Abstract
Hemoglobin (Hb) is a heme-containing protein found in the red blood cells of vertebrates. For many years, the only known Hb-like molecule in plants was leghemoglobin (Lb). The discovery that other Hb-like proteins existed in plants led to the term “nonsymbiotic Hbs (nsHbs)” to differentiate them from the Lbs. While this terminology was adequate in the early stages of research on the protein, the complexity of the research in this area necessitates a change in the definition of these proteins to delineate them from red blood cell Hb. At the 2014 XVIII Conference on Oxygen-Binding and Sensing Proteins, the group devoted to the study of heme-containing proteins, this issue was discussed and a consensus was reached on a proposed name change. We propose Phytoglobin (Phytogb) as a logical, descriptive name to describe a heme-containing (Hb-like) protein found in plants. It will be readily recognized by the research community without a prolonged explanation of the origin of the term. The classification system that has been established can essentially remain unchanged substituting Phytogb in place of nsHb. Here, we present a guide to the new nomenclature, with reference to the existing terminology and a phylogenetic scheme, placing the known Phytogbs in the new nomenclature.
Keywords: Algae, angiosperms, bryophytes, gymnosperms, legumes, nonsymbiotic, truncated
Hemoglobin (Hb) is a heme-containing protein found in the red blood cells of vertebrates 1. Hemoglobin-like proteins are also found in other tissues of vertebrates where they are given tissue-specific names that help to identify their locations and distinguish them from red blood cell Hb 2, 3. For many years, the only known Hb-like molecule in plants was leghemoglobin (Lb), a protein induced as a result of the symbiotic relationship between legume plants and nitrogen-fixing bacteria 4. The discovery that other Hb-like proteins existed in plants not capable of symbiotic relationships led to the term “nonsymbiotic Hbs (nsHbs)” to differentiate them from the Lbs 5. While this terminology was adequate in the early stages of research on the protein, the complexity of the research in this area necessitates a change in the definition of these proteins to delineate them from red blood cell Hb, in keeping with the terminology for other Hb-like proteins, such as myoglobin in muscle, neuroglobin in neuron tissue and cytoglobin in vertebrate cell cytoplasm 2, 3. In 2001 Hunt et al. 6 classified plant Hbs as globin (GLB)0, GLB1, GLB2, GLb3 and GLBS corresponding to undetermined (mostly liverwort and moss) nsHbs, angiosperm nsHbs class/type 1 and nsHbs class/type 2, truncated Hbs and symbiotic Hbs (which included Lbs), respectively. However, an epithet for plant Hbs was absent in this nomenclature and distinctive characteristics for each category were not fully defined resulting in an incomplete classification system.
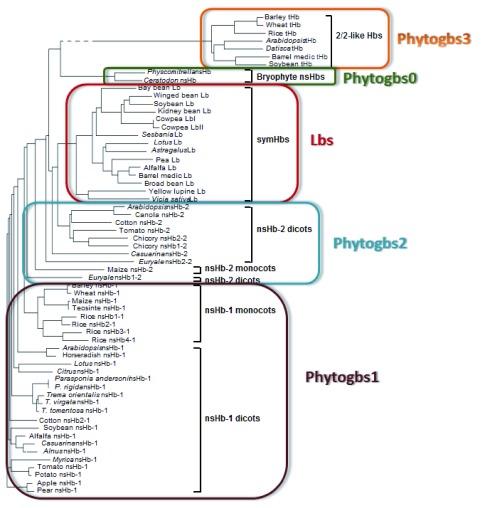
At the 2014 XVIII Conference on Oxygen-Binding and Sensing Proteins, the group devoted to the study of heme-containing proteins, the above issue was discussed and a consensus was reached on a proposed name change. Phytoglobin ( phyto, plant; globin, heme-containing protein folding structurally similar to the sperm whale myoglobin structure whose heme-Fe is invariably coordinated at the proximal site by His F8), abbreviated as Phytogb, was proposed as a logical, descriptive name to describe a heme-containing (Hb-like) protein found in plants. It will be readily recognized by the research community without a prolonged explanation of the origin of the term, as is the case for ‘nonsymbiotic hemoglobin’. The classification system that has been established can essentially remain unchanged substituting Phytogb in place of nsHb. A guide to the new nomenclature, with reference to the existing terminology, is given in Table 1. A more detailed phylogenetic scheme, placing the known Phytogbs in the new nomenclature, is shown in Figure 1. Also, we propose that acronym for the species-specific Phytogbs corresponds to the first three binomial (i.e. genus and species) letters followed by the Phytogb type and phytogb number of copy. For example, the acronym for rice ( Oryza sativa) Phytogb1.1 (see Table 1) corresponds to OrysatPhytogb1.1.
Figure 1. Phylogenetic representation of the novel nomenclature for land plant Phytogbs.
Note that Parasponia, Casuarina, Alnus and Myrica SymPhytogbs are intermediate between SymPhytogbs and Phytogbs1 and Phytogbs2 (see Table 1 for explanation). Figure modified from Garrocho-Villegas et al. 23 (reprinted with permission).
Table 1. System and characteristics of the accepted nomenclature for plant (algae + land plants) Phytoglobins (Phytogb).
| Former plant globin
name and abbreviation (in parenthesis) |
New
nomenclature a |
Plant origin | Distinctive characteristics b |
|---|---|---|---|
| Nonsymbiotic hemoglobin
(nsHb) |
Phytogb0 | Algae
c+bryophytes+
gymnosperms |
Heme-Fe either penta- or hexacoordinate.
Moderate to high affinity for O 2. Localized in any plant organ. |
| Class/type 1
nonsymbiotic hemoglobin (nsHb-1) |
Phytogb1 | Angiosperms | Heme-Fe predominantly hexacoordinated by a distal
amino acid. Extremely high affinity for O 2 mostly due to a very low O 2-dissociation rate constant ( k off). Localized in any plant organ. |
| Class/type 2
nonsymbiotic hemoglobin (nsHb-2) |
Phytogb2 | Angiosperms | Heme-Fe predominantly pentacoordinated.
Moderate to high affinity for O 2. Localized in any plant organ. |
| Symbiotic hemoglobin
(symHb) |
SymPhytogb | Non-legume N
2-fixing
plants d |
Heme-Fe predominantly pentacoordinated.
Moderate to high affinity for O 2. Specifically localized in N 2-fixing nodules of actinorhizal plants or any other non-legume land plant |
| Leghemoglobin
(Lb) |
Lb | N 2-fixing legumes d | Heme-Fe predominantly pentacoordinate.
Moderate to high affinity for O 2. Specifically localized in legume N 2-fixing nodules. |
| Class/type 3
nonsymbiotic hemoglobin/ Truncated hemoglobin (tHb) |
Phytogb3 | Algae c+land plants | Globin-domain amino acid sequence and structure
(i.e. folding into the 2/2-fold) similar to those of bacterial tHbs. Heme-Fe either penta- or hexacoordinate. Moderate to high affinity for O 2. Localized in any plant organ. |
aNumerical classification corresponds to that previously proposed by Hunt et al. 6. Proteins coded by multiple phytogb gene copy numbers within the same plant species should be indicated as the number of copy after the Phytogb numerical classification. For example, rice ( Oryza sativa) Phytogbs 1 and 2 (corresponding to the former nsHbs-1) should be indicated as rice Phytogb1.1 and Phytogb1.2, respectively (see text for a description on the species-specific Phytogbs acronym).
bHeme-Fe coordination and affinity for O 2 correspond to those from moss Phytogb0 7– 9, barley 10, rice 11 and Arabidopsis 12 Phytogb1, Arabidopsis Phytogb2 12, Casuarina SymPhytogb 13, soybean Lb 14, 15 and Arabidopsis Phytogb3 16 representative of Phytogb0, Phytogb1, Phytogb2, SymPhytogb, Lb and Phytogb3, respectively.
cAmino acid sequence of algal globins analyzed so far 17– 19 is similar to that of land plant Phytogb0 and Phytogb3, hence algal globins can be classified as Phytogb0 or Phytogb3, respectively.
dSome SymPhytogbs and Lbs (such as the Parasponia 20 and Casuarina 21 and Chamaecrista 22 globins, respectively) are intermediate between Phytogbs1 and Phytogbs2 and SymPhytogbs and Lbs 22, 23 because they exhibit amino acid sequence similarity to Phytogbs1 and Phytogbs2 ( Figure 1) and are localized in non-legume an legume nodules and apparently play a role in symbiotic N 2-fixation.
Acknowledgements
Authors wish to express their gratitude to Itzel Rojas Sánchez, Gisselle A. Fuentes and Gustavo Rodríguez-Alonso for evaluating the Phytoglobins nomenclature presented here and providing useful comments.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Dickerson RE, Geis I: Hemoglobin: structure, function, evolution, and pathology. Menlo Park, California: The Benjamin/Cummings Pub. Co., Inc.;1983;176 Reference Source [Google Scholar]
- 2. Vinogradov SN, Hoogewijs D, Bailly X, et al. : A phylogenomic profile of globins. BMC Evol Biol. 2006;6:31. 10.1186/1471-2148-6-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weber RE, Vinogradov SN: Nonvertebrate hemoglobins: functions and molecular adaptations. Physiol Rev. 2001;81(2):569–628. [DOI] [PubMed] [Google Scholar]
- 4. Appleby CA: Leghemoglobin and Rhizobium respiration. Annu Rev Plant Physiol. 1984;35:443–478. 10.1146/annurev.pp.35.060184.002303 [DOI] [Google Scholar]
- 5. Bogusz D, Appleby CA, Landsmann J, et al. : Functioning haemoglobin genes in non-nodulating plants. Nature. 1988;331(6152):178–180. 10.1038/331178a0 [DOI] [PubMed] [Google Scholar]
- 6. Hunt PW, Watts RA, Trevaskis B, et al. : Expression and evolution of functionally distinct haemoglobin genes in plants. Plant Mol Biol. 2001;47(5):677–692. 10.1023/A:1012440926982 [DOI] [PubMed] [Google Scholar]
- 7. Garrocho-Villegas V, Arredondo-Peter R: Molecular cloning and characterization of a moss ( Ceratodon purpureus) non-symbiotic hemoglobin provides insight into the early evolution of plant non-symbiotic hemoglobins. Mol Biol Evol. 2008;25(7):1482–1487. 10.1093/molbev/msn096 [DOI] [PubMed] [Google Scholar]
- 8. Smagghe BJ, Kundu S, Hoy JA, et al. : Role of phenylalanine B10 in plant non-symbiotic hemoglobins. Biochemistry. 2006;45(32):9735–9745. 10.1021/bi060716s [DOI] [PubMed] [Google Scholar]
- 9. Vázquez-Limón C, Castro-Bustos S, Arredondo-Peter R: Spectroscopic analysis of moss ( Ceratodon purpureus and Physcomitrella patens) recombinant non-symbiotic hemoglobins. Commun Integr Biol. 2012;5(6):527–530. 10.4161/cib.21473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duff SM, Wittenberg JB, Hill RD: Expression, purification, and properties of recombinant barley ( Hordeum sp.) hemoglobin. Optical spectra and reactions with gaseous ligands. J Biol Chem. 1997;272(27):16746–16752. 10.1074/jbc.272.27.16746 [DOI] [PubMed] [Google Scholar]
- 11. Arredondo-Peter R, Hargrove MS, Sarath G, et al. : Rice hemoglobins. Gene cloning, analysis, and O2-binding kinetics of a recombinant protein synthesized in Escherichia coli. Plant Physiol. 1997;115(3):1259–1266. 10.1104/pp.115.3.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trevaskis B, Watts RA, Andersson SR, et al. : Two hemoglobin genes in Arabidopsis thaliana: the evolutionary origins of leghemoglobins. Proc Natl Acad Sci USA. 1997;94(22):12230–12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fleming AI, Wittenberg JB, Wittenberg BA, et al. : The purification, characterization and ligand-binding kinetics of hemoglobins from root nodules of the non-leguminous Casuarina glauca- Frankia symbiosis. Biochim Biophys Acta. 1987;911(2):209–220. 10.1016/0167-4838(87)90010-0 [DOI] [Google Scholar]
- 14. Hargrove MS, Barry JK, Brucker EA, et al. : Characterization of recombinant soybean leghemoglobin a and apolar distal histidine mutants. J Mol Biol. 1997;266(5):1032–1042. 10.1006/jmbi.1996.0833 [DOI] [PubMed] [Google Scholar]
- 15. Wittenberg JB, Appleby CA, Wittenberg BA: The kinetics of the reactions of leghemoglobin with oxygen and carbon monoxide. J Biol Chem. 1972;247(2):527–31. [PubMed] [Google Scholar]
- 16. Watts RA, Hunt PW, Hvitved AN, et al. : A hemoglobin from plants homologous to truncated hemoglobins of microorganisms. Proc Natl Acad Sci USA. 2001;98(18):10119–10124. 10.1073/pnas.191349198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernández I, Vinogradov SN, Arredondo-Peter R: Identification and in silico characterization of a putative ancestor to land plant non-symbiotic hemoglobins from the prasinophyceae algae Micromonas and Ostreococcus. Global J Biochem. 2010;1:18–30. Reference Source [Google Scholar]
- 18. Vinogradov SN, Fernández I, Hoogewijs D, et al. : Phylogenetic relationships of 3/3 and 2/2 hemoglobins in Archaeplastida genomes to bacterial and other eukaryote hemoglobins. Mol Plant. 2011;4(1):42–58. 10.1093/mp/ssq040 [DOI] [PubMed] [Google Scholar]
- 19. Vinogradov SN, Hoogewijs D, Arredondo-Peter R: What are the origins and phylogeny of plant hemoglobins? Commun Integr Biol. 2011;4(4):443–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Appleby CA, Tjepkema JD, Trinick MJ: Hemoglobin in a nonleguminous plant, Parasponia: possible genetic origin and function in nitrogen fixation. Science. 1983;220(4600):951–953. 10.1126/science.220.4600.951 [DOI] [PubMed] [Google Scholar]
- 21. Jacobsen-Lyon K, Jensen EO, Jørgensen JE, et al. : Symbiotic and non-symbiotic hemoglobin genes of Casuarina glauca. Plant Cell. 1995;7(2):213–223. 10.1105/tpc.7.2.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gopalasubramaniam SK, Kovacs F, Violante-Mota F, et al. : Cloning and characterization of a caesalpinoid ( Chamaecrista fasciculata) hemoglobin: the structural transition from a nonsymbiotic hemoglobin to a leghemoglobin. Proteins. 2008;72(1):252–260. 10.1002/prot.21917 [DOI] [PubMed] [Google Scholar]
- 23. Garrocho-Villegas V, Gopalasubramaniam SK, Arredondo-Peter R: Plant hemoglobins: what we know six decades after their discovery. Gene. 2007;398(1–2):78–85. 10.1016/j.gene.2007.01.035 [DOI] [PubMed] [Google Scholar]