Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease in which cells and tissues undergo damage mediated by tissue-binding autoantibodies. At its onset, it may involve one organ alone or more than one organ simultaneously; over a time, additional manifestations due to the involvement of other organs may occur. Our observations have confirmed that hematological manifestations are the commonest initial presentation in SLE. The criteria used till 2012 was the American College of Rheumatology (ACR) criteria, which is only a classification criterion and not really for diagnosis; if we rely on ACR criteria, the diagnosis is often delayed. Time required for satisfying all four of the eleven criteria is variable and prolonged. Moreover, hematological manifestations are underrepresented in the ACR criteria. Based on the clinical observations made on patients evaluated in a tertiary center in North Kerala, an alternate diagnostic criterion named the Kozhikode criteria was proposed, especially for the diagnosis of SLE when it presents with hematological manifestations alone. The present study was an attempt to validate the same and to look for any association of diet and lifestyle with the disease.
Keywords: SLE, Kozhikode criteria, lifestyle, diet habits, hematological disease
Video abstract
Introduction
Systemic lupus erythematosus (SLE) is a chronic recurrent multisystem disorder, which is difficult to diagnose.1,2 There is no single diagnostic marker. It is diagnosed with the help of a set of clinical and laboratory criteria.3 Accurate diagnosis of this disease is important because early diagnosis and appropriate treatment reduces morbidity and mortality.4–12 SLE is considered to be a rheumatologic disorder, the logic of which is questionable too. Blood and blood vessels contain more variety of antigens than that of other organs, and therefore, naturally, the clinical manifestations should be more often hematological.13,14
The criteria used till 2012 for the diagnosis of SLE were the American College of Rheumatology (ACR) criteria. It is useful only in classifying the disease as SLE because four of the eleven components have to be satisfied. Most cases do not satisfy all these at presentation, and if one depends on ACR criteria for diagnosis, it takes several years to confirm the disease as SLE, and hence, prompt diagnosis and treatment is delayed in almost all the cases. Another pitfall of the ACR criteria is that it does not give adequate weightage for hematological manifestations, in spite of the fact that hematological manifestations are the commonest; it is not represented adequately, leading to missing the diagnosis of SLE.
Based on the observations made in our tertiary care center in North Kerala, it was found that hematological manifestations are the commonest, and rheumatologic manifestations are rather late at least in our set of population.13,14 Most of the cases presented to our institution were diagnostic problems rather than fully established cases, and early diagnosis could be made only with high index of suspicion. Based on these observations, we proposed the need for a practical guideline to diagnose SLE and framed a new criterion named as the Kozhikode criteria.13,14
Kozhikode criteria
Major/essential criteria
The major/essential criteria are as follows: 1) Presence of an unresolved autoimmune disorder, which is known to occur with SLE (chronic immune thrombocytopenic purpura [ITP], autoimmune hemolytic anemia, autoimmune hypothyroidism, and autoimmune hepatitis).13,14 2) No other causes identified other than autoimmunity for the said clinical problem by clinical reasoning and investigations if required.
Minor criteria
The minor criteria are as follows: 1) Another coexisting autoimmune disorder/any other evidence of autoimmunity. 2) Positive antinuclear antibody (ANA). 3) Positive antidouble-stranded DNA. 4) Sustained and definitive response to steroid and immuno suppressant even after 6 months of follow-up.
If the patient has two essential and two or more minor criteria, they can be diagnosed as SLE.
Inclusion criteria
All diagnosed cases of SLE during the period of January 2013 to December 2013 were included. The cases included newly diagnosed patients and cases already under follow-up from the Department of Medicine, Rheumatology and Dermatology at the Government Medical College, Kozhikode, Calicut. Clinical diagnosis of SLE was considered in those presenting with unsettled clinical problems and conditions that were autoimmune in nature, such as chronic ITP, hypothyroidism, autoimmune hemolytic anemia, vitiligo, and alopecia with clinical and laboratory evidence of autoimmunity.
Materials and methods
The study was an observational one with a prospective study design. Role of lifestyle and diet was assessed by a case–control study. The study was approved by the institutional ethics committee of the Govt Medical College, Kozhikode. Written informed consent was obtained from each participant in the study.
The ACR criteria and the Kozhikode criteria were applied to all the diagnosed cases of SLE. All the newly diagnosed cases that were diagnosed as SLE using the Kozhikode criteria were also subjected to the ACR criteria. They were reviewed after a period of 6 months, and the ACR criteria was applied again to see whether they satisfied it and thereby to prove whether the Kozhikode criteria helps in early diagnosis of SLE.
In addition, those who were already under follow-up were studied by reviewing their clinical features and treatment details and whether or not they satisfied the ACR criteria at the beginning and by calculating the average time required for them to satisfy the ACR criteria.
For assessing the influence of diet and lifestyle, healthy bystanders of patients in General Medicine wards were taken who were matched to cases on age and sex. One control per case was recruited. They were normal by physical examination and had normal routine laboratory investigations and were not on any kind of medications.
Data collection was by personalized interview, including presenting complaints, demographic information, reproductive history, environmental exposure, and medications. Dietary information of patients was estimated using the semi-quantitative food frequency questionnaire derived from the Integrated Disease Surveillance Project Non-Communicable Disease Survey questionnaire for risk assessment. There were six possible responses, regarding the food frequency. The data was entered using Microsoft Excel and was analyzed using SPSS software (ver 18.0, Norman H Nic, Chicago, Illinois, USA).
Results
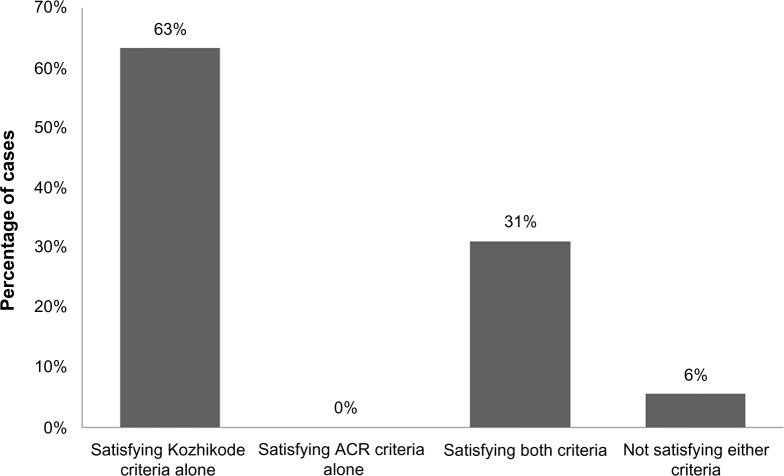
There were 71 patients diagnosed as SLE. Of them, 30 were new cases and 41 were previously diagnosed as SLE and were under follow-up. The male to female ratio was 1:9. Majority of females were in the age-group of 20–40 years, male subjects were equally distributed in all the age-groups. Of the 71 patients, 45 satisfied the Kozhikode criteria alone, 22 satisfied both ACR and the Kozhikode criteria, and four did not satisfy either criterion. Altogether 67 (94%) patients satisfied the Kozhikode criteria at the beginning of the study (Figure 1).
Figure 1.
Distribution of cases based on whether or not they satisfied the criteria.
Abbreviation: ACR, American College of Rheumatology.
There was no such group of patients who satisfied the ACR criteria alone but not satisfying the Kozhikode criteria, validating the new criterion. Both these observations are highlighting the utility of the new criterion. Of the 30 new cases, 26 individuals satisfied the Kozhikode criteria, whereas only six were satisfying the ACR criteria. The 26 newly diagnosed cases, which satisfied the Kozhikode criteria alone, were followed up for a period of 6 months, and it was observed that only two of them satisfied the ACR criteria even after 6 months. Among the 30 new cases, there were four individuals with suspected SLE who did not satisfy either of the criteria.
Of the four cases that did not satisfy either of the criteria, two of them satisfied the Kozhikode criteria at the end of 6 months. One of them had refractory oral ulcer and became ANA positive and responded well to steroids. The other patient was a case of alopecia and ITP, which responded to steroids and later became ANA positive. None of the four satisfied the ACR criteria even at the end of 6 months. Of the two patients who did not satisfy either of the criteria, one patient had refractory oral ulcers and the other patient was lost for follow-up.
Among the patients who were already under follow-up, it was found that 16 of them who all had satisfied the Kozhikode criteria in the initial diagnosis itself, had satisfied the ACR criteria only during variable periods of follow-up (Table 1).
Table 1.
Average time required to satisfy the ACR criteria among the cases
| Serial number | Diagnosis | Average time from diagnosis to satisfying ACR criteria (months) |
|---|---|---|
| 1 | ITP | 32 |
| 2 | ITP | 72 |
| 3 | ITP, hypothyroidism | 40 |
| 4 | AIHA | 7 |
| 5 | AIHA, nephrotic syndrome | 64 |
| 6 | Polyarthralgia | 4 |
| 7 | APLA, high ESR | 14 |
| 8 | CVA, ITP, high ESR | 144 |
| 9 | Anemia, splenomegaly | 6 |
| 10 | Secondary Sjögren’s syndrome | 4 |
| 11 | Thyrotoxicosis | 11 |
| 12 | Polyarthralgia | 8 |
| 13 | Polyarthralgia | 4 |
| 14 | Anemia, high ESR | 12 |
| 15 | Pancytopenia | 12 |
| 16 | MDS | 60 |
Abbreviations: ACR, American College of Rheumatology; ITP, immune thrombocytopenic purpura; AIHA, autoimmune hemolytic anemia; APLA, antiphospholipid antibody syndrome; ESR, erythrocyte sedimentation rate; CVA, cerebrovascular accident; MDS, myelodysplastic syndrome.
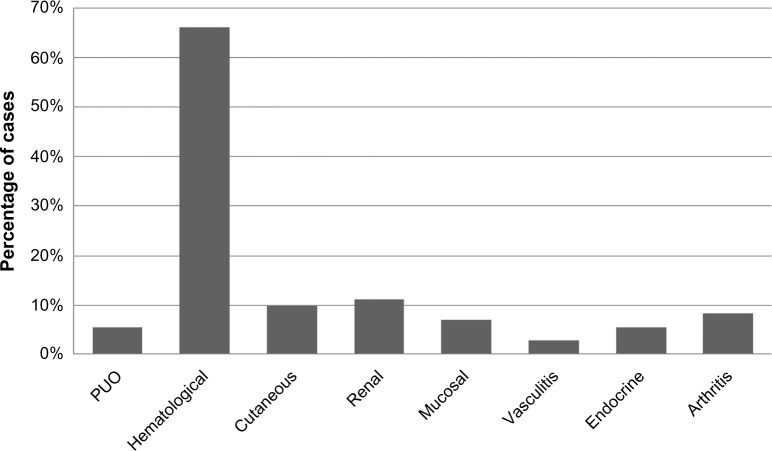
The most common clinical manifestation was hematological, and it was unusual to see any joint symptoms in this subset. Arthritis was seen in combination with other symptoms but not alone as the initial manifestation. Fever was an unusual presentation seen in our set of population (Figure 2).
Figure 2.
Clinical manifestations in patients with SLE.
Abbreviations: SLE, systemic lupus erythematosus; PUO, pyrexia of unknown origin.
Among the new cases, only 12 were ANA positive at the time of initial clinical presentation. They were diagnosed and treated as SLE due to strong index of suspicion. Those who were ANA positive were also positive for antidouble-stranded DNA. Of the ANA-negative patients among the new cases, only two became ANA positive at 6-month follow-up.
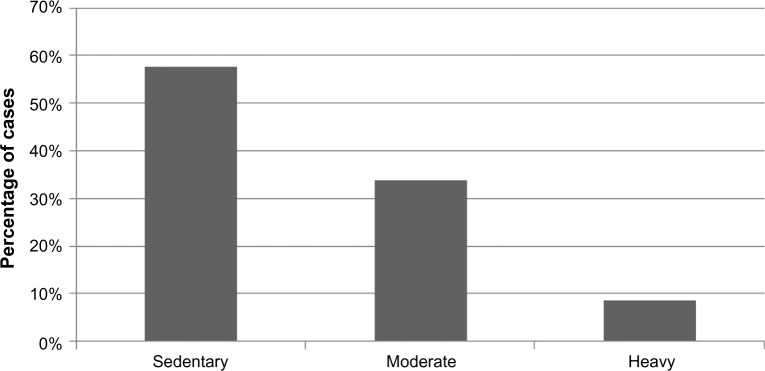
Majority of the patients belonged to moderate socioeconomic status. Among those diagnosed as SLE, most of them had a sedentary lifestyle (P<0.05) (Figure 3).
Figure 3.
Level of physical activity in the patients with SLE.
Abbreviation: SLE, systemic lupus erythematosus.
Of the 64 females, consistent use of cosmetics or nail polishes was not seen in any individuals. The patients with cutaneous or oral manifestations also did not use any cosmetics. Comparison of diet among cases and controls showed statistically significant decrease in intake of pulses, green leafy vegetables, legumes, and fruits (P=0.000). The intake of junk food and canned food was significantly higher among cases than controls (P=0.000).
Discussion
The study was undertaken to validate the Kozhikode criteria as an alternative to the ACR criteria, since for the last 3 decades, we had been observing that majority of the patients were presenting with hematological manifestations and most of them did not satisfy the existing criterion at the time of presentation. The Kozhikode criteria was developed for this reason, and we were diagnosing and managing SLE based on this criterion and wanted to share this information with others who are interested in SLE. This study was performed to validate the new criterion.
The observations of the study have shown the utility of the Kozhikode criteria for early diagnosis of SLE, and the observations mentioned are supporting the superiority of the Kozhikode criteria over the ACR criteria for early diagnosis of SLE. The study has thus validated the Kozhikode criteria as a useful tool for early diagnosis of SLE. In this study and in our earlier study, hematological manifestations were found to be the most common initial presentation of SLE.14 Hematological manifestations were the presenting complaints in 61% of individuals. This observation made in our tertiary center in Kerala was contradictory to the description of the disease in most Indian and Western text books15,16 and majority of the previously conducted studies.17,18 However, a multicentric French study on the initial presentation of childhood lupus showed that the most common presentation was hematological.19 The Indian study supporting this observation was reported from our own institution.14
We had observed that several of these patients were ANA negative at the time of diagnosis and they become ANA positive during follow-up only. All these patients were clinically diagnosed as SLE or evolving SLE, and all of them had satisfied the Kozhikode criteria and not the ACR criteria, again highlighting the usefulness of Kozhikode criteria for early diagnosis.
In 1982, Mchardy et al,20 investigating a cohort of SLE patients in Aberdeenshire, Scotland, demonstrated a 8.9% ANA-negative SLE.20 Gladman et al and Ferreiro et al in two separate studies found a prevalence of 5% cases of SLE, which were ANA negative at the time of diagnosis.21,22 All these cases could have been diagnosed as SLE, had we applied the Kozhikode criteria.
None of the patients were doing any kind of exercise on a regular basis. The level of daily physical activity of newly diagnosed cases when compared with controls showed statistically significant decrease in physical activity among cases. Although there were studies establishing the role of lack of regular exercise and sedentary lifestyle increasing the mortality in SLE,23,24 there were no studies relating physical activity as etiological factor in SLE.
The comparison between diets of cases and controls showed statistically significant difference showing poor intake of pulses, green leafy vegetables, and fruits. The intake of junk food, fried food, meat, and canned food was high among cases than controls, and the P-values were statistically significant.
The study also observed the following pitfalls of the ACR criteria. Hematological manifestations are underestimated and not given adequate representation in the ACR criteria. The dietary, lifestyle, and the ethnic factors may be responsible for more hematological manifestations of SLE in our population, and ACR criteria, which were validated on the western population, cannot be applied to our population. SLE can be diagnosed by applying ACR criteria only when more than one organ is involved, and it will usually take years for patients to satisfy four of the eleven criteria, and this results in undue delay in diagnosis and initiation of treatment. A comparison of the ACR criteria and the Kozhikode criteria clearly shows the advantages of the latter (Table 2).
Table 2.
Comparison of ACR criteria and Kozhikode criteria
| ACR criteria | Kozhikode criteria |
|---|---|
| Classification criteria only. Cannot be used for diagnosing SLE. | Diagnostic criteria. |
| Requires at least four of the eleven criteria, most of which are not present at the time of initial presentation and could be present at any time in a patient’s history – delays diagnosis. | Requires four of the six and are easily available features occurring early on, as the diagnosis of SLE is based on clinical suspicion. Useful criterion promotes early diagnosis. |
| Underrepresentation of hematological manifestations, which is more common in at least our subset of patients. | Adequate representation of hematological manifestations. |
| Presence of antinuclear antibodies is given equal weightage as other criteria. | Antinuclear antibody is only a minor criterion and helps in identification of ANA-negative SLE. |
Abbreviations: ANA, antinuclear antibody; ACR, American College of Rheumatology; SLE, systemic lupus erythematosus.
The study has highlighted the inadequacy of the ACR criteria in diagnosing SLE. If these patients were categorically diagnosed as SLE, they could have received definitive therapy. The observations prove that early diagnosis is not possible with the ACR criteria but is possible with the Kozhikode criteria.
Acknowledgments
PK Sasidharan is presently working in PVS Hospital, Kozhikode, as a consultant in Internal Medicine and Haematology. N Arathi was a junior resident in the Department of Medicine at the time of the study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Edworthy SM. Clinical manifestations of systemic lupus erythematosus. In: Ruddy S, Harris ED, Sledge CB, Kelley WN, editors. Kelley’s Textbook of Rheumatology. 6th ed. Philadelphia, PA: Saunders; 2001. pp. 1105–1119. [Google Scholar]
- 2.Guidelines for referral and management of systemic lupus erythematosus in adults American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Arthritis Rheum. 1999;42:1785–1796. doi: 10.1002/1529-0131(199909)42:9<1785::AID-ANR1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Petri M. Treatment of systemic lupus erythematosus: an update. Am Fam Physician. 1998;57:2753–2760. [PubMed] [Google Scholar]
- 4.Meinao IM, Sato EI, Andrade LE, Ferraz MB, Atra E. Controlled trial with chloroquine diphosphate in systemic lupus erythematosus. Lupus. 1996;5:237–241. doi: 10.1177/096120339600500313. [DOI] [PubMed] [Google Scholar]
- 5.Molina JF, McGrath H., Jr Longterm ultraviolet-A1 irradiation therapy in systemic lupus erythematosus. J Rheumatol. 1997;24:1072–1074. [PubMed] [Google Scholar]
- 6.McGrath H, Martinez-Osuna P, Lee FA. Ultraviolet-A1 (340–400 nm) irradiation therapy in systemic lupus erythematosus. Lupus. 1996;5:269–274. doi: 10.1177/096120339600500405. [DOI] [PubMed] [Google Scholar]
- 7.Dammacco F, Della Casa Alberighi O, Ferraccioli G, Racanelli V, Casatta L, Bartoli E. Cyclosporine-A plus steroids versus steroids alone in the 12-month treatment of systemic lupus erythematosus. Int J Clin Lab Res. 2000;30:67–73. doi: 10.1007/s005990070017. [DOI] [PubMed] [Google Scholar]
- 8.Carneiro JR, Sato EI. Double blind, randomized, placebo controlled clinical trial of methotrexate in systemic lupus erythematosus. J Rheumatol. 1999;26:1275–1279. [PubMed] [Google Scholar]
- 9.Alvarez-Nemegyei J, Cobarrubias-Cobos A, Escalante-Triay F, Sosa-Munoz J, Miranda JM, Jara LJ. Bromocriptine in systemic lupus erythematosus: a double-blind, randomized, placebo-controlled study. Lupus. 1998;7:414–419. doi: 10.1191/096120398678920334. [DOI] [PubMed] [Google Scholar]
- 10.Pollak VE, Pirani CL, Kark RM. Effect of large doses of prednisone on the renal lesions of and life span of patients with lupus glomerulonephritis. J Lab Clin Med. 1961;57:495–511. [PubMed] [Google Scholar]
- 11.Bansal VK, Beto JA. Treatment of lupus nephritis: a meta-analysis of clinical trials. Am J Kidney Dis. 1997;29:193–199. doi: 10.1016/s0272-6386(97)90029-9. [DOI] [PubMed] [Google Scholar]
- 12.Bellomio V, Spindler A, Lucero E, et al. Systemic lupus erythematosus: mortality and survival in Argentina. A multicenter study. Lupus. 2000;9:377–381. [PubMed] [Google Scholar]
- 13.Sasidharan PK. SLE as a hematological problem. In: Agarwal MB, editor. Hematology Today. Mumbai: Vikas Publications; 2010. pp. 953–966. [Google Scholar]
- 14.Sasidharan PK, Bindya M, Sajeeth Kumar KG. Hematological manifestations of SLE at initial presentation: is it underestimated? ISRN Hematol. 2012;2012:961872. doi: 10.5402/2012/961872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn BH. Systemic lupus erythematosus. In: Fauci AS, Braunwald E, Kasper DL, et al., editors. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: Mc-Graw Hill; 2008. pp. 207–2083. [Google Scholar]
- 16.Wallace DJ, Hahn BH. Dubois’ Lupus Erythematosus. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 17.Feldt V. Systemic lupus erythematosus: recognizing its various presentations. J Postgrad Med. 1995;97(4):79, 83, 86. [PubMed] [Google Scholar]
- 18.Villamin CA, Navara SV. Clinical manifestations and clinical syndromes of Filipino patients with systemic lupus erythematosus. Mod Rheumatol. 2008;18(2):161–164. doi: 10.1007/s10165-008-0029-0. [DOI] [PubMed] [Google Scholar]
- 19.Bader-Meunier B, Armengaud JB, Haddad E, et al. Initial presentation of childhood onset systemic lupus erythematosus: a French multicentre study. J Pediatr. 2005;146(5):648–653. doi: 10.1016/j.jpeds.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 20.Mchardy KC, Horne CH, Rennie J. Antinuclear antibody-negative systemic lupus erythematosus – how common? J Clin Pathol. 1982;35:1118–1121. doi: 10.1136/jcp.35.10.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gladman DD, Chalmers A, Urowitz MB. Systemic lupus erythematosus with negative LE cells and antinuclear factor. J Rheumatol. 1978;5:14. [PubMed] [Google Scholar]
- 22.Ferreiro JE, Reiter WM, Saldana MJ. Systemic lupus erythematosus presenting as chronic serositis with no demonstrable antinuclear antibodies. Am J Med. 1984;76:1100–1105. doi: 10.1016/0002-9343(84)90865-9. [DOI] [PubMed] [Google Scholar]
- 23.Kathleen J, Berkebile C, Hastings C, Yocum AD. Health status and disease activity in SLE. Arthritis Rheum. 1989;2(2):65–69. doi: 10.1002/anr.1790020208. [DOI] [PubMed] [Google Scholar]
- 24.Minami Y, Sazaki T, Arai Y, Kurisu Y, Hisamichi S. Diet and systemic lupus erythematosus: a 4 year prospective study of Japanese patients. J Rheumatol. 2003;30(4):747–754. [PubMed] [Google Scholar]