Abstract
Telomere uncapping increases with advancing age in human arteries and this telomere uncapping is associated with increased markers of senescence, independent of mean telomere length. However, whether there are sex specific differences in arterial telomere uncapping is unknown. We found that telomere uncapping (serine 139 phosphorylated histone γ-H2A.X in telomeres) in arteries was ~2.5 fold greater in post-menopausal women (n=17, 63±2 yrs) compared with premenopausal women (n=11, 30±2 yrs, p=0.02), while there was only a trend towards greater telomere uncapping in older men (n=26, 66±2 yrs) compared with young men (n=11, 31±2, p=0.11). Senescence markers, p53 bound to the p21 gene promoter and p21 gene expression, were 3-4 fold greater in post-menopausal compared with premenopausal women (p=0.01-0.02), but only 1.5-2 fold greater in older compared with young men (p=0.02-0.08). Blood glucose was related to telomere uncapping in women, while systolic blood pressure, pulse pressure and serum creatinine were related to telomere uncapping in men. Mean arterial telomere length decreased similarly in women and men with age (p<0.01). Thus, the age-related increase in arterial telomere uncapping and senescence is greater in women than men, despite similar age-related reductions in mean telomere length in both sexes.
Keywords: aging, sex differences, artery, telomere dysfunction, senescence
1. Introduction
In recent years, the relation between altered telomere function and cardiovascular disease (CVD) risk has grown to be appreciated. Although epidemiology and physiology studies typically focus on mean telomere length (Brouilette and others 2003; Samani and others 2001; Willeit and others 2010), we recently demonstrated in arteries that other features of telomere biology (i.e., the uncapping of telomere ends) are better related to markers of cellular dysfunction (Morgan and others 2013). Telomere ends form a loop structure, known as a cap, which prevents the chromosome ends from being recognized as double-stranded DNA breaks and initiating a DNA damage response (Griffith and others 1999; Stansel and others 2001; Takai and others 2003). In human arteries, telomere uncapping from loss of the loop structure increases with advancing age and is associated with hypertension (Morgan and others 2013; Morgan and others 2014). In arteries, greater telomere uncapping, independent of mean telomere length, is associated with increased markers of cellular senescence and inflammation (Morgan and others 2013). However, whether there are sex specific differences in the age-related changes in telomere biology is unknown.
Increasing age is a major risk factor for CVD in both women and men, but for women, there is a greater incidence of CVD post-menopause compared to pre-menopause independent of traditional cardiovascular risk factors (Kannel and others 1976). Previous studies of sex differences related to telomeres have typically focused on only telomere length in circulating immune cells, with most studies indicating that women have longer telomeres than men in leukocytes (Bekaert and others 2007; Fitzpatrick and others 2011; Gardner and others 2014; Willeit and others 2010). In addition, later onset of menopause and longer exposure to circulating estrogen is associated with greater leukocyte telomere length in women (Gray and others 2014; Lin and others 2011). Thus, not only is it unknown if telomere uncapping or mean telomere length are different in the arteries of women and men, but the role of menopause in women has yet to be studied.
The major consequence of telomere uncapping is the initiation of a DNA damage response and subsequent senescence signaling by the p53/p21 pathway (d'Adda di Fagagna and others 2003; Takai and others 2003). In human arteries, advancing age is associated with greater p53 bound to the p21 gene promoter as well as greater p21 gene expression (Morgan and others 2013). Importantly, cellular senescence is associated with an aging phenotype (Baker and others 2011; Herbig and others 2006) and senescent cells in arteries have been associated with CVD (Matthews and others 2006). However, it is currently unknown if markers of p53/p21 mediated senescence differ in arteries with advancing age in women and men.
Consequently, we sought to determine if telomere uncapping in resistance arteries, indicated by phosphorylation of histone γ-H2A.X at serine 139 (p-H2A.X) in telomeric chromatin, was different in women and men as well as between pre- and post-menopausal women. We also sought to determine if any elevations in telomere uncapping occurred concomitantly with greater activation of the p53/p21 senescence pathway. In addition, we examined if mean arterial telomere length and activity of telomerase differed between women and men with advancing age. Finally, we sought to determine if the clinical characteristics associated with telomere uncapping or shortening were different between women and men.
2. Materials and methods
2.1. Subjects
A total of 65 subjects undergoing a prophylactic melanoma-associated sentinel lymph node biopsy were enrolled in this study. Patients with a prior diagnosis of metastatic melanoma, prior chemotherapy treatment, and/or indication of melanoma metastasis (blood lactate dehydrogenase >618 U/L or positive sentinel lymph node biopsy) were excluded. To omit the peri-menopausal time period, subjects between the ages of 40 and 55 years were excluded. We enrolled 11 premenopausal women and 11 young men 20-39 years of age, and 17 post-menopausal women and 26 older men 56-85 years of age. The Institutional Review Boards of the University of Utah and the Salt Lake City Veteran’s Affairs Medical Center approved all protocols, and written informed consent was obtained from all subjects prior to biopsy surgery.
2.2. Subject Characteristics and Arterial Collection
Medical history and prescription medication use were obtained from medical records. Blood pressure, anthropometry and blood chemistry analysis were performed utilizing standard procedures. Arteries were collected during sentinel lymph node biopsy surgery performed at the Huntsman Cancer Hospital, University of Utah. Skeletal muscle feed arteries were excised from the inguinal (e.g., hip adductors or quadriceps femoris) or axillary (e.g. serratus anterior or latissimus dorsi) regions and were free of melanoma cells (Ives and others 2013). Arteries were identified as skeletal muscle feed arteries by entry into the muscle bed, gross anatomy, coloration, and pulsatile bleed pattern (Ives and others 2013). Arteries were cleaned of adipose and connective tissue, and washed to remove residual blood cells. The average size of each artery was approximately 2 mm in length, 0.5 mm in luminal diameter, and weighed 10-20 mg. Cleaned arteries were then snap frozen in liquid nitrogen and stored at −80°C prior to performing the following assessments. All samples were assayed in triplicate, and replicate means were used for analysis.
2.2. Telomere uncapping
Chromatin immunoprecipitation (ChIP) was used to determine the amount of p-H2A.X (Santa Cruz Biotechnology, Inc.) localized to telomeres. ChIPs were performed as previously described (Morgan and others 2013), and analyzed via qPCR for telomere content as described by Cawthon (Cawthon 2009). Final values were expressed as the ratio of background corrected starting quantity (SQ) of telomeric DNA enriched by ChIP to telomeric DNA SQ in INPUT fraction. INPUTs represented 50% of telomeric DNA present in corresponding ChIP and were used to control for tissue concentration in samples with the calculation: (γ-H2 SQ - background SQ)/INPUT SQ = final value.
2.3. p53/p21-induced senescence
ChIPs were performed to assess p53 bound to p21 gene promoter (EMD Millipore Corporation) as previously described (Morgan and others 2013), using a sequence-independent qPCR assay with FastStart SYBR Green Master (Roche Diagnostics Corporation, Roche Applied Science). Additionally, p21 mRNA expression was determined by qRT-PCR using the Quantitect Reverse Transcription kit (Qiagen, Inc.) and FastStart SYBR Green Master (Roche Diagnostics Corporation, Roche Applied Science) according to the manufacturer’s protocols. Final mRNA SQs were generated by standard curve and expressed as a ratio of target mRNA SQ to 18s rRNA SQ (18s rRNA QuantiTect Primer Assay: Qiagen, Inc.). 18s rRNA was used as a housekeeping gene transcript to control for tissue concentration in samples. p21 mRNA primers: fwd-gacctgtcactgtcttgta, rev-cctcttggagaagatcagccg.
2.4. Mean telomere length
A sequence-independent multiplex qPCR technique using a SYBR Green master mix with 0.625U AmpliTaq Gold 360 DNA polymerase (Life Technologies Corporation) was utilized to determine mean telomere length as described by Cawthon (Cawthon 2009). Telomeric DNA (T) SQs and albumin SQs, used as single copy gene (S) to control tissue concentration in samples, were generated by standard curve and relative mean telomere length was expressed as the T/S ratio.
2.5. Active telomerase (hTERT)
ChIPs were performed to assess hTERT (Abcam) bound to telomeres as described above (Morgan and others 2013; Morgan and others 2014).
2.6. Data analysis
Statistical analyses were performed with IBM SPSS (version 20, Armonk, NY). A 2x2 (age x sex) ANOVA was used to assess group differences and in cases of a significant F, post-hoc analyses were performed with a Bonferroni correction for pre-planned comparisons of age groups for each sex and sex groups for each age group. Pearson correlation analysis was used to assess bivariate relations of interest. In addition, partial correlations were used to assess the influence of subject characteristics on the relation between telomere length/uncapping and age. Variables that were not normally distributed (Shapiro-Wilk test) were log transformed. Values for telomere uncapping, senescence markers, telomere length and hTERT were normalized to the mean for the premenopausal women. Significance was set at P<0.05. Values are presented as mean±SE.
3. Results
3.1. Subject Characteristics
premenopausal women and young men were not different in terms of any clinical characteristics except for serum creatinine and none of the subjects in these groups had a history of cardiovascular diseases or were taking cardiovascular-related medications. Likewise, post-menopausal women and older men were not different in terms of any clinical characteristics except for serum creatinine. Body mass index (BMI) and blood urea nitrogen (BUN) were greater with advancing age in both men and women, while pulse pressure and serum creatinine were only significantly greater with advancing age in men. 47% of post-menopausal women and 38% of older men were hypertensive and 65% of post-menopausal women and 65% of older men were taking cardiovascular-related medications (calcium channel blockers, beta blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers or diuretics)(Table 1) .
Table 1.
Subject characteristics
| Variable | Pre- menopausal Women |
Post- menopausal Women |
Young Men | Older Men |
|---|---|---|---|---|
| n | 11 | 17 | 11 | 26 |
| Age (years) a | 30 ± 2 | 63 ± 2 * | 31 ± 2 | 66 ± 2 † |
| Body mass index (kg/m2) a | 23 ± 1 | 29 ± 2 * | 24 ± 2 | 29 ± 1† |
| Systolic blood pressure (mm Hg) a | 125 ± 5 | 132 ± 4 | 130 ± 3 | 142 ± 4 |
| Diastolic blood pressure (mm Hg) b | 71 ± 4 | 72 ± 2 | 78 ± 2 | 80 ± 2 |
| Pulse pressure (mm Hg) a | 55 ± 4 | 62 ± 3 | 52 ± 2 | 62 ± 3 † |
| Blood glucose (mg/dl) | 85 ± 5 | 91 ± 3 | 95 ± 6 | 96 ± 4 |
| Blood urea nitrogen (mg/dl) a | 12 ± 1 | 17 ± 1* | 12 ± 1 | 17 ± 1 *† |
| Serum creatinine (mg/dl) a,b | 0.76 ± 0.02 | 0.86 ± 0.04 | 0.92 ± 0.05 * | 1.04 ± 0.03 †‡ |
| Medical History | ||||
| Hypertension c | 0 (0%) | 8 (47%) | 0 (0%) | 10 (38%) |
| Coronary artery disease | 0 (0%) | 1 (6%) | 0 (0%) | 2 (8%) |
| Peripheral vascular disease | 0 (0%) | 1 (6%) | 0 (0%) | 0 (0%) |
| Diabetes mellitus | 0 (0%) | 3 (18%) | 1 (9%) | 4 (15%) |
| Chronic kidney disease | 0 (0%) | 1 (6%) | 0 (0%) | 1 (4%) |
| Medication | ||||
| Calcium channel blocker | 0 (0%) | 4 (24%) | 0 (0%) | 3 (11%) |
| Beta blocker | 0 (0%) | 2 (12%) | 0 (0%) | 1 (4%) |
| ACE inhibitor | 0 (0%) | 3 (18%) | 0 (0%) | 4 (15%) |
| Angiotensin receptor blocker | 0 (0%) | 1 (6%) | 0 (0%) | 3 (11%) |
| Diuretics | 0 (0%) | 3 (18%) | 0 (0%) | 4 (15%) |
Data are mean ± SE. ACE, angiotensin converting enzyme.
p<0.05 main effect of age,
p<0.05 main effect of sex,
p<0.05 chi-square,
P<0.05 vs. pre-menopausal women,
P<0.05 vs. young men,
P<0.05 vs. post-menopausal women
3.2. Telomere uncapping and senescence
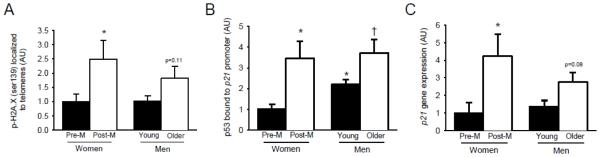
When not separated by sex, telomere uncapping, as indicated by p-H2A.X localized to telomeres, was ~2 fold greater in arteries from older subjects compared to young subjects (1.8±0.4 vs. 1.0±0.2 AU, p=0.02). When the groups were separated by sex, arterial telomere uncapping was ~2.5 fold greater in post-menopausal compared with premenopausal women (p=0.02), but only trended towards being greater in older compared with young men (p=0.11) (2x2 ANOVA main effect of age p=0.03, Figure 1A). Telomere uncapping was not different between premenopausal women and young men or between post-menopausal women and older men (p>0.05)
Figure 1. Arterial telomere uncapping and p53/p21-induced senescence.
(A) telomere uncapping indicated by p-H2A.X (ser139) localized to telomeres, (B) induction of p53 indicated by p53 bound to the p21 gene promoter, and (C) p21 gene expression in arteries from premenopausal (pre-m) women, post-menopausal (post-m) women, young men, and older men. *p<0.05 vs. pre-M women, †p<0.05 vs. young men, p-values displayed are vs. young men.
In arteries from premenopausal women, induction of p53 mediated senescence, as indicated by p53 bound to the p21 gene promoter, was about ~2 fold less compared with age-matched young men (p=0.02); however, there was no difference between post-menopausal women and age-matched older men (p=0.40, Figure 1B). p53 bound to the p21 gene promoter was ~3.5 fold greater in arteries from post-menopausal compared with premenopausal women (p=0.01) and ~1.5 fold greater in arteries from older compared with young men (p=0.02) (2x2 ANOVA main effect of age p=0.03, Figure 1B). Senescence [(log) p53 bound to the p21 gene promoter] was related to telomere uncapping [(log) p-H2A.X localized to telomeres] in both women (r=0.56, p=0.002) and men (r=0.35, p=0.02). Likewise, arterial p21 gene expression was ~4 fold greater in post-menopausal compared with premenopausal women (p=0.02), but only trended towards being greater in older compared with young men (p=0.08) (2x2 ANOVA main effect of age p=0.03, Figure 1C). p21 gene expression was not different between premenopausal women and young men or between post-menopausal women and older men (p>0.05).
3.3. Telomere length and telomerase
Mean arterial telomere length was lower in post-menopausal compared with premenopausal women (P=0.01) and lower in older compared with young men (p<0.001) (2x2 ANOVA main effect of age p<0.001. Figure 2A). Relative mean arterial telomere length was not different between premenopausal women and young men or between post-menopausal women and older men (p>0.05). Active telomerase did not differ between groups (p>0.56, Figure 2B). Mean arterial telomere length was related to senescence [(log) p53 bound to the p21 gene promoter] among women (r=-0.37, p=0.04), but not men (r=0.08, p=0.34).
Figure 2. Arterial telomere length and telomerase activity.
(A) Relative mean telomere length and (B) active telomerase (hTERT) in arteries from premenopausal (pre-m) women, post-menopausal (post-m) women, young men, and older men. *p<0.05 vs. pre-M women, †p<0.05 vs. young men.
3.4. Clinical characteristics related to telomere uncapping and length
(log) p-H2A.X localized to telomeres was related to blood glucose among women, but not men (Figure 3A,B). (log) p-H2A.X localized to telomeres was related to systolic blood pressure, pulse pressure, and serum creatinine among men, but not women (Figure 3C,D, Table 2). (log) p-H2A.X localized to telomeres was not related to BMI, diastolic blood pressure, or BUN among women or men (Table 2). Among women, the relation between age and (log) p-H2A.X localized to telomeres was no longer significant when controlling for blood glucose (partial correlation, r=0.20, p=0.18), but remained significant when controlling for systolic blood pressure (partial correlation, r=0.53, p=0.003), pulse pressure (partial correlation, r=0.45, p=0.01), or serum creatinine (partial correlation, r=0.38, p=0.04).
Figure 3. Relation between arterial telomere uncapping and blood glucose and pulse pressure.
Relation bewteen telomere uncapping, indicated by p-H2A.X (ser139) localized to telomeres, with blood glucose in (A) women and (B) men. Relation between telomere uncapping with pulse pressure in (C) women and (D) men.
Table 2.
Relation between clinical characteristics and arterial telomere uncapping ((log) p-H2A.X) and mean telomere length
| Women |
Men |
|||
|---|---|---|---|---|
| Correlate with (log) p-H2A.X | r | p | r | p |
| Body mass index | 0.09 | 0.32 | 0.18 | 0.15 |
| Systolic blood pressure | −0.15 | 0.23 | 0.28 | 0.05* |
| Diastolic blood pressure | −0.27 | 0.09 | 0.11 | 0.26 |
| Blood urea nitrogen | 0.04 | 0.43 | −0.03 | 0.42 |
| Serum creatinine | −0.08 | 0.35 | 0.30 | 0.04* |
| Correlate with mean telomere length | ||||
|
| ||||
| Body mass index | −0.26 | 0.10 | −0.26 | 0.07 |
| Systolic blood pressure | −0.07 | 0.36 | −0.25 | 0.08 |
| Diastolic blood pressure | −0.11 | 0.30 | −0.22 | 0.11 |
| Pulse pressure | −0.15 | 0.22 | −0.15 | 0.20 |
| Blood glucose | −0.45 | 0.01* | −0.03 | 0.43 |
| Blood urea nitrogen | −0.06 | 0.39 | −0.39 | 0.02* |
| Serum creatinine | 0.04 | 0.43 | −0.12 | 0.26 |
Mean arterial telomere length was related to blood glucose among women, but not men (Table 2). Mean arterial telomere length was related to BUN among men, but not women (Table 2). Mean arterial telomere length was not related to BMI, systolic or diastolic blood pressure, pulse pressure, or serum creatinine among women or men (Table 2). In both women and men, the relations between age and mean arterial telomere length remained significant even when controlling for glucose or BUN (partial correlations, p<0.05).
4. Discussion
In this study, we demonstrated, for the first time, that arteries from post-menopausal women have greater telomere uncapping and senescence markers compared with arteries from premenopausal women. Furthermore, we determined that the effect of age on arterial telomere uncapping and senescence is greater in women compared with men. As expected, mean telomere length shortens with age in arteries from both women and men, but we did not find a difference in mean arterial telomere length between the sexes in either the young or older groups. In addition, our results suggest that the clinical characteristics associated with telomere dysfunction may be different between women and men, as arterial telomere uncapping and/or mean telomere length are related to blood glucose among women, but are related to blood pressure and markers of kidney function among men. Taken together, exploration of sex differences with regards to the study of aging and telomere biology is a critical consideration.
4.1. Sex differences in telomere uncapping and senescence
We have previously demonstrated in arteries that telomere uncapping increases with advancing age in a combined group of women and men (Morgan and others 2013). In the present study, we demonstrate that arterial telomere uncapping was significantly greater with age in women, but not men. However, it should be noted that compared to our previous work, the age range for the older subjects who participated in this study was slightly different (previously >60 years, presently > 55 years) as our main goal was to exclude the perimenopausal period (Morgan and others 2013). Thus, the inclusion of some men who could be considered “middle-aged” rather than “older” in the present study may have reduced our power to detect age-related differences in this group. Nevertheless, even if statistical significance was attained, the magnitude of increase in telomere uncapping with age is still clearly greater in women than men. The increase in telomere uncapping post-menopause may indicate an influence of sex hormones on telomere capping and could suggest a role for arterial telomere dysfunction in the increased risk for CVDs in women after menopause (Kannel and others 1976).
The uncapping of telomere ends has been shown to lead to activation of the DNA damage response and cellular senescence by the p53/p21 pathway (d'Adda di Fagagna and others 2003; Takai and others 2003). The importance of cellular senescence within arteries is illustrated by the presence of senescent markers in atherosclerotic plaques and arterial cells from patients with chronic obstructive pulmonary disease, a group with elevated CVD risk (Matthews and others 2006; Noureddine and others 2011). In the present study, although the group differences in p21 gene expression mirror the differences in telomere uncapping, there appears to be an additional effect of sex on p53 activation, as differences were found between young women and men. This indicates that some factor, likely other than telomere uncapping, leads to differential arterial p53 activation between the sexes. Interestingly, there is evidence that the consequences of p53 activation may not be the same between females and males, as p53 appear to have detrimental effects on lifespan in female, but not male Drosophila (Waskar and others 2009), although this has yet to be explored in mammals. While most studies examining telomeres focus on telomere length, we demonstrate here, and previously, that in arteries, telomere uncapping is more strongly related to markers of senescence than mean telomere length. These findings highlight the importance of examining telomere characteristics, beyond simply length, in future epidemiological and physiological studies.
4.2. Sex differences in telomere length and telomerase activity
It has been demonstrated previously that telomere length declines with age in arterial tissue (Chang and Harley 1995; Ogami and others 2004; Okuda and others 2000), but these studies did not determine the influence of sex. Previous studies examining differences in telomere length between women and men typically have been performed in leukocytes and the majority of these studies find that women have longer telomeres than men (Bekaert and others 2007; Fitzpatrick and others 2011; Gardner and others 2014; Willeit and others 2010), although this might be dependent on the measurement technique utilized (Gardner and others 2014). Furthermore, a study examining mean telomere length in various human tissues found significant sex differences in leukocytes and muscle, but not in skin or fat (Daniali and others 2013). One possible mechanism for the sex difference in telomere length in leukocytes relates to telomerase activity. Specifically, there is an estrogen receptor response element on the telomerase gene promoter and estrogen has been shown to increase both telomerase expression and activity (Kyo and others 1999; Misiti and others 2000; Nanni and others 2002). Indeed, previous studies indicate that a later onset of menopause and longer exposure to circulating estrogen is associated with greater leukocyte telomere length in women (Gray and others 2014; Lin and others 2011). However, although we confirm a decline in mean telomere length with age in arteries, we did not find a sex specific difference in mean arterial telomere length. The lack of sex differences in this study is, perhaps, a reflection of the low telomerase activity in minimally proliferative tissues (Collins and Mitchell 2002), such as arteries in the post developmental period. Unfortunately, limited subject characteristics were collected for this study and we do not have data concerning the age of menopause or concentrations of circulating sex hormones in the women. Thus, we do not have any further insight into the role of estrogen in mean telomere length in arteries beyond the comparison of pre- and post-menopausal women. Nevertheless, our findings indicate that the effects of sex, and perhaps circulating hormones, on mean telomere length are not the same in arterial tissue and circulating leukocytes.
4.3. Sex differences in clinical characteristics and telomere biology
Determining the clinical characteristics that relate to telomere dysfunction is important as this may provide insight into mechanisms and potential therapeutic targets. Furthermore, as the risk factors for CVD are not always the same between women and men (Mosca and others 2011), it is important to study sex specific differences in the relation of clinical characteristics to telomere biology. In this study, we found that blood glucose related to telomere uncapping and mean telomere length among women, but not men. Similarly, in previous studies of leukocytes, glucose tolerance and/or insulin resistance have been documented to relate to telomere length among women (Aviv and others 2006) as well as a group of both women and men (Adaikalakoteswari and others 2007). This is also interesting as diabetes is more strongly related to ischemic heart disease in women than in men (Barrett-Connor and others 1991). Our results indicate that perhaps the influence of circulating glucose on telomeres and consequent senescence in arteries may contribute to this disease risk. However, it is unclear why the relation of blood glucose and arterial telomere uncapping is greater in women than men and this observation calls for further studies in the area.
Previously, in a group of both women and men, we determined that arterial telomere uncapping was greater in subjects with hypertension, which by definition includes subjects both with a blood pressure >140/90 mmHg, as well as those receiving anti-hypertensive medication (Morgan and others 2014). In the present study, in subjects that include both users and non-users of anti-hypertensive medications, we find that systolic blood pressure and pulse pressure relate to resistance artery telomere uncapping among men, but not women. Pulse pressure is an indicator of large artery stiffness and previous studies have demonstrated a relation between increased large artery stiffness and resistance artery dysfunction (Mitchell and others 2005) and the results of the present study indicate that telomere uncapping may play a role. In addition, markers of kidney dysfunction (i.e., serum creatinine or BUN) were related to both telomere uncapping and mean telomere length among men, but not women. Thus, although we cannot determine the exact causes and consequences, we demonstrate potentially important associations between telomere biology and clinical characteristics in women and men.
4.4 Limitations
There are a few important limitations of these studies to note. First, as we needed to collect tissue from live subjects to perform our measurements, we were limited to the use of peripheral arteries. While the study of coronary arteries may be more directly applicable to cardiovascular disease, there are indications that arterial dysfunction may be “systemic”. For example, endothelial dysfunction in peripheral arteries is predictive of future cardiovascular events (Heitzer and others 2001; Rossi and others 2008; Yeboah and others 2007; Yeboah and others 2009) and is correlated with endothelial dysfunction in coronary arteries (Anderson and others 1995). Thus, the changes in telomere biology occurring with sex and age in skeletal muscle feed arteries may reflect changes to other arteries as well. Second, it is of note that all individuals in this study were diagnosed with melanoma, but any individuals with evidence of metastatic melanoma were excluded. Thus, we do not believe that the melanoma had any direct effect on the arterial tissue collected. Nevertheless, these subjects may have a greater susceptibility to melanoma than the general population, which may result from differences in genetic or lifestyle risk factors that could affect telomere biology. These risk factors could influence how the clinical characteristics interact with telomere dysfunction compared to individuals less likely to develop melanoma. However, we do not believe that the susceptibility to develop melanoma had any large effects on our comparisons of sex and age differences in telomere biology, as all subjects in this study had the same diagnosis. Third, given our need to collect arteries from live human subjects, our sample size for each group is relatively small. Our lower power was not an issue for our comparisons between pre- and post-menopausal women, as we attained statistically significant group differences. Inclusion of more subjects likely would allow us to attain statistical significance between young and older men for telomere uncapping, however due to the smaller effect size between these groups we calculate that ~50 subjects/group would be needed to attain a power of 0.8. Furthermore, even without attaining statistical significance between young and older men, it is evident that the magnitude of increase in telomere uncapping with age is greater in women than men.
5. Conclusions
In this study, we demonstrate, for the first time in arteries, that telomere dysfunction, indicated by uncapping, is greater with age in women compared with men. Additionally, telomere uncapping is related to greater markers of senescence for both women and men, however in this sample the clinical factors that relate to telomere dysfunction are different between the sexes. In arteries, the increase in telomere uncapping and senescence markers post-menopause could explain some of the increased risk of CVD in older women. Furthermore, these results indicate that examining combined groups of women and men may lead to missing important features of telomere biology.
Highlights.
Arterial telomere uncapping and senescence are greater post-menopause in women.
The effect of age on arterial telomere uncapping is greater in women than men.
Mean arterial telomere length shortens with age similarly in women and men.
There are sex differences in which clinical features relate to telomere dysfunction.
Acknowledgements
This work was supported by National Institutes of Health AG040297, AG043952, AG046326, ES007032, HL091830; the Department of Veterans Affairs 1I01BX002151, RR&D E6910-R, E1697-R, E1433-P, and E9275-L; and a University of Utah Center on Aging pilot grant.
Abbreviations
- BMI
body mass index
- BUN
blood urea nitrogen
- ChIP
chromatin immunoprecipitation
- CVD
cardiovascular disease
- hTERT
telomerase
- p21
cyclin-dependent kinase inhibitor 1A
- p53
tumor suppressor p53
- p-H2A.X
phosphorylation of histone γ-H2A.X at Serine 139;
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors report no conflicts of interest. Dr. Cawthon and the University of Utah have patented telomere length measurement by QPCR, and granted an exclusive license for its commercial use to Telomere Diagnostics, Inc. of Menlo Park, California.
References
- Adaikalakoteswari A, Balasubramanyam M, Ravikumar R, Deepa R, Mohan V. Association of telomere shortening with impaired glucose tolerance and diabetic macroangiopathy. Atherosclerosis. 2007;195:83–89. doi: 10.1016/j.atherosclerosis.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- Aviv A, Valdes A, Gardner JP, Swaminathan R, Kimura M, Spector TD. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J Clin Endocrinol Metab. 2006;91:635–640. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA. 1991;265:627–631. [PubMed] [Google Scholar]
- Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M, Segers P, Cooman L, Van Damme P, Cassiman P, Van Criekinge W, Verdonck P, De Backer GG, Gillebert TC, Van Oostveldt P, Asklepios i. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6:639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic acids research. 37:e21–2009. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E, Harley CB. Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A. 1995;92:11190–11194. doi: 10.1073/pnas.92.24.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins K, Mitchell JR. Telomerase in the human organism. Oncogene. 2002;21:564–579. doi: 10.1038/sj.onc.1205083. [DOI] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, Desai K, Granick M, Aviv A. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. doi: 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Kimura M, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Hardikar S, Aviv A. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011;66:421–429. doi: 10.1093/gerona/glq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, Martin-Ruiz C, Shiels P, Sayer AA, Barbieri M, Bekaert S, Bischoff C, Brooks-Wilson A, Chen W, Cooper C, Christensen K, De Meyer T, Deary I, Der G, Diez Roux A, Fitzpatrick A, Hajat A, Halaschek-Wiener J, Harris S, Hunt SC, Jagger C, Jeon HS, Kaplan R, Kimura M, Lansdorp P, Li C, Maeda T, Mangino M, Nawrot TS, Nilsson P, Nordfjall K, Paolisso G, Ren F, Riabowol K, Robertson T, Roos G, Staessen JA, Spector T, Tang N, Unryn B, van der Harst P, Woo J, Xing C, Yadegarfar ME, Park JY, Young N, Kuh D, von Zglinicki T, Ben-Shlomo Y, Halcyon study t. Gender and telomere length: systematic review and meta-analysis. Exp Gerontol. 2014;51:15–27. doi: 10.1016/j.exger.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KE, Schiff MA, Fitzpatrick AL, Kimura M, Aviv A, Starr JR. Leukocyte telomere length and age at menopause. Epidemiology. 2014;25:139–146. doi: 10.1097/EDE.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006:311–1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- Ives SJ, Andtbacka RH, Noyes RD, Morgan RG, Gifford JR, Park SY, Symons JD, Richardson RS. alpha1-Adrenergic responsiveness in human skeletal muscle feed arteries: the impact of reducing extracellular pH. Experimental physiology. 2013;98:256–267. doi: 10.1113/expphysiol.2012.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976;85:447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, Orimo A, Inoue M. Estrogen activates telomerase. Cancer Res. 1999;59:5917–5921. [PubMed] [Google Scholar]
- Lin J, Kroenke CH, Epel E, Kenna HA, Wolkowitz OM, Blackburn E, Rasgon NL. Greater endogenous estrogen exposure is associated with longer telomeres in postmenopausal women at risk for cognitive decline. Brain Res. 2011;1379:224–231. doi: 10.1016/j.brainres.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, Goddard M, Bennett M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res. 2006;99:156–164. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- Misiti S, Nanni S, Fontemaggi G, Cong YS, Wen J, Hirte HW, Piaggio G, Sacchi A, Pontecorvi A, Bacchetti S, Farsetti A. Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells. Mol Cell Biol. 2000;20:3764–3771. doi: 10.1128/mcb.20.11.3764-3771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- Morgan RG, Ives SJ, Lesniewski LA, Cawthon RM, Andtbacka RH, Noyes RD, Richardson RS, Donato AJ. Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am J Physiol Heart Circ Physiol. 2013;305:H251–258. doi: 10.1152/ajpheart.00197.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RG, Ives SJ, Walker AE, Cawthon RM, Andtbacka RH, Noyes D, Lesniewski LA, Richardson RS, Donato AJ. Role of arterial telomere dysfunction in hypertension: relative contributions of telomere shortening and telomere uncapping. J Hypertens. 2014;32:1293–1299. doi: 10.1097/HJH.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni S, Narducci M, Della Pietra L, Moretti F, Grasselli A, De Carli P, Sacchi A, Pontecorvi A, Farsetti A. Signaling through estrogen receptors modulates telomerase activity in human prostate cancer. J Clin Invest. 2002;110:219–227. doi: 10.1172/JCI15552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noureddine H, Gary-Bobo G, Alifano M, Marcos E, Saker M, Vienney N, Amsellem V, Maitre B, Chaouat A, Chouaid C, Dubois-Rande JL, Damotte D, Adnot S. Pulmonary artery smooth muscle cell senescence is a pathogenic mechanism for pulmonary hypertension in chronic lung disease. Circ Res. 2011;109:543–553. doi: 10.1161/CIRCRESAHA.111.241299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogami M, Ikura Y, Ohsawa M, Matsuo T, Kayo S, Yoshimi N, Hai E, Shirai N, Ehara S, Komatsu R, Naruko T, Ueda M. Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol. 2004;24:546–550. doi: 10.1161/01.ATV.0000117200.46938.e7. [DOI] [PubMed] [Google Scholar]
- Okuda K, Khan MY, Skurnick J, Kimura M, Aviv H, Aviv A. Telomere attrition of the human abdominal aorta: relationships with age and atherosclerosis. Atherosclerosis. 2000;152:391–398. doi: 10.1016/s0021-9150(99)00482-7. [DOI] [PubMed] [Google Scholar]
- Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol. 2008;51:997–1002. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- Stansel RM, de Lange T, Griffith JD. T-loop assembly in vitro involves binding of TRF2 near the 3' telomeric overhang. EMBO J. 2001;20:5532–5540. doi: 10.1093/emboj/20.19.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- Waskar M, Landis GN, Shen J, Curtis C, Tozer K, Abdueva D, Skvortsov D, Tavare S, Tower J. Drosophila melanogaster p53 has developmental stage-specific and sex-specific effects on adult life span indicative of sexual antagonistic pleiotropy. Aging (Albany NY) 2009;1:903–936. doi: 10.18632/aging.100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit P, Willeit J, Brandstatter A, Ehrlenbach S, Mayr A, Gasperi A, Weger S, Oberhollenzer F, Reindl M, Kronenberg F, Kiechl S. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler Thromb Vasc Biol. 2010;30:1649–1656. doi: 10.1161/ATVBAHA.110.205492. [DOI] [PubMed] [Google Scholar]
- Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]