Abstract
Ligands for G protein–coupled receptor 88 (GPR88) have not yet been found. A new study finds that GPR88 is important in the physiology of dorsal striatal projection neurons, as well as in behaviors involving this brain region.
Finding the proper role for orphan G protein–coupled receptors (GPCRs) is often a long and painful process that brings to mind the difficult journey of Dickens’ iconic orphan Oliver Twist to find his place in the world. A case in point is GPR88, which is designated as an orphan receptor because no ligand that interacts with the receptor has as yet been identified1. Nonetheless, the enrichment of GPR88 in the striatum1,2, sensitivity of receptor expression to antidepressant treatments3, and genetic linkage to schizophrenia4, has stimulated interest in its roles in striatal physiology and behaviors involving this brain region. Dorsal striatal circuitry contains a predominance of GABAergic medium spiny neurons (MSNs) that inhibit downstream nuclei of the basal ganglia, a feature shared by other striatal-like forebrain regions that is distinct from the glutamatergic projections arising in cortical-like structures. The molecular profile of MSNs is also distinct from forebrain glutamatergic projection neurons. Indeed, neurotransmitter receptors and intracellular signaling molecules of various types are highly enriched in MSNs2. Among these are G proteins such as Golf5, signaling molecules such as REM2 (ref. 2), and the adenosine 2A (A2A) GPCR6. Defining the roles of these striatum-enriched proteins, including GPR88, should help us to identify molecular networks that act in concert to influence basal ganglia circuitry and action control.
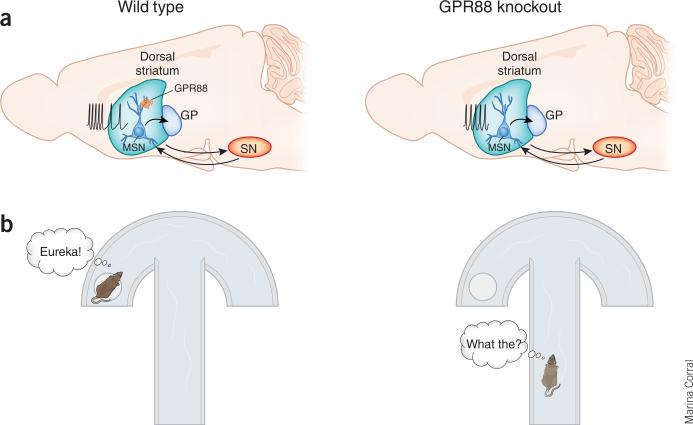
In a study appearing in this issue of Nature Neuroscience7, Quintana and colleagues used gene-targeted mice lacking GPR88 and viral-based re-expression of the protein to determine the receptor's role in MSN physiology, synaptic transmission, action control and action learning. Constitutive knockout of GPR88 led to increased locomotion, decreased performance and impaired learning on a motor skill test, and slower learning and poorer performance in cued escape behaviors (Fig. 1). When the activity of putative MSNs was examined in awake mice in a familiar environment, an overall increase in neuronal firing was observed in the knockout mice, with regular firing mainly at moderate frequencies and decreases in irregular burst firing (Fig. 1).
Figure 1.
Knockout of GPR88 leads to hyperexcitation and more regular firing patterns in striatal MSNs, as well as impairment in cued response learning. (a) Schematic diagram of forebrain showing striatal connections and MSN activity. With normal levels of GPR88 expression (left) in MSNs, firing of the neurons varies between low frequency and short bursting modes. Loss of GRP88 (right) strengthens synaptic efficacy at inputs from cortex and decreases GABAergic signaling7. MSN firing becomes stable at moderate frequencies with an overall increase in firing rate in the knockout mice. GP, globus pallidus; SN, substantia nigra. Arrows indicate MSN projections to substantia nigra and back, and to globus pallidus. (b) Schematic diagram of the cued water U maze task used by Quintana et al.7. Mice lacking GPR88 (right) show impaired task acquisition in relation to wild-type mice (left). Deficits in learning of other cued response and avoidance tasks were also observed in the GPR88 knockout mice.
Examination of MSN electrophysiology in brain slices revealed decreased tonic GABAergic inhibition and decreased responses to applied and synaptically released GABA in the GRP88 knockout mice. In contrast, glutamatergic excitatory synaptic transmission was increased in the knockout mice. Increased phosphorylation of the AMPA-type glutamate receptor subunit GluR1 was also observed in the knockout mice, suggesting a potential postsynaptic mechanism for the enhanced efficacy of glutamatergic transmission. These physiological findings indicate that loss of GPR88 renders MSNs more susceptible to synaptic excitation, likely contributing to the increased firing observed in vivo.
Inappropriately facile activation of MSNs could well contribute to the impaired learning and performance of actions in the GRP88 knockout mice, as poorly timed output from the striatum could suppress performance of desired actions while activating counterproductive actions. Interestingly, the authors found that GPR88 is expressed in both “direct pathway” and “indirect pathway” MSNs (Fig. 1). These two pathways work to enhance and suppress cortical output respectively, ultimately shaping action selection and sequencing. Inappropriate output via either pathway could lead to counterproductive actions.
Constitutive loss of GRP88 could impair striatal function by preventing proper develop ment of synapses and circuits. However, MSNs of both the direct and indirect pathways were intact in the knockout mice. Furthermore, a survey of expression of many neuronal proteins revealed a change in expression of only one protein, Regulator of G protein signaling 4 (RGS4). Thus, the cellular and molecular makeup of the striatum was not greatly altered even though the receptor was never expressed in these mice. Notably, signaling through RGS4 has been implicated in modulatory receptor effects and synaptic plasticity in striatum8,9. Receptor–G protein signals involving GPR88 and RGS4 may therefore contribute to the phenotypes observed by the authors.
The constitutive knockout approach used by Quintana et al.7 also prevents expression of GPR88 in nonstriatal brain regions. Although the protein is highly enriched in striatum, the authors found some expression in extra-striatal regions, as previously reported2. To address the problems of constitutive receptor loss, Quintana et al.7 performed elegant studies using virus-based re-expression of GRP88 in striatum of adult mice. Replacing lost GPR88 over a wide area of striatum rescued several behavioral and electrophysiological phenotypes in the knockout mice. These findings indicate that the deficits in these mice are a result of receptor loss in striatum and of ongoing GPR88 signaling in the adult animal rather than altered development.
Previous studies have identified GPR88 as a candidate gene related to schizophrenia and bipolar disorder4, and antidepressant treatment increases GRP88 expression3. This prompted an examination of behavioral phenotypes associated with psychiatric disorders in a separate line of GPR88 knockout mice10. Notably, these mice showed altered pre-pulse inhibition of startle and increased sensitivity to the dopamine receptor agonist apomorphine, phenotypes that were normalized to control levels by antipsychotic drug administration. Quintana et al.7 replicated the decreased PPI phenotype and also observed altered locomotor activity in response to dopamine receptors agonists, including increased sensitivity to a D2-like receptor agonist. Thus, the phenotypes observed following GPR88 removal may be relevant to psychiatric disorders as well as striatal-based action control and learning.
Several key questions remain to be answered concerning GPR88 location and function. Given the orphan status of the receptor, it is not clear how GPR88 activation occurs in vivo. A ligand may yet be identified, but it is also worth considering, as the authors do, that the receptor could have constitutive activity11. Another possibility is that GRP88 may dimerize with a non-orphan partner that provides the ligand specificity11. The signaling pathway(s) activated by GPR88 is also unclear. Determining which type of G protein is activated by the receptor will help to elucidate how loss of GPR88 leads to the observed electro-physiological changes.
Localization of Gpr88 mRNA and protein in rodent striatum indicates that expression is highest in the dorsolateral striatum, which is part of a sensorimotor circuit that has been implicated in habitual actions12,13. Expression is lower in the medial striatum, part of an associative circuit that participates in goal-directed actions. Age-related changes in GPR88 expression level and subregional expression pattern have also been observed13. Thus, it will be important to determine whether there is subregional specificity of effects of GPR88 knockout.
Subcellular localization of the receptor has also not been established with certainty. A previous study used immunohisto- and cytochemistry to show protein expression in postsynaptic elements of striatal MSNs, including the soma, dendritic shafts and dendritic spines, often occurring near synapses12. However, no apparent presynaptic GPR88 was detected using electron microscopy. Additional studies using immunolocalization are needed to confirm and extend these findings. The findings of Quintana et al.7 are consistent with the idea that GPR88 receptors influence the efficacy of GABAergic and glutamatergic synapses at a postsynaptic locus. Ultimately, it will be necessary to find methods to influence GPR88 function on a rapid timescale, not only to determine which responses are directly influenced by receptor activation, but also to try to develop therapeutic approaches aimed at this target.
This demonstration of the importance of GPR88 for normal striatal function and behavior suggests that other striatal-enriched receptors and proteins should be examined in this context. The A2A receptor and other striatal receptors with available ligands have prominent roles in striatal physiology and behavior14,15. The combined knockout and region-specific, viral-mediated re-expression approach used by Quintana et al.7 is a power ful approach to determine the contribution of other striatal proteins for which ligands are as yet unknown. With this sort of information in hand, we can begin to unravel the relationship between striatal molecular composition, the physiology of this crucial brain region and clinically relevant behaviors. Examination of multiple striatum-enriched proteins may also help us to identify molecules that work together to influence physiology and behavior, and may be useful for the development of therapeutic approaches to treatment of disorders involving the basal ganglia.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Mizushima K, et al. Genomics. 2000;69:314–321. doi: 10.1006/geno.2000.6340. [DOI] [PubMed] [Google Scholar]
- 2.Becker JA, et al. Neuroscience. 2008;156:950–965. doi: 10.1016/j.neuroscience.2008.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conti B, et al. Mol. Psychiatry. 2007;12:167–189. doi: 10.1038/sj.mp.4001897. [DOI] [PubMed] [Google Scholar]
- 4.Le-Niculescu H, et al. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2007;144B:129–158. doi: 10.1002/ajmg.b.30481. [DOI] [PubMed] [Google Scholar]
- 5.Hervé D, Rogard M, Lévi-Strauss M. Brain Res. Mol. Brain Res. 1995;32:125–134. doi: 10.1016/0169-328x(95)00070-9. [DOI] [PubMed] [Google Scholar]
- 6.Schiffmann SN, Jacobs O, Vanderhaeghen JJ. J. Neurochem. 1991;57:1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- 7.Quintana A, et al. Nat. Neurosci. 2012;15:1547–1555. doi: 10.1038/nn.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding J, et al. Nat. Neurosci. 2006;9:832–842. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- 9.Lerner TN, Kreitzer AC. Neuron. 2012;73:347–359. doi: 10.1016/j.neuron.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logue SF, et al. Mol. Cell. Neurosci. 2009;42:438–447. doi: 10.1016/j.mcn.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Levoye A, Dam J, Ayoub MA, Guillaume JL, Jockers R. EMBO Rep. 2006;7:1094–1098. doi: 10.1038/sj.embor.7400838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massart R, Guilloux JP, Mignon V, Sokoloff P, Diaz J. Eur. J. Neurosci. 2009;30:397–414. doi: 10.1111/j.1460-9568.2009.06842.x. [DOI] [PubMed] [Google Scholar]
- 13.Van Waes V, Tseng KY, Steiner H. Basal Ganglia. 2011;1:83–89. doi: 10.1016/j.baga.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreitzer AC. Annu. Rev. Neurosci. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- 15.Surmeier DJ, Plotkin J, Shen W. Curr. Opin. Neurobiol. 2009;19:621–628. doi: 10.1016/j.conb.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]