Abstract
BACKGROUND
In the absence of breast-feeding and its immunomodulatory factors, supplementation of starter infant formula (IF) with probiotics is currently used to support immune functions and gut development.
AIM
To assess whether immune-related beneficial effects of regular dose (107 CFU/g of powder) of the probiotic Bifidobacterium lactis CNCM I-3446 (hereafter named B. lactis) in starter IF supplementation can be maintained with starter IF containing a low dose (104 CFU/g of powder) of B. lactis.
METHOD
This trial was designed as a pilot, prospective, double-blind, randomized, single-center clinical trial of two parallel groups (n = 77 infants/group) of C-section delivered infants receiving a starter IF containing either low dose or regular dose of the probiotic B. lactis from birth to six months of age. In addition, a reference group of infants breast-fed for a minimum of four months (n = 44 infants), also born by C-section, were included. All groups were then provided follow-up formula without B. lactis up to 12 months of age. Occurrence of diarrhea, immune and gut maturation, responses to vaccinations, and growth were assessed from birth to 12 months. The effect of low-dose B. lactis formula was compared to regular-dose B. lactis formula, considered as reference for IF with probiotics, and both were further compared to breast-feeding as a physiological reference.
RESULTS
Data showed that feeding low-dose B. lactis IF provides similar effects as feeding regular-dose B. lactis IF or breast milk. No consistent statistical differences regarding early life protection against gastrointestinal infections, immune and gut maturation, microbiota establishment, and growth were observed between randomized formula-fed groups as well as with the breast-fed reference group.
CONCLUSION
This pilot study suggests that supplementing C-section born neonates with low-dose B. lactis-containing starter formula may impact immune as well as gut maturation similarly to regular-dose B. lactis, close to the breast-feeding reference.
Keywords: infant formula, c-section babies, probiotic dose, diarrhea, immune maturation, B. lactis, vaccine responses
Introduction
Neonates represent a particularly vulnerable population suscep tible to infections due to the immaturity of their immune system.1 At the time of birth, they move from an almost sterile environment within the maternal uterus into a world teeming with bacteria. Within the first days of life, mucosal surfaces of the host, including the gastrointestinal tract as well as the respiratory tract, become colonized with different bacterial communities,2,3 comprising a large spectrum of commensal and potentially pathogenic microorganisms. This complex environment contributes to the maturation of the immune system, which is able to later on fight against many potential life-threatening infections.4–6
In addition to microbial colonization, it has been demon strated that other postnatal factors, such as breast-feeding, are extremely important for the maturation of the immune system allowing its full functionality.7–9 Interestingly, even if compounds of breast milk (BM) such as antibodies, cytokines, and growth factors can directly act on the developing gut-associated lymphoid tissues,1 the impact of BM on immune maturation is also closely linked to its effects on the establishing microbiota. BM shapes the microbiota profile via a prebiotic effect of oligosaccharides or specific proteins that are able to favor beneficial gut colonization by lactobacilli and bifidobacteria.10,11 Indeed, recent findings have demonstrated that BM naturally contains small amounts of living bacteria that are transmitted to the infants.12,13 It is hypothesized that this postnatal natural bacterial inoculum is also a key for the programming of the neonatal immune system to establish oral tolerance and protection early in life.14
In the absence of breast-feeding, supplementation of infant formula (IF) with probiotics is one of the strategies commonly considered to improve early life immunity. Series of publications have shown that administration of the probiotic Bifidobacterium lactis CNCM I-3446 (hereafter named B. lactis) at regular average doses of 109 CFU per day to newborns is able to promote early life immune development and improve gastrointestinal health. Indeed, a three-week supplementation with B. lactis in breast-fed (BF) preterm infants, mostly born by C-section, was shown to increase fecal IgA and reduce calprotectin production.15 Moreover, the same intervention also modulated microbiota composition with an increase of bifidobacteria and a decrease of clostridia as well as enterobacteria.16 In another study, feeding of C-section delivered full-term infants with IF containing B. lactis enhanced responses to polio and rotavirus vaccines over the six-week intervention period.17 These effects of B. lactis feeding on immune and microbiota markers reflect a reinforcement of defenses that may lead to a beneficial impact on the outcomes of infection, such as lowering the risk of developing diarrhea.18,19
Considering the low amount of bacteria observed in human milk as described earlier, the present trial aims at exploring whether beneficial effects of B. lactis IF supplementation can be maintained in infants fed with IF containing a lowered dose of B. lactis, from regular dose 107 CFU/g of powder to low dose 104 CFU/g of powder.
In order to provide optimal exploratory conditions to address the objectives of the study, C-section delivered newborns have been selected. This target population was chosen for two main reasons: (i) C-section born babies present a defect in the development of immune defenses leading to increased susceptibility to infections in the first months of life20 and (ii) cesarean delivery induces an alteration in the early life microbiota composition, including a diminished and delayed bifidobacteria colonization in comparison to vaginally delivered babies.21,22 Thus, these newborns are expected to be more sensitive to nutritional intervention with different doses of bifidobacteria probiotics.
Methods
Clinical trial design
This trial was designed as a prospective, double-blind, randomized, single-center clinical trial of two parallel groups (low dose and regular dose of B. lactis). In addition, there was an observational reference group of BF infants followed from birth to 12 months. We considered the B. lactis regular-dose group as a reference for IF with probiotics in this study as compared to the exploratory low-dose IF group. Both groups were compared to the physiological BF reference group. This study was conducted by the team of Prof. C. Costalos in the Alexandra General Hospital, Athens, Greece, between June 2009 and March 2011. The trial was performed in accordance with the Declaration of Helsinki and compiled with good clinical practices as laid out in the International Conference on Harmonization guidelines. It was approved by the institutional ethics committees (the Board of Directors and the Scientific Council of the Alexandra General Hospital). Parents/legal guardians and investigators signed the informed consent.
All randomized infants received a starter IF (67 kcal/100 mL of reconstituted formula, 1.8 g of protein/100 kcal, developed at Nestlé Product Technology Center) which contains sufficient amounts of proteins, carbohydrates, fats, vitamins, and minerals for their normal growth from birth to six months. The study formulas contained either a low dose (3.7 ± 2.1 104 CFU/g of powder) or a regular dose (3.1 ± 1.4 107 CFU/g of powder) of probiotic B. lactis, depending on the allocated dose group, from birth to six months (Fig. 1, upper part). The two formulas were indistinguishable and were supplied in similar cans that were coded with letters and colors by the study sponsor (Nestlé). The B. lactis CFU counts were monitored in both products throughout the study. The IFs were provided to the parents during each study visit. Parents, investigators, support staff, and clinical project manager were blinded to the identity of the formulas. Then, from 6 to 12 months of age, these infants were given a follow-up formula without B. lactis (67 kcal/100 mL of reconstituted formula, 2.0 g of protein/100 kcal, developed at Nestlé Product Technology Center).
Figure 1.
Consort diagram for the two randomized and BF reference groups.
Note: *denotes analysis performed on this population.
For the BF reference group, breast-feeding was recommended for a minimum of four months. Those infants who stopped breast-feeding before four months received a starter formula without B. lactis. At weaning, the same follow-up formula without probiotics, as for randomized groups, was given up to 12 months.
Study population
The protocol was planned to recruit a total of 160 infants (80 per formulation group). Healthy full-term C-section delivered newborns, infants who had a birth weight between 2500 and 4500 g, infants whose mothers had anticipated not to breast-feed or decided to stop breast-feeding within 24 hours after delivery, and those infants with written informed consent obtained from his/her legal representative were enrolled within a maximum of 96 hours after birth.
Infants whose mothers intended to breast-feed from birth to at least four months were enrolled in a nonrandomized reference group.
Enrolled infants were vaccinated for diphtheria, Bordetella pertussis, polio, tetanus, and Heamophilus influenzae type B (HiB) (Pentavac, Sanofi Pasteur MSD, France) following the guidelines set by the Greek National Council for vaccinations of the Ministry of Health.
Infants were not enrolled in the study if they received a Rotarix® vaccine, were still BF beyond 24 hours (except for BF group), were expected to have problems with compliance, and were already participating in, or were from a mother currently participating or had participated in another clinical trial during the preceding three months prior to the inclusion in this study.
Measured outcomes
The primary outcome measure was prevalence of diarrhea, incidence of diarrhea, and total number of days with diarrhea over the study period (12 months). Diarrhea was defined as one day (24-hour period) with at least two to three watery stools. An episode of diarrhea was defined as at least one day of diarrhea followed by at least 48 hours without diarrhea.
Secondary outcomes were grouped as follows:
Immune maturation: fecal Immunoglobulin A (IgA) at one week, one month, and four months after birth.
Gut maturation: fecal calprotectin and 1-antitrypsin at one week, one month, and four months after birth, adjusted for the baseline value.
Microbiota: total counts of Bifidobacteria and the presence of B. lactis in feces at four months after birth.
Immune responses to vaccines: Antibody responses at 7 and 12 months after birth to diphtheria, B. pertussis, polio, tetanus, and HiB. In addition, for HiB, the percentage of protective response, which was estimated as the proportion of subjects who reached the protective level, ie, HiB >1 µg/mL,23 was also calculated.
Anthropometry: change in weight, length, BMI, and head circumference, during the first 4 months (1 week–4 months) and during the first year (1 week–12 months). Based on these data, z-scores were calculated for each subject and visit based on the EuroGrowth database.24–26
Serious and nonserious adverse events (System Organ Class), as well as concomitant medication, were collected through the 12-month follow-up period. Adverse events were defined as any untoward occurrence in a patient or clinical investigation subject administered an investigational product and which does not necessarily have to have a causal relationship with this treatment. Adverse events are illnesses, signs or symptoms occurring or worsening, and/or abnormal laboratory findings during the course of the study. Adverse events include occasions when the subjects contact the investigator or their private physician and are examined or given medical direction. They may or may not lead to the withdrawal of the subject from the study.
Laboratory methods
According to manufacturer’s instructions, dosages of fecal IgA (Quantitative Human IgA ELISA; ZeptoMetrix Corporation, ref. 0801197), fecal calprotectin (Calprotectin ELISA; Bühlmann Laboratories AG, ref. EK-CAL), fecal α1-antitrypsin (α1-Antitrypsin ELISA Kit; Immundiagnostik AG, ref. K6750), plasma IgG titers anti-diphtheria (diphtheria IgG ELISA; IBL International, ref. RE56191), anti-B. pertussis (B. pertussis IgG ELISA; IBL International, ref. RE56141), anti-polio (Poliomyelitis IgG ELISA Kit; HYCOR, ref. POL-01), anti-tetanus (tetanus IgG ELISA; IBL International, ref. RE56901), and anti-HiB (VaccZyme™ Human Anti-Haemophilus influenzae type b Enzyme Immunoassay Kit; The Binding Site Group Ltd., ref. MK016) were performed at the Harokopio University of Athens.
Total counts of bifidobacteria and the presence of B. lactis in feces were obtained from aliquots of ~1 g of stool transferred into a cryotube of 5 mL and frozen ideally at −80 °C after addition of 10% glycerol. Measurements were performed following the AAT internal protocol for B. lactis detection (Advanced Analytical Technologies Srl) that consisted in plating of the samples on Bifidobacterium spp. selective medium, counting CFU before scraping of plates surface and recovery of grown colonies, cells disruption of the plates triplicate by means of Maxwell protocol_AAT procedure. This later allowed assessment of the presence of the probiotic B. lactis by strain-specific polymerase chain reaction (detection limit 103 CFU/g feces). Specific primers used were (Sequence 5′–3′): sense GAGCTGATCGACGACCTGAC and anti-sense CCGAGAAAATCTGGGATGAG.
Statistics
Sample size
A total number of 160 infants (80 per randomized group) were planned to be recruited into the study. In addition, 30 infants were to be recruited in the BF reference group. The sample size was not determined by a formal power calculation given the exploratory nature of the study.
Randomization
Randomization was done by using an electronic program (TrialSys, developed by Nestlé) ensuring dynamic randomization via Internet with minimization technique.
Statistical methods
Primary outcome was analyzed in both the intention-to-treat (ITT) and per protocol populations, and secondary outcomes were analyzed in the ITT population. The ITT population consisted of all infants who were randomized and received any formula intake. The statistical significance level was set at 0.05, and no adjustment was applied due to the exploratory nature of the study. The effects of low dose versus regular dose of probiotic on diarrhea incidence, episode, and duration were analyzed using generalized linear Binomial model, Poisson model, and ANOVA, respectively. Outcomes of fecal IgA, gut maturation, vaccinations, and anthropometry parameters were compared between the two probiotic doses utilizing mixed models. Microbiota and morbidity data were analyzed using Fisher’s exact test. Only descriptive statistics (mean ± SD or 25th–75th percentile) were used to compare data from randomized groups versus those from physiological BF reference group (no P values were calculated, comparison was made on numerical trends).
Results
Disposition of subjects
In total, 208 infants were recruited in the study. One hundred sixty-four infants were randomly allocated to either the low-dose (n = 84 infants) or regular-dose (n = 80 infants) B. lactis starter formula groups. In both groups, 77 infants actually started consumption of study product (Fig. 1). All 44 infants recruited in the reference BF group started the study (Fig. 1).
Demographics and baseline data
Gender was equally distributed between the two randomized groups with just over 50% of males in each group (Table 1). In the BF group, 64% were males. At enrollment in the study (ie, randomization), the mean age was two days for the randomized groups with a range from zero to four days. The mean age at enrollment for the BF group was three days with a range from one to four days. All infants were in good health at birth with a median APGAR score ≥9, at 1, 5, and 10 minutes after birth. The median body weight at enrollment was the same for infants randomized in the low-dose and the regular-dose (2.9 kg) groups. The mean birth weight for the BF group (3.0 kg) was similar to the randomized group infants. The majority of randomized infants were not BF at all (87% and 74% for low- and regular-dose groups, respectively). Infants from the BF reference group were exclusively BF for an average of 5.3 ± 4.1 (SD) months.
Table 1.
Demographic data of the study population.
| RANDOMIZED INFANT FORMULA GROUPS | REFERENCE GROUP | ||
|---|---|---|---|
| LOW DOSE B. lactis | REGULAR DOSE B. lactis | BREAST MILK | |
| Gender [n (%)] | |||
| Male | 39 (50.6) | 39 (50.6) | 28 (63.6) |
| Female | 38 (49.4) | 38 (49.4) | 16 (36.4) |
| Age at enrolment (Visit 0) [days, median (n; 25th–75th percentile)] | |||
| 2 (77; 2–3) | 2 (77; 2–3) | 3 (44; 2–3) | |
| Body weight [kg, median (n; 25th–75th percentile)] | |||
| 2.9 (77; 2.7–3.3) | 2.9 (77; 2.7–3.2) | 3.0 (44; 2.8–3.3) | |
| Body length [cm, median (n; 25th–75th percentile)] | |||
| 50.0 (77; 49.0–51.0) | 50.0 (77; 48.0–51.0) | 51.0 (44; 49.4–52.0) | |
| Head circumference [cm, median (n; 25th–75th percentile)] | |||
| 34.0 (77; 33.2–34.5) | 34.0 (77; 33.5–35.0) | 35.0 (44; 34.0–35.5) | |
| Body mass index [kg/m2, median (n; 25th–75th pecentile)] | |||
| 11.8 (77; 11.3–12.8) | 11.7 (77; 11.1–12.5) | 11.8 (44; 11.3–12.5) | |
| Number of children living in the same household [number, median (n; 25th–75th pecentile)] | |||
| 1 (74; 0–1) | 1 (75; 0–1) | 1 (44; 1–1) | |
| Breastfeeding since birth and up to visit V0 [number (%; duration in days)] | |||
| No | 67 (87.0; 0) | 57 (74.0; 0) | 2 (4.5; 0) |
| Yes | 10 (13.0; 1) | 20 (26.0; 1) | 42 (95.5; 2) |
Primary outcome: diarrhea prevention
During the 12-month follow-up of infants, no statistically significant difference could be observed between the low and regular probiotic dose groups with respect to prevalence of diarrhea (20.8% vs. 23.4%, respectively, P = 0.70). Incidence (0.26 ± 0.57 episodes vs. 0.25 ± 0.46 episodes) or mean number of days with diarrhea per infant (0.72 ± 1.84 days vs. 1.17 ± 2.72 days) were also similar in both groups (P = 0.83 and P = 0.58, respectively; Table 2). No major difference could be seen as well between the randomized groups and the BF reference group regarding diarrhea status (prevalence: 18.2%; incidence: 0.30 ± 0.70 episodes; mean number of days with diarrhea: 1.09 ± 3.08 days).
Table 2.
Diarrhea incidence, total counts, and duration at one year (ITT population).
| DIARRHEA | RANDOMIZED INFANT FORMULA GROUPS | REFERENCE GROUP | P-VALUES | |
|---|---|---|---|---|
| LOW DOSE B. lactis (N = 77) | REGULAR DOSE B. lactis (N = 77) | BREAST MILK (N = 44) | LOW VS. REGULAR DOSE | |
| Prevalence during study period (12 months) [%] | 20.78 | 23.38 | 18.18 | 0.6977 |
| Incidence [total counts/infant/12 months, mean ± SD] | 0.26 ± 0.57 | 0.25 ± 0.46 | 0.30 ± 0.70 | 0.8279 |
| Number of days of diarrhea/infant [days, mean ± SD] | 0.72 ± 1.84 | 1.17 ± 2.72 | 1.09 ± 3.08 | 0.5811 |
Secondary outcomes
Immune and gut maturation
There were no statistically significant differences between the low-dose group and the regular-dose group with respect to fecal IgA, calprotectin, and α1-antitrypsin levels for any of the defined time points (one week, one month and four months after birth; Table 3). As expected, fecal IgA level was numerically higher in the BF reference group compared to IF groups. However, there was no difference between the randomized groups and the BF reference group for the two other markers.
Table 3.
Immune and gut maturation up to four months (ITT population).
| OUTCOME | RANDOMIZED INFANT FORMULA GROUPS | REFERENCE GROUP | P-VALUES | |
|---|---|---|---|---|
| LOW DOSE B. lactis | REGULAR DOSE B. lactis | BREAST MILK | LOW vs. REGULAR DOSE | |
| Immune maturation | ||||
| Fecal IgA [µg/ml, median (n; 25th–75th percentile)] | ||||
| 1 week after birth | 15.65 (76; 7.19–106.37) | 16.21 (77; 7.30–58.34) | 103.45 (43; 50.24–1390.21) | 0.9013 |
| 1 month after birth | 75.38 (74; 36.81–631.74) | 49.25 (75; 32.63–265.99) | 117.56 (40; 57.60–532.86) | 0.1342 |
| 4 montsh after birth | 61.15 (67; 35.17–478.78) | 57.93 (71; 36.68–108.72) | 105.01 (38; 46.17–385.36) | 0.4294 |
| Gut maturation (change fram baseline, Visit 0) | ||||
| Fecal calprotectin [µg/mL, median (n; 25th–75th percentile)] | ||||
| 1 week after birth | 51.22 (75; −32.69–95.61) | 33.53 (77; −66.69–79.00) | 36.87 (43; −42.54–89.11) | 0.2345 |
| 1 month after birth | 63.54 (74; −31.11–129.57) | 59.69 (75; −80.00–121.00) | 63.27 (40; −72.09–121.00) | 0.5223 |
| 4 montsh after birth | 38.83 (67; −51.98–107.54) | 33.30 (71; −86.47–116.33) | 11.81 (38; −112.24–120.11) | 0.4356 |
| Fecal α1-antitrypsin [mg/dL, median (n; 25th/75th percentile)] | ||||
| 1 week after birth | 2.98 (75; −1.67–4.98) | 3.17 (77; 2.01–6.00) | 2.32 (43; 0.66–7.00) | 0.2197 |
| 1 month after birth | 6.94 (73; 4.29–10.48) | 6.35 (75; 3.34–12.42) | 5.44 (40; 3.47–8.94) | 0.8962 |
| 4 montsh after birth | 9.89 (66; 4.59–14.59) | 10.98 (71; 6.34–15.42) | 8.77 (39; 4.12–15.51) | 0.1911 |
Microbiota – total bifidobacteria counts and B. lactis detection in feces
There was no statistically significant difference between the low- and regular-dose groups with respect to the total bifidobacteria counts in feces (median log CFU/g [with 25th/75th percentile] of 6.6 [5.8/7.5] and 6.7 [5.6/7.6], respectively, P = 0.78). There was also no substantial difference between the randomized groups and the BF group having a total bifidobacteria count of 7.1 (6.2/7.9).
Approximately 85% of infants randomized to the regular-dose group were colonized with B. lactis, ie, with positive detection of B. lactis in their feces, while only 47% of them were positive in the low-dose group. This difference was statistically significant (P < 0.0001). Noteworthy, a background of 16% of positive detection was observed in the BF reference group.
Immune responses to vaccinations
There were no statistically significant differences between the low-dose and regular-dose groups, as well as between the two randomized and BF groups, with respect to the response to diphtheria and B. pertussis at any of the specified time points (7 and 12 months after birth; Table 4).
Table 4.
Immune responses to vaccination at 7 and 12 months (ITT population).
| RANDOMIZED INFANT FORMULA GROUPS | REFERENCE GROUP | P-VALUES | ||
|---|---|---|---|---|
| LOW DOSE B. lactis | REGULAR DOSE B. lactis | BREAST MILK | LOW vs. REGULAR DOSE | |
| Response to Diphteria [lU/mL, median (n; 25th–75th percentile)] | ||||
| 7 months after birth | 1.98 (48; 1.15–2.35) | 1.92 (55; 1.21–2.30) | 1.80 (20; 1.41–2.32) | 0.9895 |
| 12 months after birth | 1.72 (41; 1.16–2.20) | 1.66 (47; 1.04–2.34) | 1.69 (18; 1.43–2.28) | 0.5359 |
| Response to Bordetella pertussis [lU/mL, median (n; 25th–75th percentile)] | ||||
| 7 months after birth | 21.16 (47; 6.39–89.40) | 44.34 (56; 13.92–132.75) | 12.24 (19; 8.67–84.15) | 0.1013 |
| 12 months after birth | 25.75 (42; 7.52–72.43 | 34.69 (48; 13.07–143.82) | 22.40 (18; 10.10–185.53) | 0.1847 |
| Response to Tetanus [lU/mL, median (n; 25th–75th percentile)] | ||||
| 7 months after birth | 2.62 (48; 1.48–4.30) | 3.13 (56; 2.42–3.84) | 3.05 (20; 1.45–3.90) | 0.3169 |
| 12 months after birth | 2.20 (42; 1.04–2.78) | 2.58 (48; 1.61–3.52) | 1.84 (17; 1.15–3.51) | 0.0411 |
| Response to Polio [U/mL, media (n; 25th–75th percentile)] | ||||
| 7 months after birth | 11.63 (48; 1.04–72.40) | 25.40 (56; 3.88–72.65) | 2.62 (20; 0.46–11.98) | 0.2719 |
| 12 months after birth | 12.71 (42; 4.34–47.84) | 10.11 (47; 2.91–45.41) | 4.22 (18; 1.01–13.46) | 0.4088 |
| Response to Heamophilus influenza B [µg/mL, median (n; 25th–75th percentile)] | ||||
| 7 months after birth | 2.23 (47; 0.22–4.48) | 2.20 (56; 0.70–4.49) | 0.73 (20; 0.37–2.12) | 0.2849 |
| 12 months after birth | 4.25 (42; 1.50–5.51) | 1.58 (48; 0.35–4.64) | 0.70 (18; 0.21–5.10) | 0.0186 |
| Infants who reached anti-HiB protective antibody over the 12 months | ||||
| Titers (>1 µg/mL) [% (n)] 79.6 (49) | 81 (58) | 59.1 (22) | 0.8515 | |
Note: Significant statistical differences (P < 0.05) are highlighted in bold.
Immune response to the tetanus vaccine was significantly higher in the regular-dose group compared to the low-dose group only at 12 months after birth, while no difference could be observed between the randomized groups and the BF group (Table 4).
Regarding response to polio vaccination, no statistically significant difference could be observed between the low- and regular-dose groups at any of the specified time points (Table 4). Absolute titer values of Ig response to polio vaccine in both IF groups appeared substantially higher than in BF infants.
Finally, immune response to the HiB vaccine appeared to be higher in the low-dose group than in the regular-dose group at 12 months after birth (Table 4). When compared to BF infants, immune response to HiB was found to be noticeably higher in the regular-dose group at 7 months after birth, but this difference was not seen at 12 months. In the low-dose group, this difference was higher only when the infants were 12 months old, as it was the case for response to polio vaccine. Besides, 79.6% and 81% of the infants in low-dose and regular-dose probiotic, respectively, reached the protective level of HiB antibodies (ie, anti-HiB >1 µg/mL23) at any time during the 12-month follow-up (P > 0.05; Table 4). Noteworthy, this protective level of antibodies against HiB was only reached by 59.1% of the BF infants.
Anthropometrics
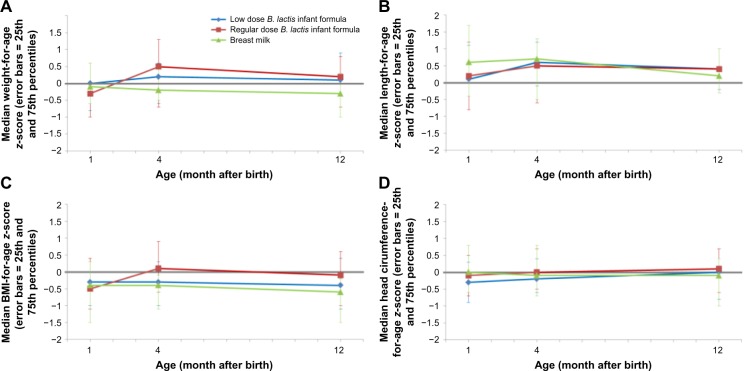
No consistent significant difference in growth parameters could be observed between the low- and regular-dose groups (Fig. 2). Compared with EuroGrowth database standards, infants in all groups grew normally throughout the study. Mean values for all growth measures through age four months were within 0.5 SD of the median value.27
Figure 2.
Weight-for-age (A), Length-for-age (B), BMI-for-age (C) and head circumference-for-age (D) z-scores (EuroGrowth database).
Serious adverse events
Serious adverse events reported throughout the study were <5% in all groups (3.9%, 1.3%, and 2.3% for low-dose, regular-dose, and BF groups, respectively). No statistically significant difference was observed between the low-dose and regular-dose groups, as well as between the two randomized and BF groups.
Discussion
We hypothesized that the beneficial effects on neonatal immune maturation might be achieved with a low dose of the probiotic B. lactis.
At this preliminary exploratory stage, we emphasized the comparison of low dose with regular dose, considered as reference for IF with probiotics, for which several earlier studies already support a functional effect on diarrhea and immune functions.15,17–19,28,29 Both formula groups were further compared to a BF physiological reference. In that regard, a group that was fed a formula without B. lactis was not considered in the study design. We recognize that this could represent a weakness in our study. However, we still believe that this approach will be useful to pave the way toward further research in this area.
The number of diarrhea episodes, as well as total number of days with diarrhea, per infant per year, were comparable in the three arms of the present study. The observed low incidence of ~0.25–0.3 reflects a discrepancy between data from observational studies (~2.8 in Western Europe)30 and the ones from interventional studies (0.2–0.5).19 Moreover, the fact that the present study population was not attending day care centers may also account for the low incidence of diarrhea. Nevertheless, bringing together the recognized evidence that breast-feeding protects against diarrhea31 and the previously documented beneficial effect of regular dose of B. lactis in reducing incidence/duration of diarrhea in infants,18,19,28,29 it may be postulated that low dose of B. lactis in starter IF may also provide benefit in such a population of neonates. Note that full assessment of noninferiority between both formulas would have required a sample size of 5421 infants per group as retrospectively calculated from the results of this pilot study with a statistical power of 80% and a noninferiority margin of 10%.
Regarding immune maturation, no difference could be observed in intestinal IgA production, measured as fecal IgA, between both randomized groups at any of the defined time points. As the effect of a regular dose of B. lactis in increasing fecal IgA production has been previously reported,15 one can hypothesize that low-dose B. lactis might be as efficient as regular dose in promoting neonatal gut immune maturation. The relatively poor gut microbial environment in C-section born babies may have offered a favorable niche for low amount of bacteria to exert their function.
Moreover, plausibility of the effect of low-dose B. lactis could be supported by the fact that, in reality, the small intestine microbiota is far less dense (103–107 CFU/g of intestinal content) and diverse when compared to the colon (1011–1012 CFU/g of intestinal content).32 As most of the gut-associated immune system is located in the small intestine, such scarce bacterial population is still sufficient to interact with the mucosa and trigger immune functions.33
Moreover, recent advances in human milk analysis and understanding of its property show that it contains living microorganisms, including bifidobacteria, in small amounts (102–104 CFU/mL).12,13 These bacteria and/or bacterial signatures likely contribute to postnatal immune education.14,34 These indications jointly support a rational for using a low amount of physiologically relevant bacterial inoculum with probiotics during the first weeks of life.
The absence of statistically significant difference between the three groups with respect to the total bifidobacteria counts further favors our initial hypothesis of a positive effect of low dose of B. lactis. Indeed, this observation can be brought together with the recognized bifidogenic effect of BM35 and the reported capacity of regular dose of B. lactis in IF to restore BM-like levels of bifidobacteria in the gut of infants.36 Interestingly, it was recently reported that relative abundance of commensal bifidobacteria and lactobacilli correlated with reduced risk of diarrhea, further suggesting that low dose of B. lactis may beneficially impact this latter outcome.37 Noteworthy, this similar effect can be observed despite a lower rate of infants positive for fecal B. lactis in the low-dose group compared with the rate of the regular-dose group, which was here comparable to previous studies with regular dose. This lower rate reflects the difference in B. lactis feeding load between both groups that may lead B. lactis fecal levels below the detection limit.
Protection against infections early in life may be related to multifactorial parameters, such as quality of the microbiota, as already mentioned, and/or normal immune maturation. Response to vaccination is also currently accepted by expert panels (ILSI,38 EFSA39) as a valuable marker reflecting the evolution of the immune system responsiveness to foreign antigens. Demonstration of the efficacy of regular dose of B. lactis supplementing IF on vaccine responses in a C-section population has been recently reported in a placebo-controlled study.17 Fecal anti-rotavirus-specific and anti-poliovirus-specific IgA levels postvaccination were both definitely increased in the B. lactis-supplemented group in comparison to IF without B. lactis. In the present study, no consistent differences could be observed between the three groups regarding antibody responses to the five different vaccines. Interestingly, when scarce statistical significant differences could be observed between the randomized groups and BF reference group, they were always in favor of a better vaccine response in the formula groups. In the particular case of HiB vaccine, this later observation can lead to clinical relevance as a protective antibody level threshold has been defined by the WHO (>1 µg/mL).23 Indeed, the percentage of infants who reached anti- HiB protective antibody titers was substantially higher in infants fed with either IF containing regular or low doses B. lactis in comparison to those in the BF physiological reference groups (81.0% or 79.6% versus 51.9% respectively).
Finally, besides the already discussed defense-related outcomes of the host, we also investigated parameters addressing more physiological read-outs, such as gut maturation (fecal calprotectin and α1-antitrypsin) and growth (anthropometric z-scores). Both B. lactis-supplemented IF and BF reference groups behaved similarly. No safety concern is to be mentioned here.
In conclusion, the present study suggests that effects observed with regular-dose B. lactis-containing starter formula on diarrhea outcomes and immune responsiveness could be reached by feeding C-section born infants early in life with IF containing a lower dose of B. lactis.
Acknowledgments
The authors wish to thank Shahram Emady-Azar for statistical analysis and Sara Lamberti for data management. The authors are also grateful to Lionel Philippe and Delphine Egli for clinical study management and to Nanda de Groot for her expertise and manuscript review.
Footnotes
ACADEMIC EDITOR: Praveen Kumar, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1623 words, excluding any confidential comments to the academic editor.
FUNDING: This research was funded by Nestlé Nutrition.
COMPETING INTERESTS: LB, SG, KS, SZ and CC disclose personal fees received from Nestlé related to this study. LF, SP and JB are employees of Nestec Ltd.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: SP, JB, CC. Analysed the data: LF, SP, JB, CC. Wrote the first draft of the manuscript: LF, SP. Contributed to the writing of the manuscript: LF, SP, JB, CC. Agree with manuscript results and conclusions: LB, SG, KS, SZ, LF, SP, JB, CC. Jointly developed the structure and arguments for the paper: LF, SP, JB. Made critical revisions and approved final version: LF, SP, JB, CC. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.M’Rabet L, Vos AP, Boehm G, et al. Breast-feeding and its role in early development of the immune system in infants: consequences for health later in life. J Nutr. 2008;138:1782S–90S. doi: 10.1093/jn/138.9.1782S. [DOI] [PubMed] [Google Scholar]
- 2.Favier CF, Vaughan EE, De Vos WM, et al. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol. 2002;68:219–26. doi: 10.1128/AEM.68.1.219-226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jost T, Lacroix C, Braegger CP, et al. New insights in gut microbiota establishment in healthy breast fed neonates. PLoS One. 2012;7:e44595. doi: 10.1371/journal.pone.0044595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collado MC, Cernada M, Bauerl C, et al. Microbial ecology and hostmicrobiota interactions during early life stages. Gut Microbes. 2012;3:352–65. doi: 10.4161/gmic.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan JL, Shi HN, Walker WA. The role of microbes in developmental immunologic programming. Pediatr Res. 2011;69:465–72. doi: 10.1203/PDR.0b013e318217638a. [DOI] [PubMed] [Google Scholar]
- 6.Tourneur E, Chassin C. Neonatal immune adaptation of the gut and its role during infections. Clin Dev Immunol. 2013;2013:270301. doi: 10.1155/2013/270301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calder PC, Krauss-Etschmann S, de Jong EC, et al. Early nutrition and immunity – progress and perspectives. Br J Nutr. 2006;96:774–90. [PubMed] [Google Scholar]
- 8.Jacobi SK, Odle J. Nutritional factors influencing intestinal health of the neonate. Adv Nutr. 2012;3:687–96. doi: 10.3945/an.112.002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer AC. Nutritionally mediated programming of the developing immune system. Adv Nutr. 2011;2:377–95. doi: 10.3945/an.111.000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012;22:1147–62. doi: 10.1093/glycob/cws074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liepke C, Adermann K, Raida M, et al. Human milk provides peptides highly stimulating the growth of bifidobacteria. Eur J Biochem. 2002;269:712–8. doi: 10.1046/j.0014-2956.2001.02712.x. [DOI] [PubMed] [Google Scholar]
- 12.Jeurink PV, van BJ, Jimenez E, et al. Human milk: a source of more life than we imagine. Benef Microbes. 2013;4:17–30. doi: 10.3920/BM2012.0040. [DOI] [PubMed] [Google Scholar]
- 13.Jost T, Lacroix C, Braegger C, et al. Assessment of bacterial diversity in breast milk using culture-dependent and culture-independent approaches. Br J Nutr. 2013;110:1253–62. doi: 10.1017/S0007114513000597. [DOI] [PubMed] [Google Scholar]
- 14.Perez PF, Dore J, Leclerc M, et al. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics. 2007;119:e724–32. doi: 10.1542/peds.2006-1649. [DOI] [PubMed] [Google Scholar]
- 15.Mohan R, Koebnick C, Schildt J, et al. Effects of Bifidobacterium lactis Bb12 supplementation on body weight, fecal pH, acetate, lactate, calprotectin, and IgA in preterm infants. Pediatr Res. 2008;64:418–22. doi: 10.1203/PDR.0b013e318181b7fa. [DOI] [PubMed] [Google Scholar]
- 16.Mohan R, Koebnick C, Schildt J, et al. Effects of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm infants: a double-blind, placebo-controlled, randomized study. J Clin Microbiol. 2006;44:4025–31. doi: 10.1128/JCM.00767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holscher HD, Czerkies LA, Cekola P, et al. Bifidobacterium lactis Bb12 enhances intestinal antibody response in formula-fed infants: a randomized, double-blind, controlled trial. JPEN J Parenter Enteral Nutr. 2012;36:106S–17S. doi: 10.1177/0148607111430817. [DOI] [PubMed] [Google Scholar]
- 18.Chouraqui JP, Van Egroo LD, Fichot MC. Acidified milk formula supplemented with Bifidobacterium lactis: impact on infant diarrhea in residential care settings. J Pediatr Gastroenterol Nutr. 2004;38:288–92. doi: 10.1097/00005176-200403000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics. 2005;115:5–9. doi: 10.1542/peds.2004-1815. [DOI] [PubMed] [Google Scholar]
- 20.Chang JH, Hsu CY, Lo JC, et al. Comparative analysis of neonatal morbidity for vaginal and caesarean section deliveries using hospital charge. Acta Paediatr. 2006;95:1561–6. doi: 10.1080/08035250600711066. [DOI] [PubMed] [Google Scholar]
- 21.Hakansson S, Kallen K. Caesarean section increases the risk of hospital care in childhood for asthma and gastroenteritis. Clin Exp Allergy. 2003;33:757–64. doi: 10.1046/j.1365-2222.2003.01667.x. [DOI] [PubMed] [Google Scholar]
- 22.Laubereau B, Filipiak-Pittroff B, von BA, et al. Caesarean section and gastrointestinal symptoms, atopic dermatitis, and sensitisation during the first year of life. Arch Dis Child. 2004;89:993–7. doi: 10.1136/adc.2003.043265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kayhty H, Peltola H, Karanko V, et al. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983;147:1100. doi: 10.1093/infdis/147.6.1100. [DOI] [PubMed] [Google Scholar]
- 24.Haschke F, van’t Hof MA. Euro-Growth references for length, weight, and body circumferences. Euro-Growth Study Group. J Pediatr Gastroenterol Nutr. 2000;31(suppl 1):S14–38. doi: 10.1097/00005176-200007001-00003. [DOI] [PubMed] [Google Scholar]
- 25.van’t Hof MA, Haschke F. Euro-Growth references for body mass index and weight for length. Euro-Growth Study Group. J Pediatr Gastroenterol Nutr. 2000;31(suppl 1):S48–59. doi: 10.1097/00005176-200007001-00005. [DOI] [PubMed] [Google Scholar]
- 26.van’t Hof MA, Haschke F, Darvay S. Euro-Growth references on increments in length, weight, and head and arm circumferences during the first 3 years of life. Euro-Growth Study Group. J Pediatr Gastroenterol Nutr. 2000;31(suppl 1):S39–47. doi: 10.1097/00005176-200007001-00004. [DOI] [PubMed] [Google Scholar]
- 27.Grathwohl D, Macé K, Fichot MC, et al. 1216 Growth of infants fed with NAN is in good agreement with the WHO growth standard: a meta-analysis. Pediatr Res. 2010;68:602. [Google Scholar]
- 28.Correa NB, Peret Filho LA, Penna FJ, et al. A randomized formula controlled trial of Bifidobacterium lactis and Streptococcus thermophilus for prevention of antibiotic-associated diarrhea in infants. J Clin Gastroenterol. 2005;39:385–9. doi: 10.1097/01.mcg.0000159217.47419.5b. [DOI] [PubMed] [Google Scholar]
- 29.Saavedra JM, Bauman NA, Oung I, et al. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet. 1994;344:1046–9. doi: 10.1016/s0140-6736(94)91708-6. [DOI] [PubMed] [Google Scholar]
- 30.Walker CL, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–16. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamberti LM, Fischer Walker CL, Noiman A, et al. Breastfeeding and the risk for diarrhea morbidity and mortality. BMC Public Health. 2011;11(suppl 3):S15. doi: 10.1186/1471-2458-11-S3-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sekirov I, Russell SL, Antunes LC, et al. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 33.El AS, van den Bogert B, Kleerebezem M. The small intestine microbiota, nutritional modulation and relevance for health. Curr Opin Biotechnol. 2014;32C:14–20. doi: 10.1016/j.copbio.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Martin R, Langa S, Reviriego C, et al. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr. 2003;143:754–8. doi: 10.1016/j.jpeds.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 35.Coppa GV, Zampini L, Galeazzi T, et al. Prebiotics in human milk: a review. Dig Liver Dis. 2006;38(suppl 2):S291–4. doi: 10.1016/S1590-8658(07)60013-9. [DOI] [PubMed] [Google Scholar]
- 36.Langhendries JP, Detry J, Van HJ, et al. Effect of a fermented infant formula containing viable bifidobacteria on the fecal flora composition and pH of healthy full-term infants. J Pediatr Gastroenterol Nutr. 1995;21:177–81. doi: 10.1097/00005176-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Solano-Aguilar G, Fernandez KP, Ets H, et al. Characterization of fecal micro-biota of children with diarrhea in 2 locations in Colombia. J Pediatr Gastroenterol Nutr. 2013;56:503–11. doi: 10.1097/MPG.0b013e318282aa12. [DOI] [PubMed] [Google Scholar]
- 38.Albers R, Bourdet-Sicard R, Braun D, et al. Monitoring immune modulation by nutrition in the general population: identifying and substantiating effects on human health. Br J Nutr. 2013;110(suppl 2):S1–30. doi: 10.1017/S0007114513001505. [DOI] [PubMed] [Google Scholar]
- 39.EFSA on Dietetic Products Nutrition and Allergies (NDA); guidance on the scientific requirements for health claims related to gut and immune function. EFSA J. 2011;9(4) [Google Scholar]