin and may play a role in regulating doxorubicin-induced apoptosis. Its activation is necessary for the phosphorylation of FOXO3 responsible for its nuclear translocation following doxorubicin treatment in breast cancer cells [70]. MK5, a downstream target of p38, is upregulated in HCC cells and downregulated by doxorubicin. Overexpression of MK5 decreased doxorubicin-induced apoptosis [94].
• Sirtuins
The sirtuin family of NAD-dependent deacetylases is also known to play a crucial regulatory role in the cellular response to stress, apoptosis, metabolism and aging [95]. There are seven members of the sirtuin family in humans, SIRT1–7, and the expression of several SIRTs are altered in HCC, some with pro-tumorigenic and some with anti-tumorigenic effects [95–98]. SIRT1 is consistently found to be overexpressed in HCC [99], and was shown to inhibit doxorubicin-induced apoptosis in HCC cells [95]. The mechanism for SIRT1–mediated inhibition of doxorubicin sensitivity in HCC is unknown but it may involve the deacetylation of p53 [100], FOXO3 [101] or YAP2 [102], where deacetylation of each of these factors has been shown to inhibit its apoptotic activity. Additionally, in breast cancer cells with acquired resistance to epirubicin, a doxorubicin homolog, SIRT4, 5, 6 and 7 were found to be upregulated, particularly SIRT6, which was shown to mediate epirubicin resistance by deacetylation and inhibition of FOXO3 [103].
• MicroRNAs
Noncoding RNAs have been a recent focus in attempts to understand the mechanisms of chemotherapy resistance. Although noncoding RNAs have diverse functions, much recent focus has been on miRNAs, particularly examining their ability to modulate known mechanisms of resistance through suppression of mRNA translation. By comparing miRNA expression patterns in doxorubicin-sensitive and doxorubicin-resistant tumors a number of resistance-associated miRNA changes have been observed [104,105]. Some of these have been shown to cause resistance while others have a plausible mechanism for inducing resistance that has not yet been proven. There are many other associations for which there is as yet no evidence of a causative connection between the miRNA profile and the resistance phenotype. A summary of miRNA changes that have been shown to have a causative link to doxorubicin resistance is presented in Table 1.
Table 1. . miRNAs associated with doxorubicin resistance.
| miRNA | Change in resistant tumor | Cell type | Target gene(s) | Ref. |
|---|---|---|---|---|
| miR-215 | ↑ | Hepatocyte | DHFR, TS | [111] |
| miR-26b | ↓ | Hepatocyte | TAK1, TAB3 | [58] |
| miR-122 | ↑ | Hepatocyte | Cyclin G1 | [106] |
| miR-199a–3p | ↓ | Hepatocyte | mTOR, c-Met | [110] |
| miR-519d | ↑ | Hepatocyte | P21, PTEN, AKT3, TIMP2 | [112] |
| miR-101 | ↓ | Hepatocyte | EZH2 | [109] |
| miR-223 | ↓ | Hepatocyte | ABCB1 | [108] |
| Let–7a | ↑ | Hepatocyte, SCC | Caspase–3 | [125] |
| miR-138 | ↓ | HNSCC | MDR1 | [126] |
| miR-21 | ↑ | Breast | PTEN | [127] |
| miR-760 | ↓ | Breast | RHOB, ANGPTL4, ABCA1 | [128] |
| miR-218 | ↓ | Breast | Survivin | [129] |
| miR-298 | ↓ | Breast | MDR1 | [130] |
| miR-450b–3p | ↓ | Breast | HER3 | [131] |
| miR-451 | ↓ | Breast | MDR1 | [132] |
| miR-452 | ↓ | Breast | IGF–1R | [133] |
| miR-200c | ↓ | Breast | ZEB1 | [134] |
| miR-34a | ↓ | Breast | NOTCH1 | [135] |
| miR-34c | ↓ | Osteosarcoma | NOTCH1, LEF1 | [136] |
| miR-382 | ↓ | Osteosarcoma | HIPK3 | [137] |
| miR-301a | ↑ | Osteosarcoma | AMPKα1 | [138] |
| miR-708 | ↓ | Ewing sarcoma | EYA3 | [139] |
| miR-125b | ↑ | Ewing sarcoma | P53, BAK | [140] |
| miR-522 | ↓ | Colon | ABCB5 | [141] |
| miR-101 | ↓ | Colon | SphK1 | [142] |
| miR-195 | ↓ | Colon | Bcl-w | [143] |
| miR-103/107 | ↓ | Gastric | Cav–1 | [144] |
| miR-508–5p | ↓ | Gastric | ABCB1, ZNRD1 | [145] |
| miR-331–5p | ↓ | Leukemia | MDR1 | [146] |
| miR-27a | ↓ | Leukemia | MDR1 | [146] |
DHFR: Dihydrofolate reductase; TS: Thymididylate synthase; Cav–1: Caveolin–1; SCC: Squamous cell carcinoma; HNSCC: Head and neck SCC.
Several miRNA changes are both associated with doxorubicin resistance in HCC and have also been demonstrated to produce resistance in model systems. miR-122, the liver-specific miRNA that represents a large proportion of total miRNAs expressed in the liver, is frequently reduced in HCC [106]. Two separate studies show that restoration of miR-122 in HCC cells increases their sensitivity to doxorubicin due to suppression of cell cycle progression, anti-apoptosis effectors and ABC transporter proteins [106,107]. miR-223 was also shown to increase doxorubicin sensitivity by suppressing MDR1 expression in HCC cells [108]. miR-26b is another miRNA that is downregulated in HCC. It normally suppresses TAK1 and TAB3 which are NF-κB activating proteins. Thus the result of miR-26b suppression is the increased activation of NF-κB, which then promotes resistance. Overexpression of miR-26b in HCC cells inhibits NF-κB activation increasing doxorubicin sensitivity [58]. The downregulation of miR-101 [109], miR-199a–3p [110] and miR-215 [111] is also associated with doxorubicin resistance in HCC and restoring their expression has been shown to increase doxorubicin sensitivity. miR-519d, is overexpressed in HCC and has been shown to inhibit apoptosis and promote tumor cell growth by reducing the expression of several tumor suppressor proteins including p21 and PTEN. Significantly, overexpression of miR-519d in HCC cells was shown to decrease sensitivity to doxorubicin [112].
• Role of cancer stem cells
An important consideration in understanding HCC chemoresistance is to identify whether resistance is primarily a property of the bulk tumor cells or a subpopulation. The cancer stem cell model proposes that there is a limited population of cancer stem cells (CSCs) within a tumor with properties of stem/progenitor cells including self-renewal, and multipotency that are responsible for initiation and maintenance of the tumor [113]. The existence of CSCs in human HCC has been experimentally validated through isolation and xenotransplantation assays in immunodeficient mice, and CSCs have proven to have a pivotal role in the development and progression of HCC [114]. CSCs have been shown to be particularly resistant to chemotherapy [115] and cells surviving in the region of trans-arterial embolization-treated tumors display the CSC marker, CD13 [116], suggesting they are a major source of treatment failure and recurrence. Other markers of CSC expression in human HCC, specifically EpCAM and CD133, were found to be increased in TACE-doxorubicin-treated HCC and associated with tumor recurrence after transplant [117]. The mechanisms for doxorubicin resistance in HCC CSCs appear to be multiple including an increased expression of MDR transporters [118,119] and increased Akt and Bcl-2 cell survival signaling [120], possibly resulting from altered miRNA expression [121]. Treatments specifically targeting proteins and pathways differentially expressed in HCC CSCs, such as anti-CD13 antibodies, have shown promise in increasing the efficacy of doxorubicin treatment [116].
Conclusion
Poor response to doxorubicin-based loco-regional chemotherapy is a major obstacle to treatment of patients with HCC. Resistance of tumor cells to doxorubicin itself is an important component of clinical treatment failure and recurrence. Multiple mechanisms are responsible for doxorubicin resistance and these include the presence of drug efflux transporters, alterations in the ability of doxorubicin to form DSBs in DNA, and alterations in downstream apoptosis signaling triggered by DNA damage. Many of these changes result from miRNA-mediated changes in protein abundance and are prominent in tumor cells possessing a stem-cell like phenotype.
Future perspective
Arterially targeted chemotherapy in the form of TACE-administered doxorubicin is a mainstay of management of HCC patients prior to liver transplantation and although rationally designed targeted therapies for early and intermediate stage HCC continue to be pursued, it is likely to remain an important therapeutic modality for the foreseeable future. Tumor resistance to doxorubicin is the key limitation of this treatment, responsible for treatment failures and recurrences, as well as the need to use high doses of drug, thus limiting the ability to treat patients with the most advanced liver disease. The ability to counteract resistance mechanisms would be a major clinical advance. Attempts have been made to augment doxorubicin sensitivity of HCCs and other tumors by employing inhibitors of MDR transporters [122], PI3K/Akt [86] and sirtuins [99] using small molecule inhibitors or RNAi approaches. None have yet to demonstrate benefit in clinical trials. In experimental models of HCC, doxorubicin chemosensitization has been achieved with PP2A inhibitors [123], CD13 antibodies [116] or different approaches to inhibit MDR transporters including small molecule inhibitors and antisense constructs [33,124]. Further understanding of the mechanisms responsible for doxorubicin resistance will thus be important to make chemosensitization a routine part of the TACE treatment protocol.
Footnotes
Financial & competing interests disclosure
Work from the authors’ lab was supported by grant AA012863 from the National Institute on Alcoholism and Alcohol Abuse. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaugrand M, N'Kontchou G, Seror O, Ganne N, Trinchet JC. Local/regional and systemic treatments of hepatocellular carcinoma. Semin. Liver Dis. 2005;25:201–211. doi: 10.1055/s-2005-871199. [DOI] [PubMed] [Google Scholar]
- 3.Burroughs A, Hochhauser D, Meyer T. Systemic treatment and liver transplantation for hepatocellular carcinoma: two ends of the therapeutic spectrum. Lancet Oncol. 2004;5:409–418. doi: 10.1016/S1470-2045(04)01508-6. [DOI] [PubMed] [Google Scholar]
- 4.Ganne-Carrie N, Trinchet JC. Systemic treatment of hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2004;16:275–281. doi: 10.1097/00042737-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Kalyan A, Nimeiri H, Kulik L. Systemic therapy of hepatocellular carcinoma: current and promising. Clin. Liver Dis. 2015;19:421–432. doi: 10.1016/j.cld.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat. Rev. Clin. Oncol. 2015;12:408–424. doi: 10.1038/nrclinonc.2015.103. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 9.Fong ZV, Tanabe KK. The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence-based comparison and review. Cancer. 2014;120:2824–2838. doi: 10.1002/cncr.28730. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 12.Malagari K, Pomoni M, Kelekis A, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc. Intervent. Radiol. 2010;33:541–551. doi: 10.1007/s00270-009-9750-0. [DOI] [PubMed] [Google Scholar]
- 13.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc. Intervent. Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984;226:466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]; •• First demonstration that doxorubicin-induced double-strand DNA breaks (DSBs) in DNA are a result of its inhibition of TOP2A.
- 15.Chen SH, Chan NL, Hsieh TS. New mechanistic and functional insights into DNA topoisomerases. Annu. Rev. Biochem. 2013;82:139–170. doi: 10.1146/annurev-biochem-061809-100002. [DOI] [PubMed] [Google Scholar]
- 16.Roos WP, Kaina B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013;332:237–248. doi: 10.1016/j.canlet.2012.01.007. [DOI] [PubMed] [Google Scholar]; • Reviews the cellular mechanisms responsible for determining cell fate following different types of DNA lesions.
- 17.Wilson TR, Johnston PG, Longley DB. Anti-apoptotic mechanisms of drug resistance in cancer. Curr. Cancer Drug Targets. 2009;9:307–319. doi: 10.2174/156800909788166547. [DOI] [PubMed] [Google Scholar]
- 18.Swift LP, Rephaeli A, Nudelman A, Phillips DR, Cutts SM. Doxorubicin-DNA adducts induce a non-topoisomerase II-mediated form of cell death. Cancer Res. 2006;66:4863–4871. doi: 10.1158/0008-5472.CAN-05-3410. [DOI] [PubMed] [Google Scholar]
- 19.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 20.Bonin S, Pascolo L, Croce LS, Stanta G, Tiribelli C. Gene expression of ABC proteins in hepatocellular carcinoma, perineoplastic tissue, and liver diseases. Mol. Med. 2002;8:318–325. [PMC free article] [PubMed] [Google Scholar]
- 21.Scotto KW. Transcriptional regulation of ABC drug transporters. Oncogene. 2003;22:7496–7511. doi: 10.1038/sj.onc.1206950. [DOI] [PubMed] [Google Scholar]
- 22.Johnson RA, Ince TA, Scotto KW. Transcriptional repression by p53 through direct binding to a novel DNA element. J. Biol. Chem. 2001;276:27716–27720. doi: 10.1074/jbc.C100121200. [DOI] [PubMed] [Google Scholar]
- 23.Osborn MT, Chambers TC. Role of the stress-activated/c-Jun NH2–terminal protein kinase pathway in the cellular response to adriamycin and other chemotherapeutic drugs. J. Biol. Chem. 1996;271:30950–30955. doi: 10.1074/jbc.271.48.30950. [DOI] [PubMed] [Google Scholar]
- 24.Kuo MT, Liu Z, Wei Y, et al. Induction of human MDR1 gene expression by 2–acetylaminofluorene is mediated by effectors of the phosphoinositide 3–kinase pathway that activate NF-kappaB signaling. Oncogene. 2002;21:1945–1954. doi: 10.1038/sj.onc.1205117. [DOI] [PubMed] [Google Scholar]
- 25.Fantappie O, Solazzo M, Lasagna N, Platini F, Tessitore L, Mazzanti R. P-glycoprotein mediates celecoxib-induced apoptosis in multiple drug-resistant cell lines. Cancer Res. 2007;67:4915–4923. doi: 10.1158/0008-5472.CAN-06-3952. [DOI] [PubMed] [Google Scholar]
- 26.Mazzanti R, Platini F, Bottini C, Fantappie O, Solazzo M, Tessitore L. Down-regulation of the HGF/MET autocrine loop induced by celecoxib and mediated by P-gp in MDR-positive human hepatocellular carcinoma cell line. Biochem. Pharmacol. 2009;78:21–32. doi: 10.1016/j.bcp.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Ng IO, Liu CL, Fan ST, Ng M. Expression of P-glycoprotein in hepatocellular carcinoma. A determinant of chemotherapy response. Am. J. Clin. Pathol. 2000;113:355–363. doi: 10.1309/AC1M-4TY4-U0TN-EN7T. [DOI] [PubMed] [Google Scholar]
- 28.Nies AT, Konig J, Pfannschmidt M, Klar E, Hofmann WJ, Keppler D. Expression of the multidrug resistance proteins MRP2 and MRP3 in human hepatocellular carcinoma. Int. J. Cancer. 2001;94:492–499. doi: 10.1002/ijc.1498. [DOI] [PubMed] [Google Scholar]
- 29.Silverman JA, Thorgeirsson SS. Regulation and function of the multidrug resistance genes in liver. Prog. Liver Dis. 1995;13:101–123. [PubMed] [Google Scholar]
- 30.Solazzo M, Fantappie O, D'Amico M, et al. Mitochondrial expression and functional activity of breast cancer resistance protein in different multiple drug-resistant cell lines. Cancer Res. 2009;69:7235–7242. doi: 10.1158/0008-5472.CAN-08-4315. [DOI] [PubMed] [Google Scholar]
- 31.Ueda K, Cardarelli C, Gottesman MM, Pastan I. Expression of a full-length cDNA for the human MDR1 gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc. Natl Acad. Sci. USA. 1987;84:3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung ST, Cheung PF, Cheng CK, Wong NC, Fan ST. Granulin-epithelin precursor and ATP-dependent binding cassette (ABC)B5 regulate liver cancer cell chemoresistance. Gastroenterology. 2011;140:344–355. doi: 10.1053/j.gastro.2010.07.049. [DOI] [PubMed] [Google Scholar]; • Demonstrates the role of GEP in regulating ABCB5 expression in liver cancer stem cells and its effects on doxorubicin sensitivity.
- 33.Shiraga K, Sakaguchi K, Senoh T, et al. Modulation of doxorubicin sensitivity by cyclosporine A in hepatocellular carcinoma cells and their doxorubicin-resistant sublines. J. Gastroenterol. Hepatol. 2001;16:460–466. doi: 10.1046/j.1440-1746.2001.02457.x. [DOI] [PubMed] [Google Scholar]
- 34.Kerr DJ, Graham J, Cummings J, et al. The effect of verapamil on the pharmacokinetics of adriamycin. Cancer Chemother. Pharmacol. 1986;18:239–242. doi: 10.1007/BF00273394. [DOI] [PubMed] [Google Scholar]
- 35.Ferry DR, Traunecker H, Kerr DJ. Clinical trials of P-glycoprotein reversal in solid tumours. Eur. J. Cancer. 1996;32A:1070–1081. doi: 10.1016/0959-8049(96)00091-3. [DOI] [PubMed] [Google Scholar]
- 36.Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarvinen TA, Tanner M, Rantanen V, et al. Amplification and deletion of topoisomerase IIalpha associate with ErbB–2 amplification and affect sensitivity to topoisomerase II inhibitor doxorubicin in breast cancer. Am. J. Pathol. 2000;156:839–847. doi: 10.1016/s0002-9440(10)64952-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Press MF, Sauter G, Buyse M, et al. Alteration of topoisomerase II-alpha gene in human breast cancer: association with responsiveness to anthracycline-based chemotherapy. J. Clin. Oncol. 2011;29:859–867. doi: 10.1200/JCO.2009.27.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000;11:265–283. doi: 10.1016/s0928-0987(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 40.Panvichian R, Tantiwetrueangdet A, Angkathunyakul N, Leelaudomlipi S. TOP2A amplification and overexpression in hepatocellular carcinoma tissues. Biomed. Res. Int. 2015;2015:381602. doi: 10.1155/2015/381602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pang E, Hu Y, Chan KY, et al. Karyotypic imbalances and differential gene expressions in the acquired doxorubicin resistance of hepatocellular carcinoma cells. Lab. Invest. 2005;85:664–674. doi: 10.1038/labinvest.3700254. [DOI] [PubMed] [Google Scholar]
- 42.Wong N, Yeo W, Wong WL, et al. TOP2A overexpression in hepatocellular carcinoma correlates with early age onset, shorter patients survival and chemoresistance. Int. J. Cancer. 2009;124:644–652. doi: 10.1002/ijc.23968. [DOI] [PubMed] [Google Scholar]
- 43.Okada Y, Tosaka A, Nimura Y, Kikuchi A, Yoshida S, Suzuki M. Atypical multidrug resistance may be associated with catalytically active mutants of human DNA topoisomerase II alpha. Gene. 2001;272:141–148. doi: 10.1016/s0378-1119(01)00554-6. [DOI] [PubMed] [Google Scholar]
- 44.Bartke T, Siegmund D, Peters N, et al. p53 upregulates cFLIP, inhibits transcription of NF-kappaB-regulated genes and induces caspase–8–independent cell death in DLD–1 cells. Oncogene. 2001;20:571–580. doi: 10.1038/sj.onc.1204124. [DOI] [PubMed] [Google Scholar]
- 45.Ryan KM, Phillips AC, Vousden KH. Regulation and function of the p53 tumor suppressor protein. Curr. Opin. Cell Biol. 2001;13:332–337. doi: 10.1016/s0955-0674(00)00216-7. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J. Biol. Chem. 2002;277:3247–3257. doi: 10.1074/jbc.M106643200. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y, Mehew JW, Heckman CA, Arcinas M, Boxer LM. Negative regulation of Bcl-2 expression by p53 in hematopoietic cells. Oncogene. 2001;20:240–251. doi: 10.1038/sj.onc.1204067. [DOI] [PubMed] [Google Scholar]
- 48.Bunz F, Hwang PM, Torrance C, et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J. Clin. Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstration of the differential effects of p53 disruption on sensitization to different chemotherapy agents.
- 49.Khanna KK, Jackson SP. DNA double-strand breaks: signaling repair and the cancer connection. Nat. Genet. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 50.Zheng T, Wang J, Song X, et al. Nutlin–3 cooperates with doxorubicin to induce apoptosis of human hepatocellular carcinoma cells through p53 or p73 signaling pathways. J. Cancer Res. Clin. Oncol. 2010;136:1597–1604. doi: 10.1007/s00432-010-0817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guan YS, La Z, Yang L, He Q, Li P. p53 gene in treatment of hepatic carcinoma: status quo. World J. Gastroenterol. 2007;13:985–992. doi: 10.3748/wjg.v13.i7.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao HC, Zhang Q, Yang Y, et al. p53–expressing conditionally replicative adenovirus CNHK500–p53 against hepatocellular carcinoma in vitro. World J. Gastroenterol. 2007;13:683–691. doi: 10.3748/wjg.v13.i5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu H, Chen W, Liang C, et al. WP1130 increases doxorubicin sensitivity in hepatocellular carcinoma cells through usp9x-dependent p53 degradation. Cancer Lett. 2015;361:218–225. doi: 10.1016/j.canlet.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Lee TK, Lau TC, Ng IO. Doxorubicin-induced apoptosis and chemosensitivity in hepatoma cell lines. Cancer Chemother. Pharmacol. 2002;49:78–86. doi: 10.1007/s00280-001-0376-4. [DOI] [PubMed] [Google Scholar]
- 55.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat. Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 56.Fan Y, Dutta J, Gupta N, Fan G, Gelinas C. Regulation of programmed cell death by NF-kappaB and its role in tumorigenesis and therapy. Adv. Exp. Med. Biol. 2008;615:223–250. doi: 10.1007/978-1-4020-6554-5_11. [DOI] [PubMed] [Google Scholar]
- 57.Perkins ND. The diverse and complex roles of NF-kappaB subunits in cancer. Nat. Rev. Cancer. 2012;12:121–132. doi: 10.1038/nrc3204. [DOI] [PubMed] [Google Scholar]
- 58.Zhao N, Wang R, Zhou L, Zhu Y, Gong J, Zhuang SM. MicroRNA–26b suppresses the NF-kappaB signaling and enhances the chemosensitivity of hepatocellular carcinoma cells by targeting TAK1 and TAB3. Mol. Cancer. 2014;13:35. doi: 10.1186/1476-4598-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ni W, Chen B, Zhou G, et al. Overexpressed nuclear BAG–1 in human hepatocellular carcinoma is associated with poor prognosis and resistance to doxorubicin. J. Cell. Biochem. 2013;114:2120–2130. doi: 10.1002/jcb.24560. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y, Lou G, Wu W, et al. Involvement of the NF-kappaB pathway in multidrug resistance induced by HBx in a hepatoma cell line. J. Viral Hepat. 2011;18:e439–446. doi: 10.1111/j.1365-2893.2011.01463.x. [DOI] [PubMed] [Google Scholar]
- 61.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 62.Paik JH, Kollipara R, Chu G, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fei M, Lu M, Wang Y, et al. Arsenic trioxide-induced growth arrest of human hepatocellular carcinoma cells involving FOXO3a expression and localization. Med. Oncol. 2009;26:178–185. doi: 10.1007/s12032-008-9105-8. [DOI] [PubMed] [Google Scholar]
- 64.He L, Yang X, Cao X, Liu F, Quan M, Cao J. Casticin induces growth suppression and cell cycle arrest through activation of FOXO3a in hepatocellular carcinoma. Oncol. Rep. 2013;29:103–108. doi: 10.3892/or.2012.2076. [DOI] [PubMed] [Google Scholar]
- 65.Wang JG, Zheng XX, Zeng GY, Zhou YJ, Yuan H. Purified vitexin compound 1 induces apoptosis through activation of FOXO3a in hepatocellular carcinoma. Oncol. Rep. 2014;31:488–496. doi: 10.3892/or.2013.2855. [DOI] [PubMed] [Google Scholar]
- 66.Xu D, He X, Chang Y, et al. Inhibition of miR-96 expression reduces cell proliferation and clonogenicity of HepG2 hepatoma cells. Oncol. Rep. 2013;29:653–661. doi: 10.3892/or.2012.2138. [DOI] [PubMed] [Google Scholar]
- 67.Lu M, Ma J, Xue W, et al. The expression and prognosis of FOXO3a and Skp2 in human hepatocellular carcinoma. Pathol. Oncol. Res. 2009;15:679–687. doi: 10.1007/s12253-009-9171-z. [DOI] [PubMed] [Google Scholar]
- 68.Xie C, Song LB, Wu JH, et al. Upregulator of cell proliferation predicts poor prognosis in hepatocellular carcinoma and contributes to hepatocarcinogenesis by downregulating FOXO3a. PLoS ONE. 2012;7:e40607. doi: 10.1371/journal.pone.0040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen J, Gomes AR, Monteiro LJ, et al. Constitutively nuclear FOXO3a localization predicts poor survival and promotes Akt phosphorylation in breast cancer. PLoS ONE. 2010;5:e12293. doi: 10.1371/journal.pone.0012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ho KK, McGuire VA, Koo CY, et al. Phosphorylation of FOXO3a on Ser–7 by p38 promotes its nuclear localization in response to doxorubicin. J. Biol. Chem. 2012;287:1545–1555. doi: 10.1074/jbc.M111.284224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang K, Li PF. Foxo3a regulates apoptosis by negatively targeting miR-21. J. Biol. Chem. 2010;285:16958–16966. doi: 10.1074/jbc.M109.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dieudonne FX, Marion A, Marie PJ, Modrowski D. Targeted inhibition of T-cell factor activity promotes syndecan–2 expression and sensitization to doxorubicin in osteosarcoma cells and bone tumors in mice. J. Bone Miner. Res. 2012;27:2118–2129. doi: 10.1002/jbmr.1650. [DOI] [PubMed] [Google Scholar]
- 73.Li S, Zhou Y, Wang R, Zhang H, Dong Y, Ip C. Selenium sensitizes MCF–7 breast cancer cells to doxorubicin-induced apoptosis through modulation of phospho-Akt and its downstream substrates. Mol. Cancer Ther. 2007;6:1031–1038. doi: 10.1158/1535-7163.MCT-06-0643. [DOI] [PubMed] [Google Scholar]
- 74.Obrador-Hevia A, Serra-Sitjar M, Rodriguez J, Villalonga P. Fernandez de Mattos S. The tumour suppressor FOXO3 is a key regulator of mantle cell lymphoma proliferation and survival. Br. J. Haematol. 2012;156:334–345. doi: 10.1111/j.1365-2141.2011.08951.x. [DOI] [PubMed] [Google Scholar]
- 75.Hagenbuchner J, Kuznetsov A, Hermann M, Hausott B, Obexer P, Ausserlechner MJ. FOXO3–induced reactive oxygen species are regulated by BCL2L11 (Bim) and SESN3. J. Cell. Sci. 2012;125:1191–1203. doi: 10.1242/jcs.092098. [DOI] [PubMed] [Google Scholar]
- 76.Li Z, Zhao Z, Tikhanovich I, et al. Serine 574 phosphorylation alters transcriptional programming of FOXO3 by selectively enhancing apoptotic gene expression. Cell Death Differ. 2015 doi: 10.1038/cdd.2015.125. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Obexer P, Hagenbuchner J, Unterkircher T, et al. Repression of BIRC5/survivin by FOXO3/FKHRL1 sensitizes human neuroblastoma cells to DNA damage-induced apoptosis. Mol. Biol. Cell. 2009;20:2041–2048. doi: 10.1091/mbc.E08-07-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hedrick SM. The cunning little vixen: Foxo and the cycle of life and death. Nat. Immunol. 2009;10:1057–1063. doi: 10.1038/ni.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hui RC, Gomes AR, Constantinidou D, et al. The forkhead transcription factor FOXO3a increases phosphoinositide–3 kinase/Akt activity in drug-resistant leukemic cells through induction of PIK3CA expression. Mol. Cell. Biol. 2008;28:5886–5898. doi: 10.1128/MCB.01265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hui RC, Francis RE, Guest SK, et al. Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol. Cancer Ther. 2008;7:670–678. doi: 10.1158/1535-7163.MCT-07-0397. [DOI] [PubMed] [Google Scholar]
- 81.Cox J, Vittal A, O'Neil M, et al. Nuclear to cytoplasmic translocation of FOXO3 Determines the sensitivity of human hepatocellular carcinoma to doxorubicin. Hepatology. 2014;60 Abstract A880. [Google Scholar]
- 82.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt FoxO and regulation of apoptosis. Biochim. Biophys. Acta. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 83.McCubrey JA, Steelman LS, Abrams SL, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv. Enzyme Regul. 2006;46:249–279. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 84.Schmitz KJ, Wohlschlaeger J, Lang H, et al. Activation of the ERK and AKT signalling pathway predicts poor prognosis in hepatocellular carcinoma and ERK activation in cancer tissue is associated with hepatitis C virus infection. J. Hepatol. 2008;48:83–90. doi: 10.1016/j.jhep.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 85.Cusimano A, Puleio R, D'Alessandro N, et al. Cytotoxic activity of the novel small molecule AKT inhibitor SC66 in hepatocellular carcinoma cells. Oncotarget. 2015;6:1707–1722. doi: 10.18632/oncotarget.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simioni C, Martelli AM, Cani A, et al. The AKT inhibitor MK–2206 is cytotoxic in hepatocarcinoma cells displaying hyperphosphorylated AKT–1 and synergizes with conventional chemotherapy. Oncotarget. 2013;4:1496–1506. doi: 10.18632/oncotarget.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kessler SM, Pokorny J, Zimmer V, et al. IGF2 mRNA binding protein p62/IMP2–2 in hepatocellular carcinoma: antiapoptotic action is independent of IGF2/PI3K signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;304:G328–G336. doi: 10.1152/ajpgi.00005.2012. [DOI] [PubMed] [Google Scholar]
- 88.Wei W, Chua MS, Grepper S, So SK. Soluble Frizzled–7 receptor inhibits Wnt signaling and sensitizes hepatocellular carcinoma cells towards doxorubicin. Mol. Cancer. 2011;10:16. doi: 10.1186/1476-4598-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang CL, Jiang FQ, Xu F, Jiang GX. ADAM10 overexpression confers resistance to doxorubicin-induced apoptosis in hepatocellular carcinoma. Tumour Biol. 2012;33:1535–1541. doi: 10.1007/s13277-012-0405-4. [DOI] [PubMed] [Google Scholar]
- 90.Choi J, Yip-Schneider M, Albertin F, Wiesenauer C, Wang Y, Schmidt CM. The effect of doxorubicin on MEK-ERK signalling predicts its efficacy in HCC. J. Surg. Res. 2008;150:219–226. doi: 10.1016/j.jss.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 91.Huether A, Hopfner M, Sutter AP, Schuppan D, Scherubl H. Erlotinib induces cell cycle arrest and apoptosis in hepatocellular cancer cells and enhances chemosensitivity towards cytostatics. J. Hepatol. 2005;43:661–669. doi: 10.1016/j.jhep.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 92.Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 93.Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304:2154–2160. doi: 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 94.Zhou J, Wan B, Liu XM, Li R, Wang Y, Yu L. MK5 is degraded in response to doxorubicin and negatively regulates doxorubicin-induced apoptosis in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2012;427:581–586. doi: 10.1016/j.bbrc.2012.09.101. [DOI] [PubMed] [Google Scholar]
- 95.Chen HC, Jeng YM, Yuan RH, Hsu HC, Chen YL. SIRT1 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma and its expression predicts poor prognosis. Ann. Surg. Oncol. 2012;19:2011–2019. doi: 10.1245/s10434-011-2159-4. [DOI] [PubMed] [Google Scholar]
- 96.Kim HS, Vassilopoulos A, Wang RH, et al. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell. 2011;20:487–499. doi: 10.1016/j.ccr.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marquardt JU, Fischer K, Baus K, et al. Sirtuin–6–dependent genetic and epigenetic alterations are associated with poor clinical outcome in hepatocellular carcinoma patients. Hepatology. 2013;58:1054–1064. doi: 10.1002/hep.26413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang JX, Yi Y, Li YW, et al. Down-regulation of sirtuin 3 is associated with poor prognosis in hepatocellular carcinoma after resection. BMC Cancer. 2014;14:297. doi: 10.1186/1471-2407-14-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen J, Zhang B, Wong N, et al. Sirtuin 1 is upregulated in a subset of hepatocellular carcinomas where it is essential for telomere maintenance and tumor cell growth. Cancer Res. 2011;71:4138–4149. doi: 10.1158/0008-5472.CAN-10-4274. [DOI] [PubMed] [Google Scholar]; • Exploration of the functional effects of SIRT1 increases and decreases on doxorubicin sensitivity in hepatocellular carcinoma.
- 100.Luo J, Nikolaev AY, Imai S, Chen D, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 101.Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 102.Mao B, Hu F, Cheng J, et al. SIRT1 regulates YAP2–mediated cell proliferation and chemoresistance in hepatocellular carcinoma. Oncogene. 2014;33:1468–1474. doi: 10.1038/onc.2013.88. [DOI] [PubMed] [Google Scholar]
- 103.Khongkow M, Olmos Y, Gong C, et al. SIRT6 modulates paclitaxel and epirubicin resistance and survival in breast cancer. Carcinogenesis. 2013;34:1476–1486. doi: 10.1093/carcin/bgt098. [DOI] [PubMed] [Google Scholar]
- 104.Zhang J, Wang Y, Zhen P, et al. Genome-wide analysis of miRNA signature differentially expressed in doxorubicin-resistant and parental human hepatocellular carcinoma cell lines. PLoS ONE. 2013;8:e54111. doi: 10.1371/journal.pone.0054111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhuo L, Liu J, Wang B, Gao M, Huang A. Differential miRNA expression profiles in hepatocellular carcinoma cells and drug-resistant sublines. Oncol. Rep. 2013;29:555–562. doi: 10.3892/or.2012.2155. [DOI] [PubMed] [Google Scholar]
- 106.Fornari F, Gramantieri L, Giovannini C, et al. miR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2009;69:5761–5767. doi: 10.1158/0008-5472.CAN-08-4797. [DOI] [PubMed] [Google Scholar]
- 107.Xu Y, Xia F, Ma L, et al. MicroRNA–122 sensitizes HCC cancer cells to adriamycin and vincristine through modulating expression of MDR and inducing cell cycle arrest. Cancer Lett. 2011;310:160–169. doi: 10.1016/j.canlet.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 108.Yang T, Zheng ZM, Li XN, et al. miR-223 modulates multidrug resistance via downregulation of ABCB1 in hepatocellular carcinoma cells. Exp. Biol. Med. (Maywood) 2013;238:1024–1032. doi: 10.1177/1535370213497321. [DOI] [PubMed] [Google Scholar]
- 109.Xu L, Beckebaum S, Iacob S, et al. MicroRNA–101 inhibits human hepatocellular carcinoma progression through EZH2 downregulation and increased cytostatic drug sensitivity. J. Hepatol. 2014;60:590–598. doi: 10.1016/j.jhep.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 110.Fornari F, Milazzo M, Chieco P, et al. miR-199a–3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2010;70:5184–5193. doi: 10.1158/0008-5472.CAN-10-0145. [DOI] [PubMed] [Google Scholar]; • Clear mechanistic exploration linking specific miRNA targets to the resistance phenotype.
- 111.Wang L, Wang YM, Xu S, et al. MicroRNA–215 is upregulated by treatment with Adriamycin and leads to the chemoresistance of hepatocellular carcinoma cells and tissues. Mol. Med. 2015;12:5274–5280. doi: 10.3892/mmr.2015.4012. [DOI] [PubMed] [Google Scholar]
- 112.Fornari F, Milazzo M, Chieco P, et al. In hepatocellular carcinoma miR-519d is up-regulated by p53 and DNA hypomethylation and targets CDKN1A/p21, PTEN, AKT3 and TIMP2. J. Pathol. 2012;227:275–285. doi: 10.1002/path.3995. [DOI] [PubMed] [Google Scholar]
- 113.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells cancer and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 114.Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. J. Clin. Invest. 2013;123:1911–1918. doi: 10.1172/JCI66024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vu NB, Nguyen TT, Tran LC, et al. Doxorubicin and 5-fluorouracil resistant hepatic cancer cells demonstrate stem-like properties. Cytotechnology. 2013;65:491–503. doi: 10.1007/s10616-012-9511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haraguchi N, Ishii H, Mimori K, et al. CD13 is a therapeutic target in human liver cancer stem cells. J. Clin. Invest. 2010;120:3326–3339. doi: 10.1172/JCI42550. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstration that a stem cell directed therapeutic approach is able to sensitize hepatocellular carcinoma to doxorubicin.
- 117.Zeng Z, Ren J, O'Neil M, et al. Impact of stem cell marker expression on recurrence of TACE-treated hepatocellular carcinoma post liver transplantation. BMC Cancer. 2012;12:584. doi: 10.1186/1471-2407-12-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang XQ, Ongkeko WM, Chen L, et al. Octamer 4 (Oct4) mediates chemotherapeutic drug resistance in liver cancer cells through a potential Oct4–AKT-ATP-binding cassette G2 pathway. Hepatology. 2010;52:528–539. doi: 10.1002/hep.23692. [DOI] [PubMed] [Google Scholar]
- 119.Zhu Z, Hao X, Yan M, et al. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int. J. Cancer. 2010;126:2067–2078. doi: 10.1002/ijc.24868. [DOI] [PubMed] [Google Scholar]
- 120.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 121.Sukowati CH, Anfuso B, Pascut D, Tiribelli C. Multidrug resistance in hepatic cancer stem cells: the emerging role of miRNAs. Expert Rev. Gastroenterol. Hepatol. 2015;9:723–725. doi: 10.1586/17474124.2015.1041509. [DOI] [PubMed] [Google Scholar]
- 122.Abdallah HM, Al-Abd AM, El-Dine RS, El-Halawany AM. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: a review. J. Adv. Res. 2015;6:45–62. doi: 10.1016/j.jare.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rincon R, Cristobal I, Zazo S, et al. PP2A inhibition determines poor outcome and doxorubicin resistance in early breast cancer and its activation shows promising therapeutic effects. Oncotarget. 2015;6:4299–4314. doi: 10.18632/oncotarget.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huesker M, Folmer Y, Schneider M, Fulda C, Blum HE, Hafkemeyer P. Reversal of drug resistance of hepatocellular carcinoma cells by adenoviral delivery of anti-MDR1 ribozymes. Hepatology. 2002;36:874–884. doi: 10.1053/jhep.2002.35619. [DOI] [PubMed] [Google Scholar]
- 125.Tsang WP, Kwok TT. Let–7a microRNA suppresses therapeutics-induced cancer cell death by targeting caspase–3. Apoptosis. 2008;13:1215–1222. doi: 10.1007/s10495-008-0256-z. [DOI] [PubMed] [Google Scholar]
- 126.Zhao X, Yang L, Hu J, Ruan J. miR-138 might reverse multidrug resistance of leukemia cells. Leuk. Res. 2010;34:1078–1082. doi: 10.1016/j.leukres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 127.Wang ZX, Lu BB, Wang H, Cheng ZX, Yin YM. MicroRNA–21 modulates chemosensitivity of breast cancer cells to doxorubicin by targeting PTEN. Arch. Med. Res. 2011;42:281–290. doi: 10.1016/j.arcmed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 128.Lv J, Fu Z, Shi M, et al. Systematic analysis of gene expression pattern in has-miR-760 overexpressed resistance of the MCF–7 human breast cancer cell to doxorubicin. Biomed. Pharmacother. 2015;69:162–169. doi: 10.1016/j.biopha.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 129.Hu Y, Xu K, Yague E. miR-218 targets survivin and regulates resistance to chemotherapeutics in breast cancer. Breast Cancer Res. Treat. 2015;151:269–280. doi: 10.1007/s10549-015-3372-9. [DOI] [PubMed] [Google Scholar]
- 130.Bao L, Hazari S, Mehra S, Kaushal D, Moroz K, Dash S. Increased expression of P-glycoprotein and doxorubicin chemoresistance of metastatic breast cancer is regulated by miR-298. Am. J. Pathol. 2012;180:2490–2503. doi: 10.1016/j.ajpath.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao Z, Li R, Sha S, Wang Q, Mao W, Liu T. Targeting HER3 with miR-450b–3p suppresses breast cancer cells proliferation. Cancer Biol. Ther. 2014;15:1404–1412. doi: 10.4161/cbt.29923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kovalchuk O, Filkowski J, Meservy J, et al. Involvement of microRNA–451 in resistance of the MCF–7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol. Cancer Ther. 2008;7:2152–2159. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- 133.Hu Q, Gong JP, Li J, et al. Down-regulation of miRNA–452 is associated with adriamycin-resistance in breast cancer cells. Asian Pac. J. Cancer Prev. 2014;15:5137–5142. doi: 10.7314/apjcp.2014.15.13.5137. [DOI] [PubMed] [Google Scholar]
- 134.Chen Y, Sun Y, Chen L, et al. miRNA–200c increases the sensitivity of breast cancer cells to doxorubicin through the suppression of E-cadherin-mediated PTEN/Akt signaling. Mol. Med. Rep. 2013;7:1579–1584. doi: 10.3892/mmr.2013.1403. [DOI] [PubMed] [Google Scholar]
- 135.Park EY, Chang E, Lee EJ, et al. Targeting of miR34a-NOTCH1 axis reduced breast cancer stemness and chemoresistance. Cancer Res. 2014;74:7573–7582. doi: 10.1158/0008-5472.CAN-14-1140. [DOI] [PubMed] [Google Scholar]
- 136.Xu M, Jin H, Xu CX, Bi WZ, Wang Y. miR-34c inhibits osteosarcoma metastasis and chemoresistance. Med. Oncol. 2014;31:972. doi: 10.1007/s12032-014-0972-x. [DOI] [PubMed] [Google Scholar]
- 137.Xu M, Jin H, Xu CX, et al. miR-382 inhibits tumor growth and enhance chemosensitivity in osteosarcoma. Oncotarget. 2014;5:9472–9483. doi: 10.18632/oncotarget.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang Y, Duan G, Feng S. MicroRNA–301a modulates doxorubicin resistance in osteosarcoma cells by targeting AMP-activated protein kinase alpha 1. Biochem. Biophys. Res. Commun. 2015;459:367–373. doi: 10.1016/j.bbrc.2015.02.101. [DOI] [PubMed] [Google Scholar]
- 139.Robin TP, Smith A, McKinsey E, Reaves L, Jedlicka P, Ford HL. EWS/FLI1 regulates EYA3 in Ewing sarcoma via modulation of miRNA–708, resulting in increased cell survival and chemoresistance. Mol. Cancer Res. 2012;10:1098–1108. doi: 10.1158/1541-7786.MCR-12-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Iida K, Fukushi J, Matsumoto Y, et al. miR-125b develops chemoresistance in Ewing sarcoma/primitive neuroectodermal tumor. Cancer Cell Int. 2013;13:21. doi: 10.1186/1475-2867-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang G, Jiang O, Ling D, et al. MicroRNA–522 reverses drug resistance of doxorubicin-induced HT29 colon cancer cell by targeting ABCB5. Mol. Med. Rep. 2015;12:3930–3936. doi: 10.3892/mmr.2015.3890. [DOI] [PubMed] [Google Scholar]
- 142.Chen MB, Yang L, Lu PH, et al. MicroRNA–101 down-regulates sphingosine kinase 1 in colorectal cancer cells. Biochem. Biophys. Res. Commun. 2015;463:954–960. doi: 10.1016/j.bbrc.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 143.Qu J, Zhao L, Zhang P, et al. MicroRNA–195 chemosensitizes colon cancer cells to the chemotherapeutic drug doxorubicin by targeting the first binding site of BCL2L2 mRNA. J. Cell. Physiol. 2015;230:535–545. doi: 10.1002/jcp.24366. [DOI] [PubMed] [Google Scholar]
- 144.Zhang Y, Qu X, Li C, et al. miR-103/107 modulates multidrug resistance in human gastric carcinoma by downregulating Cav–1. Tumour Biol. 2015;36:2277–2285. doi: 10.1007/s13277-014-2835-7. [DOI] [PubMed] [Google Scholar]
- 145.Shang Y, Zhang Z, Liu Z, et al. miR-508–5p regulates multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1. Oncogene. 2014;33:3267–3276. doi: 10.1038/onc.2013.297. [DOI] [PubMed] [Google Scholar]
- 146.Feng DD, Zhang H, Zhang P, et al. Down-regulated miR-331–5p and miR-27a are associated with chemotherapy resistance and relapse in leukaemia. J. Cell. Mol. Med. 2011;15(10):2164–2175. doi: 10.1111/j.1582-4934.2010.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]