Abstract
OBJECTIVES: To study whether linguistic analysis and changes in information leaflets can improve readability and understanding. DESIGN: Randomised, controlled study. Two information leaflets concerned with trials of drugs for conditions/diseases which are commonly known were modified, and the original was tested against the revised version. SETTING: Denmark. PARTICIPANTS: 235 persons in the relevant age groups. MAIN MEASURES: Readability and understanding of contents. RESULTS: Both readability and understanding of contents was improved: readability with regard to both information leaflets and understanding with regard to one of the leaflets. CONCLUSION: The results show that both readability and understanding can be improved by increased attention to the linguistic features of the information.
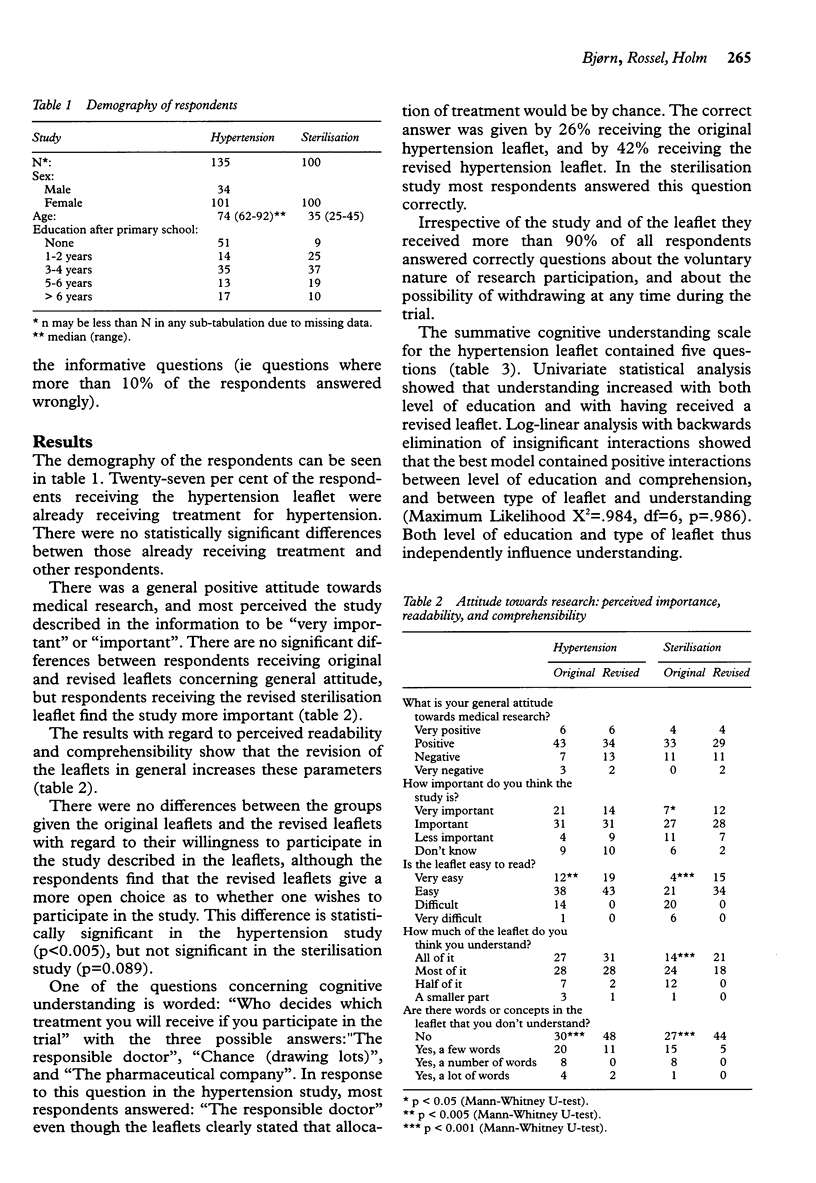
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur V. A. Written patient information: a review of the literature. J Adv Nurs. 1995 Jun;21(6):1081–1086. doi: 10.1046/j.1365-2648.1995.21061081.x. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt D. E., Keane M. A. Institutional Review Board (IRB) review lacks impact on the readability of consent forms for research. Am J Med Sci. 1992 Dec;304(6):348–351. doi: 10.1097/00000441-199212000-00003. [DOI] [PubMed] [Google Scholar]
- Handelsman M. M., Martin W. L., Jr Effects of readability on the impact and recall of written informed consent material. Prof Psychol Res Pr. 1992 Dec;23(6):500–503. doi: 10.1037/0735-7028.23.6.500. [DOI] [PubMed] [Google Scholar]
- Hochhauser Mark. Some overlooked aspects of consent form readability. IRB. 1997 Sep-Oct;19(5):5–9. [PubMed] [Google Scholar]
- Holm S. Skriftlig patientinformation. En analyse af danske biomedicinske forsøgsplaner. Ugeskr Laeger. 1992 Aug 31;154(36):2432–2435. [PubMed] [Google Scholar]
- Hopper K. D., Lambe H. A., Shirk S. J. Readability of informed consent forms for use with iodinated contrast media. Radiology. 1993 Apr;187(1):279–283. doi: 10.1148/radiology.187.1.8451429. [DOI] [PubMed] [Google Scholar]
- Mackillop W. J., Johnston P. A. Ethical problems in clinical research: the need for empirical studies of the clinical trials process. J Chronic Dis. 1986;39(3):177–188. doi: 10.1016/0021-9681(86)90022-6. [DOI] [PubMed] [Google Scholar]
- Mumford M. E. A descriptive study of the readability of patient information leaflets designed by nurses. J Adv Nurs. 1997 Nov;26(5):985–991. doi: 10.1046/j.1365-2648.1997.00455.x. [DOI] [PubMed] [Google Scholar]
- Priestley K. A., Campbell C., Valentine C. B., Denison D. M., Buller N. P. Are patient consent forms for research protocols easy to read? BMJ. 1992 Nov 21;305(6864):1263–1264. doi: 10.1136/bmj.305.6864.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera R., Reed J. S., Menius D. Evaluating the readability of informed consent forms used in contraceptive clinical trials. Int J Gynaecol Obstet. 1992 Jul;38(3):227–230. doi: 10.1016/0020-7292(82)90133-3. [DOI] [PubMed] [Google Scholar]
- Stanley B., Sieber J. E., Melton G. B. Empirical studies of ethical issues in research. A research agenda. Am Psychol. 1987 Jul;42(7):735–741. doi: 10.1037//0003-066x.42.7.735. [DOI] [PubMed] [Google Scholar]
- Tarnowski K. J., Allen D. M., Mayhall C., Kelly P. A. Readability of pediatric biomedical research informed consent forms. Pediatrics. 1990 Jan;85(1):58–62. [PubMed] [Google Scholar]
- Wager E., Tooley P. J., Emanuel M. B., Wood S. F. How to do it. Get patients' consent to enter clinical trials. BMJ. 1995 Sep 16;311(7007):734–737. doi: 10.1136/bmj.311.7007.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C. J., Zwitter M. Informed consent in European multicentre randomised clinical trials--are patients really informed? Eur J Cancer. 1994;30A(7):907–910. doi: 10.1016/0959-8049(94)90111-2. [DOI] [PubMed] [Google Scholar]



