Abstract
Purpose
To review outcomes of locally advanced pancreatic cancer (LAPC) patients treated with dose-escalated intensity modulated radiation therapy (IMRT) with curative intent.
Methods and Materials
A total of 200 patients with LAPC were treated with induction chemotherapy followed by chemoradiation between 2006 and 2014. Of these, 47 (24%) having tumors >1 cm from the luminal organs were selected for dose-escalated IMRT (biologically effective dose [BED] >70 Gy) using a simultaneous integrated boost technique, inspiration breath hold, and computed tomographic image guidance. Fractionation was optimized for coverage of gross tumor and luminal organ sparing. A 2- to 5-mm margin around the gross tumor volume was treated using a simultaneous integrated boost with a microscopic dose. Overall survival (OS), recurrence-free survival (RFS), local-regional and distant RFS, and time to local-regional and distant recurrence, calculated from start of chemoradiation, were the outcomes of interest.
Results
Median radiation dose was 50.4 Gy (BED = 59.47 Gy) with a concurrent capecitabine-based (86%) regimen. Patients who received BED >70 Gy had a superior OS (17.8 vs 15.0 months, P = .03), which was preserved throughout the follow-up period, with estimated OS rates at 2 years of 36% versus 19% and at 3 years of 31% versus 9% along with improved local-regional RFS (10.2 vs 6.2 months, P = .05) as compared with those receiving BED ≤70 Gy. Degree of gross tumor volume coverage did not seem to affect outcomes. No additional toxicity was observed in the high-dose group. Higher dose (BED) was the only predictor of improved OS on multivariate analysis.
Conclusion
Radiation dose escalation during consolidative chemoradiation therapy after induction chemotherapy for LAPC patients improves OS and local-regional RFS.
Introduction
Pancreatic cancer is the third most common gastrointestinal (GI) malignancy and the fourth leading cause of cancer-related death in the Western world (1). Currently, margin-negative surgical resection offers the only means of cure, but unfortunately only 10% to 15% of patients present with resectable disease. Combined chemotherapy and radiation therapy is the mainstay of treatment for the remaining subset of patients presenting with either locally advanced or borderline resectable disease (2–5).
Historically, the prognosis of patients with locally advanced pancreatic cancer (LAPC) has been poor, with a median survival of 9 to 13 months and a 5-year survival rate of <5%. Distant metastases are a dominant cause of disease progression. However, a recent autopsy series has highlighted the importance of achieving local control by demonstrating that close to one-third of patients with pancreatic cancer die from locally destructive disease rather than distant metastasis (6). This supports the contention that local control or a delay in local progression is paramount in preventing tumor progression and improving overall survival (OS) (3). Although induction chemotherapy followed by radiation therapy (45–50.4 Gy in 25–30 fractions) with concurrent capecitabine is generally practiced in the United States as standard treatment for LAPC, the recent report of the randomized LAP07 trial showing no significant survival advantage with the addition of radiation to systemic chemotherapy calls into question the role of radiation therapy (7).
Radiation dose escalation offers a potential strategy for improving local control and has been shown to influence clinical outcomes in other malignancies, such as prostate cancer and head and neck cancer (8, 9). However, the tolerance of the surrounding GI tract has historically limited delivering high doses of radiation therapy to the gross tumor. With recent advances in planning and delivery of radiation therapy like intensity modulated radiation therapy (IMRT) and image guided radiation therapy, it is now technically possible to treat selected patients with locally advanced disease with dose escalation. Furthermore, the universal availability of “dose painting” within IMRT workflows provides the opportunity to dose escalate discrete portions of the tumor (such as the retroperitoneal margin or gross tumor farther away from GI mucosa) while maintaining normal tissue dose-volume constraints for adjacent structures.
The purpose of this study was to compare the clinical outcomes between LAPC patients treated with curative intent using dose-escalated IMRT with daily image guided soft-tissue registration and those treated with standard fractionation radiation therapy regimens at our center.
Methods and Materials
Patient identification and selection
We performed a retrospective cohort study of consecutive patients with biopsy-proven LAPC treated with definitive chemoradiation at MD Anderson Cancer Center between 2006 and 2014. A tumor was considered locally advanced on the basis of review of computerized tomography (CT) images if it extended to the superior mesenteric artery (>180° encasement), the celiac axis, or the aorta, or caused occlusion of the superior mesenteric venous-portal venous confluence (4). The study was approved by the MD Anderson Cancer Center institutional review board.
Clinical evaluation and treatment
All patients were evaluated by a medical oncologist and a radiation oncologist. Selected patients were subsequently seen by surgical oncologists. All patients were subjected to a dedicated pancreatic cancer protocol contrast-enhanced dual phase abdominal and pelvic CT scan. Pretreatment evaluation also included baseline hematologic and biochemical parameters. All patients received induction chemotherapy before chemoradiation. Induction chemotherapy included FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, and oxaliplatin) or gemcitabine-based regimens. Concurrent chemotherapy included gemcitabine-based or capecitabine-based regimens. The median radiation dose administered was 50.4 Gy. The choice of chemotherapy was based on physician preference. Dose-escalated radiation therapy was administered using either an IMRT technique exclusively (n = 41), or a 3-dimensional conformal technique (3D-CRT) followed by an IMRT boost (n = 5), or an IMRT technique followed by a proton therapy boost (n = 1). Standard dose radiation therapy was administered using 3D-CRT (n = 140) or IMRT (n = 13). Patients were scheduled for initial follow-up visits at approximately 4 to 6 weeks (after completion of chemoradiation) and every 2 to 4 months thereafter. To compare dose effects across various fractions (Table 1), biologically effective dose (BED) was calculated using the following formula:
where n is the number of fractions, d is the dose per fraction, and α/β for tumors = 10.
Table 1.
Dose fractionation schedules, biologically effective dose, and dose-volume constraints
| Dose and no. of fractions | Biologically effective dose (Gy) |
No. of patients | Average stomach V50 (cm3) or maximum point dose if V50 = 0 cm3 |
Average duodenum V50 (cm3) or maximum point dose if V50 = 0 cm3 |
|---|---|---|---|---|
| 63 Gy in 28 fx | 77.2 | 14 | 25.5 | 22.8 |
| 70 Gy in 28 fx | 87.5 | 11 | 25 | 27.6 |
| 67.5 Gy in 15 fx | 97.9 | 7 | 0; 44.8 Gy | 0; 44.9 Gy |
| 60 Gy in 10 fx | 96.0 | 1 | 0; 41.3 Gy | 0; 43 Gy |
| 50 Gy in 5 fx | 100.0 | 1 | 0; 26.3 Gy | 0; 36.1 |
| 51.3–70.4 Gy in 13–39 fx | 70.4–84.3 | 13 | 33.9 | 15.2 |
Abbreviations: fx = fractions; V50 = volume of organ receiving >50 Gy.
α/β = 10 for calculation of biologically effective dose.
The BEDs for the standard fractionation regimens of 50.4 Gy in 28 fractions and 50 Gy in 25 fractions are 59.47 Gy and 60 Gy, respectively, whereas that for a dose-escalated regimen of 57.25 Gy in 25 fractions is 70.36 Gy. Thus a BED of 70 Gy was chosen as a cut-off to stratify patients into low- and high-dose groups.
Radiation dose escalation
Patients with unresectable tumors more than 1 cm from the closest GI mucosa were selected for treatment with dose-escalated radiation therapy with curative intent (Fig. 1). The most common technique used was an inspiratory breath-hold technique using daily CT-on-rails or cone-beam CT for alignment to soft tissues to ensure adequate coverage of gross tumor volume (GTV) and sparing of the surrounding bowel (stomach, duodenum, and jejunum) (10). The choice of dose fractionation was individualized and dictated by the trade-off between optimum minimum dose coverage of the GTV and planning target volume and achieving normal tissue dose constraints. A more fractionated regimen was used when tumors were closer to GI mucosa. Relative reproducibility of mucosal geometry was ensured by consistently simulating and treating patients at least 3 hours after their last meal. A 2- to 5-mm margin around the GTV served as the boost target volume for a simultaneous in-field boost (SIB) within the larger planning target volume expansion of approximately 1.5 cm. Acute toxicity was defined within 90 days from the start of radiation therapy in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
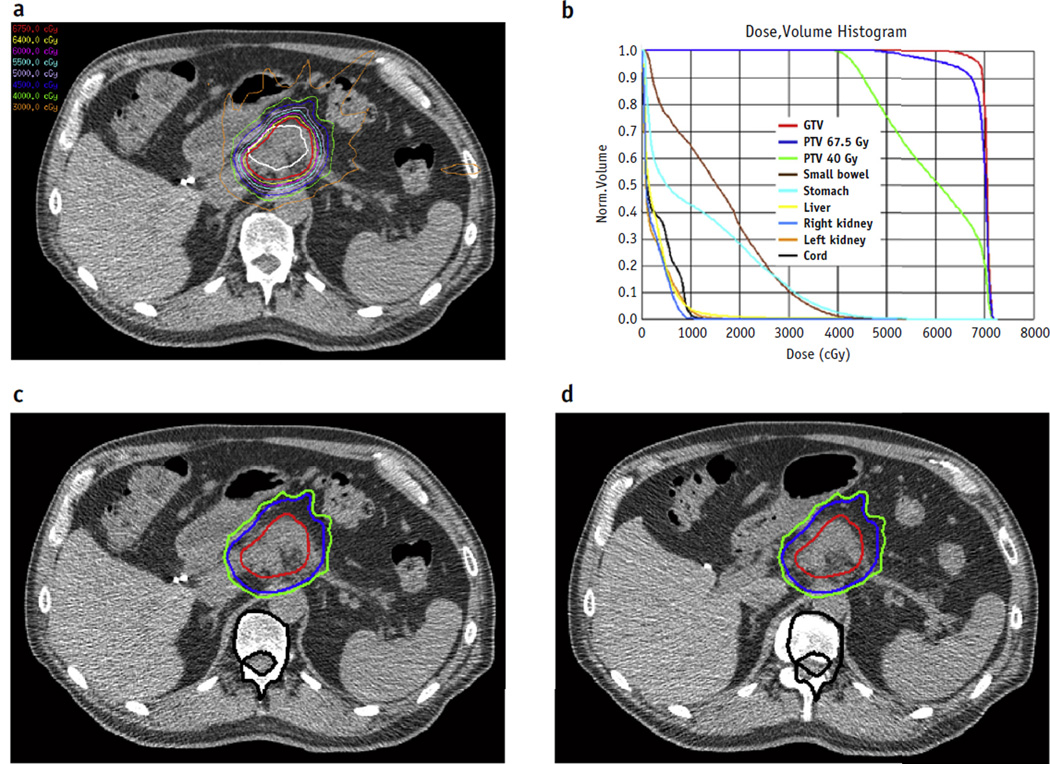
Fig. 1.
A representative plan of a patient treated with radiation dose escalation (biologically effective dose >70 Gy). (a) Axial isodose plots and (b) dose-volume histogram corresponding to a planned course of treatment. The patient was aligned daily by mapping soft-tissue anatomy and isodose lines between (c) a reference computed tomography scan obtained at simulation and (d) a computed tomography scan obtained on the day of treatment. Black contours are vertebral bodies; red isodose lines indicate 67.5 Gy, blue 45 Gy, and green 40 Gy. As noted here, alignment to bony landmarks would have resulted in an increased dose to the stomach wall. Abbreviations: GTV Z gross tumor volume; PTV Z planning target volume. A color version of this figure is available at www.redjournal.org.
Dosimetry considerations
Volumes of the stomach and duodenum (and the jejunum, when in close proximity) receiving more than 50, 55, and 60 Gy were determined for most patients and used for determination of dose fractionation. The percentage of GTV that received the SIB prescription was recorded for all patients. In patients in whom the SIB targeted primarily the retroperitoneal margin and/or the GTV away from GI mucosa, this value of fractional GTV receiving SIB dose was lower.
Endpoints
The major endpoint of our study was OS. In addition, we also examined local-regional and distant recurrence. Local-regional recurrence and distant recurrence were retrospectively determined using formal interpretations of all follow-up radiographic imaging data with lesions reported as indeterminate not being coded as progression. Evidence of any progression at or adjacent to the primary site or in the surrounding regional lymph nodes on abdominopelvic CT scans was considered a local-regional recurrence. Evidence of distant recurrence included the development of malignant ascites or radiographically visible metastasis in the peritoneum, liver, lung, bone, or any other distant site.
Statistical considerations
Categorical variables were summarized by frequency and percentage. Continuous variables were summarized by median and range. The differences between groups were compared using a χ2 test or Fisher exact test for categorical variables, and Wilcoxon rank sum test for continuous variables. Overall survival was defined as the time interval between chemoradiation start date and death date, and was censored at last follow-up date for patients who were alive. Local-regional recurrence-free survival, distant recurrence-free survival, and recurrence-free survival were defined as the time interval between chemoradiation start date and the date of local-regional recurrence, distant recurrence, and any recurrence or death date, and was censored at last follow-up date for patients who were alive and did not have local-regional recurrence, distant recurrence, and any recurrence, respectively. Time to local-regional recurrence and distant recurrence was defined as the time interval between chemoradiation start date and the date of local-regional recurrence and distant recurrence, and was censored at last follow-up date for patients who did not have local-regional recurrence and distant recurrence, respectively. Survival probabilities were estimated nonparametrically using Kaplan-Meier’s product limit method, and the log-rank test was used to compare the difference between groups. Univariate and multivariate Cox proportional hazards models were used to assess the association between potential predictors and survival time. Predictors significant in the univariate model at the 0.2 level were included in a multivariate model. Then backward elimination was implemented until all remaining covariates had a P value of <.05.
The method of inverse probability of treatment weighting using the propensity score was applied to reduce potential selection bias. The propensity score for each patient was calculated according to a multivariate logistic regression of dose group using the potential predictors of Karnofsky performance status, race, percent weight loss, baseline hemoglobin, tumor location, choice of induction chemotherapy, and choice of concurrent chemotherapy. The standardized differences were used to evaluate the balance of these potential predictors between dose groups before and after propensity score adjustment. After adjustment, survival probabilities were estimated using the weighted Kaplan-Meier methods. The weighted Cox proportional hazards regression models were applied to assess the association between potential predictors and survival time. All statistical analysis was performed using SAS 9.3 (SAS Institute, Cary NC), and figures were created in R (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient and tumor characteristics
A total of 200 patients were diagnosed and treated using chemoradiation during the study period. The median age at diagnosis was 64 years (range, 37–88 years), and the majority were men (57%). The median pretreatment CA19-9 level was 198 U/mL (range, 1–12,440 U/mL), which decreased to 98 U/mL (range, 1– 9597 U/mL) after induction chemotherapy (P<.0001), with a median reduction of 34%. The median tumor size in the largest dimension was 3.5 cm (range, 0.9–8 cm). The majority of tumors were adenocarcinomas (95%), located predominantly in the head of the pancreas (61%).
Treatment characteristics
The median time for initiation of any form of treatment was 22 days (range, 1–146 days) from the date of diagnosis. The systemic chemotherapy was administered for a median duration of 3.5 months (range, 1–13 months) using either FOLFIRINOX (n = 42, 21%) or gemcitabine-based (n = 158, 79%) regimens. The median time for initiation of chemoradiation was 138 days from diagnosis. The median radiation dose was 50.4 Gy in 28 fractions (range, 25.2–70.4 Gy) administered over a median duration of 38 days (interquartile range, 37–39 days). Concurrent chemotherapy included either 5-fluorouracil (n = 3, 1.5%), vorinostat (n = 1, <1%), gemcitabine-based (n = 24, 12%), or capecitabine-based (n = 167, 86%) therapy. Forty-seven patients (24%) were selected for treatment with dose-escalated IMRT (BED >70 Gy) according to the abovementioned criteria. After chemoradiation, 11 patients were considered for resection. Table 2 summarizes the patient, tumor, and treatment characteristics.
Table 2.
Patient, tumor, and treatment characteristics
| Characteristic | Overall cohort | Weighted cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| All patients (n = 200) |
BED >70 Gy (n = 47) |
BED ≤70 Gy (n = 153) |
P | Std diff (%) |
BED >70 Gy | BED ≤70 Gy | Std diff (%) |
|
| Patient characteristics | ||||||||
| Age (y), median (range) | 64 (37–88) | 64 (38–82) | 64 (37–88) | .92 | 66 (38–82) | 64 (37–87) | ||
| Age (y), n (%) | .60 | 8.75 | 0.66 | |||||
| >60 | 134 (67) | 30 (64) | 104 (68) | (68) | (68) | |||
| ≤60 | 66 (33) | 17 (36) | 49 (32) | (32) | (32) | |||
| Gender, n (%) | .63 | 8.14 | 0.50 | |||||
| Male | 113 (57) | 28 (60) | 85 (56) | (56) | (56) | |||
| Female | 87 (44) | 19 (40) | 68 (44) | (44) | (44) | |||
| Race, n (%) | .17 | 24.11 | 12.67 | |||||
| White | 146 (73) | 38 (81) | 108 (71) | (67) | (73) | |||
| Other | 54 (27) | 9 (19) | 45 (29) | (33) | (27) | |||
| KPS, n (%) | .45 | 15.25 | 1.05 | |||||
| ≥80 | 174 (88) | 43 (91) | 131 (87) | (88) | (88) | |||
| <80 | 24 (12) | 4 (9) | 20 (13) | (12) | (12) | |||
| Baseline CA19-9 (U/mL), median (range) | 198 (1–12,440) | 355 (1–4999) | 178 (1–12,440) | .2 | 363 (1–4999) | 183 (1–12,440) | ||
| % Weight loss, median (range) | 9.04 (0–34.97) | 7.85 (0–34.97) | 9.62 (0–29.7) | .05 | 9.04 (0–34.97) | 8.98 (0–29.70) | ||
| Weight loss, n (%) | .05 | 34.62 | 3.89 | |||||
| >10% | 88 (46) | 14 (33) | 74 (49) | (46) | (44) | |||
| ≤10% | 105 (54) | 29 (67) | 76 (51) | (54) | (56) | |||
| Baseline hemoglobin (g/dL), median (range) | 12.85 (8.2–18) | 13.15 (10.3–16.3) | 12.8 (8.2–18) | .59 | 12.3 (10.3–16.3) | 12.9 (8.2–18) | ||
| Baseline hemoglobin (g/dL), n (%) | .25 | 19.98 | 10.35 | |||||
| >12 | 133 (69) | 35 (76) | 98 (67) | (65) | (70) | |||
| ≤12 | 59 (31) | 11 (24) | 48 (33) | (35) | (30) | |||
| Tumor characteristics | ||||||||
| Tumor size (cm), median (range) | 3.5 (0.9–8) | 3.2 (0.9–8) | 3.6 (0.9–7.5) | .16 | 3.2 (0.9–8) | 3.6 (0.9–7.5) | ||
| Tumor location, n (%) | .26 | 19 | 6.04 | |||||
| Head | 118 (61) | 24 (53) | 94 (63) | (64) | (61) | |||
| Other | 77 (39) | 21 (47) | 56 (37) | (36) | (39) | |||
| Treatment characteristics | ||||||||
| Induction chemotherapy, n (%)* | .04 | 33.51 | 0.71 | |||||
| Gemcitabine-based | 158 (79) | 32 (68) | 126 (82) | (79) | (79) | |||
| FOLFIRINOX | 42 (21) | 15 (32) | 27 (18) | (21) | (21) | |||
| Concurrent chemotherapy, n (%)† | .16‡ | 22.74‡ | 3.08‡ | |||||
| 5-Fluorouracil | 3 (2) | 1 (2) | 2 (1) | (2) | (1) | |||
| Vorinostat | 1 (1) | 1 (2) | 0 (0) | (2) | (0) | |||
| Gemcitabine-based | 24 (12) | 7 (16) | 17 (11) | (12) | (13) | |||
| Capecitabine-based | 167 (86) | 34 (79) | 133 (88) | (84) | (86) | |||
| Biologically effective dose, median (range) | 59.47 (29.74–100) | 83.11 (70.36–100) | 59.47 (29.74–60) | 83.44 (70.36–100) | 59.47 (29.74–60) | |||
| Radiation technique | <.001 | |||||||
| IMRT | 58 (29) | 45 (96) | 13 (9) | (97) | (9) | |||
| Conventional 3D conformal | 141 (70) | 1 (2) | 140 (91) | (1) | (91) | |||
| IMRT + proton boost | 1 (1) | 1 (2) | 0 (0) | (2) | (0) | |||
| Change in CA 19-9 after induction chemotherapy, median (range) | 34.08 (−20,380 to 99.36) | 41.37 (−41.93 to 96.38) | 34.08 (−20,380 to 99.36) | .51 | 53.65 (−41.93 to 96.38) | 34.74 (−20,380 to 99.36) | ||
| Change in CA 19-9 after induction chemotherapy, n (%) | 1 | 0 | 0.04 | |||||
| >34 | 81 (50) | 13 (50) | 68 (50) | (50) | (50) | |||
| ≤34 | 81 (50) | 13 (50) | 68 (50) | (50) | (50) | |||
Abbreviations: BED = biologically effective dose; IMRT = intensity modulated radiation therapy; KPS = Karnofsky performance status; Std diff = standardized difference.
Gemcitabine-based induction therapy consisted of gemcitabine alone or in combination with paclitaxel, cisplatin, oxaliplatin, erlotinib, bevacizumab, or cetuximab.
Gemcitabine-based concurrent therapy consisted of gemcitabine alone or in combination with erlotinib; capecitabine-based concurrent therapy consisted of capecitabine alone or in combination with bevacizumab, erlotinib, or cetuximab; where numbers do not add to 200, insufficient data were available in remaining patients.
Calculated when concurrent chemotherapy was dichotomized as capecitabine versus others.
Recurrence and survival analysis
The median clinical follow-up time was 9.6 months (range, 1–86 months), and radiologic follow-up time was 8.5 months (range, 1–85 months). Overall, 151 patients had died at the time of final analysis. The median OS from the start of chemoradiation was 15.3 months (95% confidence interval [CI] 12.5–16.9 months), and estimated survival rates at the end of 1, 2, and 3 years were 60%, 22%, and 13%, respectively. Median recurrence-free survival was 5.5 months (95% CI 4.6–7.4 months), local-regional recurrence-free survival was 7.3 months (95% CI 5.5–8.8 months), and distant recurrence-free survival was 8.3 months (95% CI 5.5–10.2 months). Median time to local-regional recurrence was 11.2 months (95% CI 8.9–13.5 months) and to distant recurrence was 13.4 months (95% CI 10.3–18 months).
Predictors of outcome
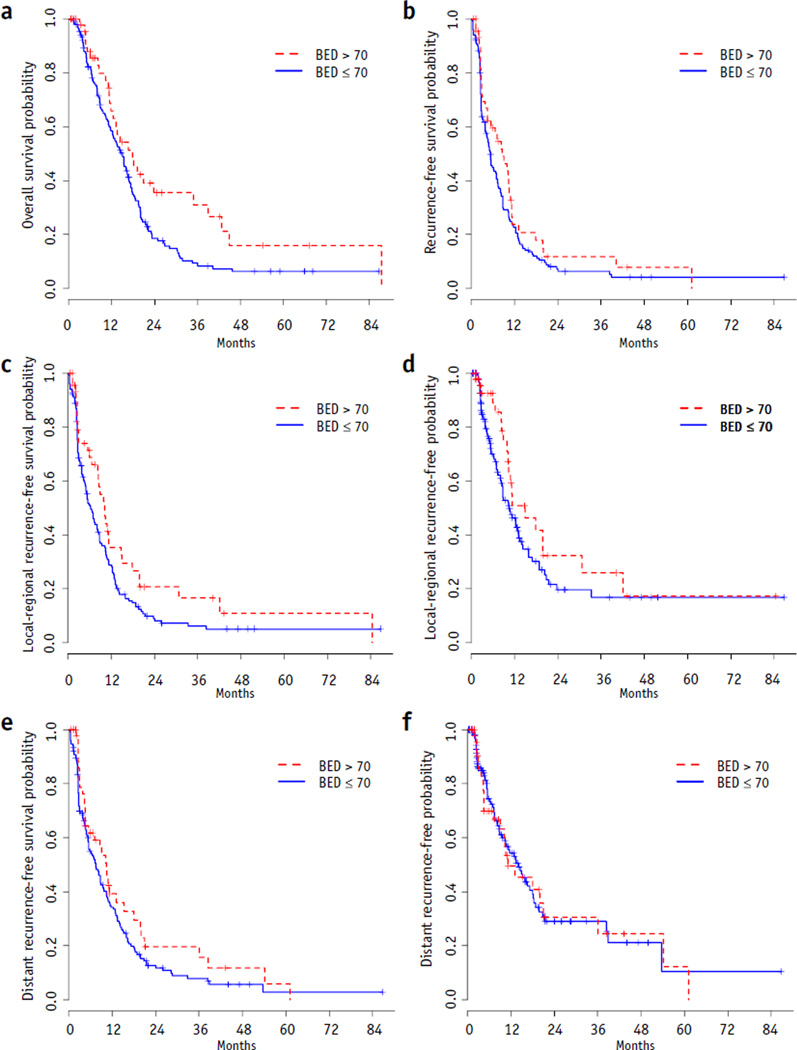
Patient characteristics such as age, gender, race, percent weight loss, baseline Karnofsky performance status, and baseline hemoglobin; tumor characteristics such as location; and treatment characteristics such as choice of induction chemotherapy, biochemical response to induction chemotherapy (change in CA19-9), choice of concurrent chemotherapy, and radiation dose (BED) were evaluated for their impact on OS. Table 3 summarizes the results of Cox proportional hazards models for OS. Only BED was significantly associated with OS in the univariate analysis. Patients who received BED >70 Gy had a superior median OS (17.8 vs 15.0 months, P = .03) (Fig. 2a) as compared with those with BED ≤70 Gy. This benefit was preserved throughout the follow-up period, with estimated OS rates of 36% versus 19% at 2 years and 31% versus 9% at 3 years for high-BED versus low-BED patients, respectively. The median local-regional recurrence-free survival (10.2 vs 6.2 months, P = .05; Fig. 2c) was prolonged in patients receiving BED >70 Gy, with local-regional recurrence-free survival rates of 21% and 17% at the end of 1 and 2 years, respectively, compared with patients receiving BED ≤70 Gy, with local-regional recurrence-free survival rates of 9% and 6% at the end of 1 and 2 years, respectively. However, dose escalation did not significantly alter the median recurrence-free survival (8.6 vs 5.3 months, P = .17; Fig. 2b), time to local-regional recurrence (14.9 vs 10.5 months, P = .14; Fig. 2d), distant recurrence-free survival (10.3 vs 7.4 months, P = .16; Fig. 2e), or the time to distant recurrence (11.2 vs 14.1 months, P = .92; Fig. 2f). On multivariate analysis, BED (hazard ratio 0.63, P = .03) was the only independent predictors of OS.
Table 3.
Cox proportional hazard models for overall survival
| Prognostic factor | Overall cohort | Weighted cohort | ||
|---|---|---|---|---|
| P | Hazard ratio (CI) | P | Hazard ratio (CI) | |
| Univariate | ||||
| Age (>60 vs ≤60 y) | .30 | 1.20 (0.85–1.69) | .06 | 1.27 (0.99–1.63) |
| Gender (female vs male) | .80 | 0.96 (0.69–1.33) | .77 | 0.97 (0.76–1.23) |
| Race (white vs other) | .38 | 0.85 (0.59–1.22) | .004 | 0.67 (0.52–0.88) |
| KPS (≥80 vs <80) | .07 | 0.66 (0.42–1.04) | .12 | 0.75 (0.51–1.08) |
| % Weight loss (<10% vs ≥10%) | .48 | 0.89 (0.64–1.23) | .13 | 0.83 (0.65–1.06) |
| Baseline hemoglobin (>12 vs ≤12g/dL) | .29 | 0.82 (0.58–1.18) | .53 | 0.92 (0.71–1.19) |
| Tumor location (head vs other) | .90 | 1.02 (0.73–1.42) | .54 | 1.08 (0.85–1.37) |
| Induction chemotherapy (gemcitabine vs others) | .54 | 1.17 (0.71–1.92) | .53 | 1.12 (0.78–1.61) |
| Concurrent chemotherapy (capecitabine vs others) | .18 | 0.74 (0.47–1.15) | .06 | 0.74 (0.54–1.01) |
| Change in CA19-9 after induction chemotherapy (>34% vs ≤ 34%) | .45 | 0.87 (0.62–1.24) | .69 | 1.06 (0.81–1.38) |
| BED (>70 vs ≤70 Gy) | .03 | 0.63 (0.41–0.95) | .01 | 0.71 (0.56–0.90) |
| Multivariate efull model | ||||
| Age (>60 vs ≤60 y) | .002 | 1.56 (1.18–2.08) | ||
| Race (white vs other) | .002 | 0.64 (0.48–0.85) | ||
| KPS (≥80 vs <80) | .15 | 0.71 (0.45–1.13) | .12 | 0.74 (0.50–1.08) |
| % Weight loss (<10% vs ≥10%) | .94 | 1.01 (0.78–1.30) | ||
| Concurrent chemotherapy (capecitabine vs others) | .17 | 0.72 (0.45–1.15) | .01 | 0.65 (0.47–0.91) |
| BED (>70 vs ≤70 Gy) | .02 | 0.59 (0.38–0.91) | .02 | 0.75 (0.59–0.94) |
| Multivariate ereduced model | ||||
| Age (>60 vs ≤60 y) | .003 | 1.52 (1.16–2.00) | ||
| Race (white vs other) | .002 | 0.64 (0.48–0.85) | ||
| Concurrent chemotherapy (capecitabine vs others) | .01 | 0.64 (0.46–0.89) | ||
| BED (>70 vs ≤70 Gy) | .03 | 0.63 (0.41–0.95) | .01 | 0.74 (0.58–0.94) |
Abbreviations: BED = biologically effective dose; CI = 95% confidence interval; KPS = Karnofsky performance status.
Fig. 2.
Kaplan- Meier plots. (a) overall survival, (b) recurrence-free survival, (c) local-regional recurrence-free survival, (d) time to local-regional recurrence, (e) distant recurrence-free survival, and (f) time to distant recurrence by biologically effective dose (BED).
Dose escalation was not associated with smaller tumor size, suggesting that BED is not a surrogate for smaller local disease burden. The median tumor size was 3.2 cm in the high-BED group versus 3.6 cm in the low-BED group (P = .16). There was no difference in terms of tumor location between patients who underwent dose escalation compared with those who did not (head, 53% vs 63%; P = .26), which suggests that individual anatomy and spatial orientation of luminal organs in relation to the tumor influenced the decision to escalate dose more than classic definition of tumor location. The concurrent chemotherapy regimen was similar in high-BED and low-BED patients (capecitabine-based chemotherapy, 79% vs 88%; P = .16). Two-thirds of all patients who received a high BED (31 of 47 patients) began their treatment in or after 2010 because this treatment paradigm was invoked more recently. In the same time period there were no major changes in quality of imaging, aggressiveness of surgery, quality of immobilization, or other such variables that might be surrogates for improvement in quality of care. The median time to initiate induction chemotherapy from diagnosis (20 vs 24 days, respectively; P = .16), duration of induction chemotherapy (4.1 vs 3 months, respectively; P = .06), and the duration of chemoradiation (38 vs 38 days, respectively; P = .69) were similar in high- and low-BED patients, which suggests that the inferior outcomes seen in low-dose patients were not due to delay in the initiation of treatment or incomplete treatments/treatment breaks in that subgroup. The median clinical and radiographic follow-up times were 9.8 and 9.1 months in the high-BED group versus 9.1 and 7.9 months in the low-BED group (P = .84 and .58, respectively). This suggests that shorter follow-up duration in the more recently treated high-BED patients may not explain the observed differences in OS. Last, the frequency of resections performed was comparable between the high-BED group (2 of 47, 4.3%) and the low-BED group (9 of 153, 5.9%), suggesting that the better outcomes were not due to more patients undergoing surgical resection.
Among patients receiving high BED, the extent of GTV coverage by the high dose prescription was variable according to attempts to boost the entire tumor, portions of the tumor further away from GI mucosa, and largely the retroperitoneal margin. The median GTV coverage in patients receiving higher BED was 96% (range, 30%–100%). Higher GTV coverage, dichotomized at 90%, 80%, and 70%, was not associated with improved OS. However, these numbers are not large enough to further stratify high dose patients into those with heterogeneity and those without heterogeneity, and thus very strong conclusions cannot be drawn.
Inverse probability of treatment-weighted analysis
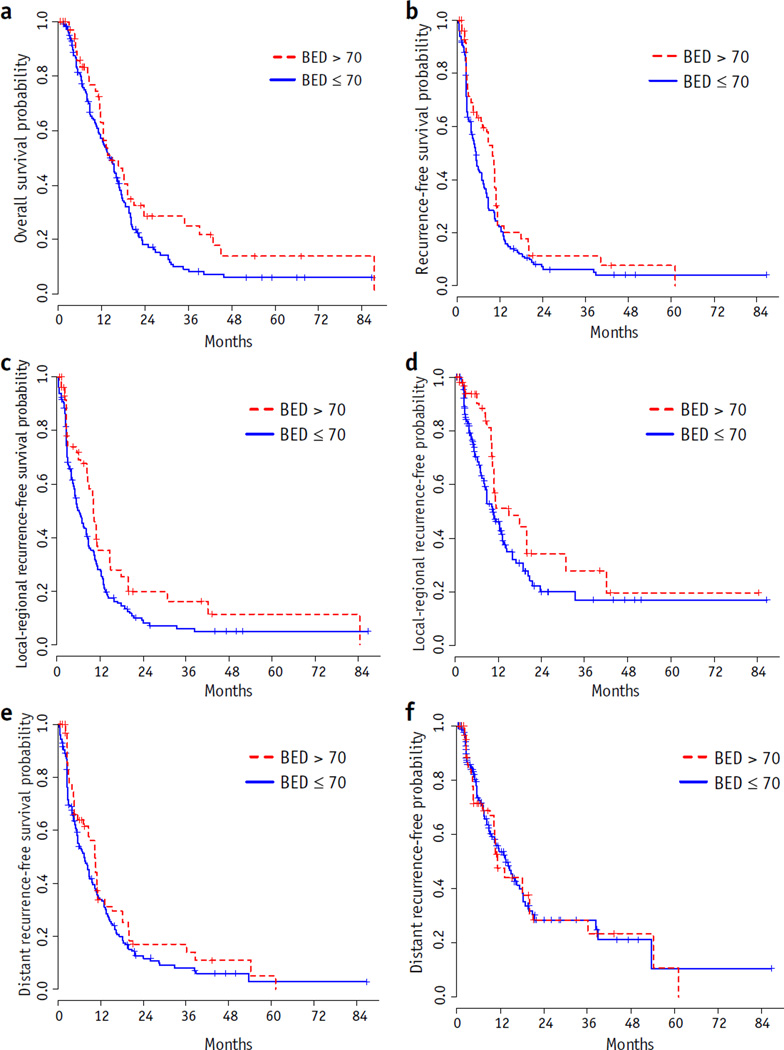
Potential predictors before and after inverse probability of treatment-weighted adjustment by propensity score were compared in Table 2. All absolute values of standardized differences were between 0.04 and 12.67, implying that these predictors across dose groups were comparable. After adjustment, the median survival is 14.4months (95%CI 12.9–16.5 months). The 1- and 2-year survival rates were 60% and 23%, respectively. Figure 3a shows the weighted Kaplan-Meier curves for OS by dose group. The median survival was 14.4 months (95% CI 12.5–18.2 months) for patients receiving BED >70 Gy and 14.4 months (95% CI 11.7–16.1 months) for patients receiving BED ≤70 Gy. Table 3 provides the results of Cox proportional hazard models of OS after adjustment. Race (P = .004) and BED (P = .01) were significantly associated with OS. In the multivariate analysis, age, race, choice of concurrent chemotherapy, and BED were associated with OS. Older and non-white patients with concurrent chemotherapy other than capecitabine and lower BED were at higher risk of death. Similarly, dose escalation was found to be significantly associated with recurrence-free survival (P = .005; Fig. 3b), local-regional recurrence-free survival (P<.001; Fig. 3c), time to local-regional recurrence-free survival (P = .002; Fig. 3d), and distant recurrence-free survival (P = .03; Fig. 3e), but no significant association was found between time to distant recurrence and BED (P = .90; Fig. 3f).
Fig. 3.
Weighted Kaplan-Meier plots. (a) Overall survival, (b) recurrence-free survival, (c) local-regional recurrence-free survival, (d) time to local-regional recurrence, (e) distant recurrence-free survival, and (f) time to distant recurrence by biologically effective dose (BED) (after adjustment).
Toxicity of dose escalation
Acute severe toxicity was uncommon and minor in patients treated with dose escalation. Grade 1 nausea, vomiting, diarrhea, or fatigue was seen in 37 patients (80%); grade 2 abdominal pain, diarrhea, anorexia, nausea, or fatigue in 13 patients (28%); and grade 3 diarrhea in 1 patient (2%). Four patients (13%) required transfusion for anemia, whereas 1 patient had an episode of GI bleed possibly related to treatment. This low rate of toxicity was not related to high-BED patients receiving localized radiation to the tumor while omitting elective regional nodal coverage, which is consistent with the shift in treatment guidelines away from elective irradiation of clinically uninvolved regional nodal basins. Our group had not treated regional nodal volume electively for almost the entire duration of this study period.
Discussion
Our data suggest that dose escalation is feasible and tolerable in a subset of patients with favorably located tumors (>1 cm from the closest luminal organ) where modern techniques allow conformal avoidance of dose-limiting organs. Higher dose (BED) was a strong independent predictor of improved OS in these patients. This did not seem to be due to tumors being smaller, follow-up being shorter, changes in therapeutic approach, or greater frequency of surgical resection in the high-BED group. The degree of conformality of SIB radiation dose to the GTV was not a predictor of better outcomes among patients receiving higher BED. However, given the limited patient numbers in these subset analyses, this would need to be independently validated in a larger cohort of patients. Nonetheless, a 3-fold increase in the 3-year OS rate is a significant advance in our ability to improve treatment outcomes for patients with LAPC. In many instances, it compares favorably with the outcomes achievable with surgical resection.
Early evidence of a potential benefit to dose escalation dates back to the 1970s and 1980s, with the Gastrointestinal Tumor Study Group (GITSG) protocol GITSG 9273 noting 1-year OS rates of 10%, 35%, and 46% in patients randomly assigned to split-course radiation therapy 60 Gy, bolus 5-fluorouracil (5-FU) with split-course radiation therapy 40 Gy, or bolus 5-FU with split-course radiation therapy 60 Gy, respectively (11) and a single-institution study demonstrating an improvement in OS in small unresectable pancreatic tumors receiving intraoperative radiation boost (12). More recently, dose escalation has been embraced again as a therapeutic paradigm for LAPC, largely as a consequence of greater adoption of IMRT, image guided radiation therapy, and stereotactic body radiation therapy (SBRT) in clinical practice. A phase 1/2 trial combining full-dose fixed-dose rate gemcitabine with escalating doses of radiation documented that the maximum tolerated dose was 55 Gy in 25 fractions, and the median OS was 15 months (13). This compared favorably with earlier attempts to escalate the dose per fraction during a 3-week course of 3D conformal radiation therapy in which the maximum tolerated dose was 36 Gy in 15 fractions at 2.4 Gy per fraction with weekly full-dose gemcitabine (14). Recent reports of fractionated, as opposed to single-fraction, SBRT suggest that OS durations of 15 months are achievable with SBRT (15). The choice of concurrent chemotherapy has an impact on the ability to escalate radiation dose for pancreatic cancer patients. Dose escalation is generally easier with concurrent capecitabine than gemcitabine; or cisplatin and 5-FU; or 5-FU and mitomycin C (16–18).
As with any retrospective analysis, there are important caveats to interpretation of and generalization of the results of our study. First, there is a possibility of selection bias because we only included patients who were referred to the radiation oncology department after induction chemotherapy. Thus patients who developed distant metastases and/or significant toxicity while on induction chemotherapy and were consequently not considered candidates for chemoradiation were excluded up front. In the patients selected by induction chemotherapy presenting to us, to the extent possible, the inclusion criteria were kept stringent and the potential confounders were evaluated. The effect of higher BED on overall outcomes was independent of these possible biases. However, that does not exclude the possibility that intangible factor(s) may have influenced the results. It does not seem biologically plausible, however, that one of these factors is tumor location (ie, merely greater spatial separation from GI mucosa can improve treatment outcomes for patients who were now eligible for dose escalation).
Second, the assessment of toxicity in a retrospective analysis inherently underestimates risks owing to incomplete reporting of side effects in clinic notes, recall bias, and lack of continuity of follow-up at 1 institution to capture all possible adverse events.
Third, there was a large range of dose fractionations used. This was a consequence of efforts to account for and mitigate against the risk of GI toxicity. Tumors closer to the stomach and duodenum were treated with more protracted courses of radiation therapy, and those further away were treated to higher total doses and at higher doses per fraction. On the basis of our previous analysis of GI toxicity with dose escalation and the current understanding of safety and tolerability of high BED, we anticipate that, going forward, some consistency with choice of fraction size and total dose can be formalized. The choice of 63 Gy in 28 fractions on the Radiation Therapy Oncology Group trial is an attempt to find such a compromise and consensus. Despite this, adaptations in dose and treatment may need to be made if treatment setup is not reproducible, for example owing to shifting of stomach/duodenum during daily treatments compared with simulation images or earlier on-treatment images.
Fourth, because all patients in the high-dose group received IMRT, it is possible that IMRT contributed to the better outcomes in these patients. It is hard to elucidate the contribution of IMRT to outcomes owing to the small number of patients in the low-dose group that received IMRT. Acknowledging this limitation, there did not seem to be a statistically significant difference in median OS (21.6 vs 19.2 months, P = .24) between the low-dose group patients receiving IMRT (n = 13) and those receiving 3D-CRT (n = 140).
Last, the impact of SMAD4 status on outcomes with localized treatment intensification could not be evaluated owing to the lack of adequate tissue from fine needle aspirates. A prospective study would more adequately address this question.
In summary, our data suggest that intensified chemoradiation may be beneficial in selected patients and support the prospective investigation of this approach (63 Gy in 28 fractions; BED of 77.175 Gy) in the ongoing Radiation Therapy Oncology Group/NRG Oncology 1201 phase 2 randomized trial for unresectable pancreatic cancer.
Summary.
Radiation dose escalation has not been explored extensively in the treatment of locally advanced pancreatic cancer, owing to proximity of the tumor to critical luminal mucosa. We demonstrate in a large retrospective analysis that newer radiation delivery techniques permit delivery of a simultaneous integrated boost to the tumor, and this translates to an improvement in local control and overall survival.
Acknowledgments
This work was supported in part by The MD Anderson Cancer Center support grant P30 CA16672 and the John E. and Dorothy J. Harris Endowed Professorship (to S.K.).
Conflict of interest: S.K. reports grants from the National Institutes of Health, US Department of Defense, MD Anderson Cancer Center, Focused Ultrasound Surgery Foundation, Shell Oil, Malaysian Palm Oil Board, Dunn Foundation, and Elekta, and royalties from Taylor and Francis, outside the submitted work; additionally, S.K. has a patent pending for radiosensitization with nanoparticles licensed to self (from MD Anderson Cancer Center).
Footnotes
Presented in part at the 2015 Gastrointestinal Cancers Symposium, January 15–17, 2015, San Francisco, CA.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Willett CG, Czito BG, Bendell JC, et al. Locally advanced pancreatic cancer. J Clin Oncol. 2005;23:4538–4544. doi: 10.1200/JCO.2005.23.911. [DOI] [PubMed] [Google Scholar]
- 3.Gutt R, Liauw SL, Weichselbaum RR. The role of radiotherapy in locally advanced pancreatic carcinoma. Nat Rev Gastroenterol Hepatol. 2010;7:437–447. doi: 10.1038/nrgastro.2010.98. [DOI] [PubMed] [Google Scholar]
- 4.Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: The importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. discussion 846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47–55. doi: 10.1002/cncr.22735. [DOI] [PubMed] [Google Scholar]
- 6.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammel P, Huguet F, Van Laethem JL, et al. Comparison of chemoradiotherapy (CRT) and chemotherapy (CT) in patients with a locally advanced pancreatic cancer (LAPC) controlled after 4 months of gemcitabine with or without erlotinib: Final results of the international phase III LAP 07 study. J Clin Oncol. 2013;31:LBA4003. [Google Scholar]
- 8.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: Results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 9.Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet. 2006;368:843–854. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 10.Nelson C, Starkschall G, Balter P, et al. Respiration-correlated treatment delivery using feedback-guided breath hold: A technical study. Med Phys. 2005;32:175–181. doi: 10.1118/1.1836332. [DOI] [PubMed] [Google Scholar]
- 11.Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: A randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Willett CG, Del Castillo CF, Shih HA, et al. Long-term results of intraoperative electron beam irradiation (IOERT) for patients with unresectable pancreatic cancer. Ann Surg. 2005;241:295–299. doi: 10.1097/01.sla.0000152016.40331.bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Josef E, Schipper M, Francis IR, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2012;84:1166–1171. doi: 10.1016/j.ijrobp.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGinn CJ, Zalupski MM, Shureiqi I, et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2001;19:4202–4208. doi: 10.1200/JCO.2001.19.22.4202. [DOI] [PubMed] [Google Scholar]
- 15.Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys. 2013;86:516–522. doi: 10.1016/j.ijrobp.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Cohen SJ, Dobelbower R, Jr, Lipsitz S, et al. A randomized phase III study of radiotherapy alone or with 5-fluorouracil and mitomycin-C in patients with locally advanced adenocarcinoma of the pancreas: Eastern Cooperative Oncology Group study E8282. Int J Radiat Oncol Biol Phys. 2005;62:1345–1350. doi: 10.1016/j.ijrobp.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 17.Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee S, Hurt CN, Bridgewater J, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): A multicentre, randomised, phase 2 trial. Lancet Oncol. 2013;14:317–326. doi: 10.1016/S1470-2045(13)70021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]