Abstract
This study was conducted to isolate endophytic bacteria possessing anti-infective property from Kalmegh (Andrographis paniculata Nees.), a well-known medicinal plant. A total of 23 strains were isolated from this plant among which the strain KL1, isolated from surface-sterilized leaf of this medicinal herb, showed broad-spectrum antagonism against an array of Gram-positive and -negative bacterial pathogens. Ethyl acetate extract of KL1-fermented media yielded a greenish amorphous substance retaining anti-infective property. Solvent-extracted crude material was separated by thin-layer chromatography, and the active ingredient was located by autobiogram analysis. The purified anti-infective compound was found as anthracene derivative as analyzed by ultraviolet and Fourier transform infrared spectroscopy. The strain was identified as Bacillus thuringiensis KL1 from cultural, physiochemical, and molecular aspects. The above results indicate the pharmaceutical potential of the candidate isolate.
Keywords: Andrographis paniculata, endophyte, Bacillus thuringiensis, antimicrobial, anthracene, pharmaceutical
Introduction
From the very beginning, pharmaceutical research is significantly dependent on traditional health-care practices with medicinal plants. Application of plants for medicinal purposes in India has been recognized in ancient literature.1 However, systemized studies on traditional medicinal plants, especially herbs, were noticed in 1956 and soon achieved recognition due to loss of traditional knowledge and declining plant population.2 At least 7,000 medicinal compounds in the modern pharmacopoeia are of plant origin. Among the 120 plant-derived bioactive compounds widely used in modern medicine, 80% exhibit a positive correlation between their modern therapeutic use and the traditional use of source plants.3 Andrographis paniculata Nees, belonging to Acanthaceae family, is a herb traditionally used as a medicine in ancient oriental, including Indian Ayurveda. Phytochemical exploration revealed diverse pharmacoactive compounds, including labdane diterpenoid lactones, flavonoids, and others.4 The leaf extract of this plant is very well known as a therapy for infectious diseases, fever-causing diseases, diarrhea colic pain, and loss of appetite.5
New classes of bioactive compounds are ever demanding, and we know that the probability of occurrence of such compounds is higher in any unexplored source. Apart from plants, microorganisms are also considered as a reservoir of bioactive compounds produced as secondary metabolites. Presently, pharmaceutical exploration of microbial origin is aimed at endophytic microorganisms. Endophytic microbes are yet less-explored population and presently gaining significant field in medicinal research for mankind.6 They reside symptomless within the plant’s internal tissues. Some of them act synergistically by producing secondary metabolites antagonistic to plant pathogens.7 Both fungi and bacteria are common endophytes, and many new antimicrobial compounds have been already discovered from such class of microorganisms.8 In most cases, the antimicrobial compounds produced by such groups of microbes are relatively less toxic as the plant itself serves as a natural selection system.7 Many bioactive substances that an endophyte produces were found relatively new to us. Therefore, there is a huge potential to screen novel, highly active, and low-toxicity antimicrobial compounds from endophytic microorganisms. A large number of endophytic bacteria have already been isolated possessing antibacterial or antifungal activity.9 Endophytic microorganisms also have great contribution in the production of antidiabetic,10 anticancerous,7 anti-insecticidal,11 antiviral,12 and even immunosuppressive compounds.13
This study was aimed to isolate endophytic bacteria from A. paniculata Nees. and evaluate their anti-infective property against some bacterial pathogens. One of the isolated strains identified as Bacillus thuringiensis KL1 from 16s rRNA gene homology and other characteristics showed potential anti-infectivity. Three bioactive metabolites were isolated from this endophytic bacterium and one of them was characterized after spectral analysis.
Materials and Methods
Sampling and endophyte isolation
A. paniculata is native to peninsular India. This herb grows as a bush and is a dominant species in the district of Paschim Medinipur, West Bengal, India. Healthy twigs were collected from different localities of Paschim Medinipur (22.57N–87.11E) and stored in refrigerator till isolation of endophytes. Leaves were separated from twigs and washed under gentle tap water. These parts of the plant were aseptically surface sterilized following standard method.14 Samples were placed on ISP2 agar media supplemented with actidione (50°µg/mL) and incubated at 30°C for five days. Endophytic bacteria, which came out from surface-sterilized part of the leaves, were immediately pure cultured and preserved with 30% glycerol at −20°C.
Evaluation of anti-infectivity
Isolated endophytic strains were individually grown in tryptone yeast extract glucose liquid media [bacto tryptone, 0.5%; yeast extract, 0.2%; glucose, 1%; K2HPO4, 0.1%, and MgSO4 7H2O, 0.05%], pH 7.2 for three days at 30°C in a shaker incubator (150 rpm).15 Cultures were taken in a centrifuge tube, and cells were pelleted by centrifugation at 6,000 g for eight minutes. Cell-free liquid media were evaluated for pathogen growth inhibition by agar well diffusion method. Six pathogens available in the laboratory were grown overnight in tryptic soy broth. Pathogens included Bacillus subtilis (ATCC 11774), Bacillus cereus (ATCC 14579), Vibrio parahaemolyticus (ATCC 1782), Aeromonas caviae (ATCC 15468), Proteus vulgaris (ATCC 12453), and Pseudomonas aeruginosa (ATCC 9027). Each pregrown pathogenic strain was seeded on Müller-Hinton agar (MHA) media separately, and culture filtrates were applied in the wells on the MHA plates.16 The MHA plates were incubated for another 24 hours, and the zone of inhibition produced was recorded.
Characterization of potential strain
Endophytic isolated strain KL1 was characterized from morphological and biochemical aspects. The strain was observed under compound and scanning electron microscope. Bacterial smear (18 hours grown) was prepared on poly-l-lysine-coated glass slide and fixed with 0.25% glutaraldehyde (prepared in Na-phosphate, pH 7.2). Cells were then dehydrated for 15 minutes with different grades of ethanol (30%–100%) and dried up to the critical point after which slides were attached to the stub.17 The materials were used for imaging through a scanning electron microscope (Vega© TESCAN) after gold coating. Colony and cellular details were recorded, and various extracellular enzyme productions were analyzed for the strain. Sugar utilization patterns were also determined. The endophytic strain was cultured in TYG medium at pH 7 and 30°C incubation temperature with a shaking speed of 150 rpm to study its growth pattern. The growth pattern in batch fashion was determined after plotting the optical density of the cell suspension against time.
Genomic DNA isolation and 16s rDNA amplification
The strain was grown in Luria Bertini for 24 hours and centrifuged for 10 minutes at 10,000 rpm. Cell pellets were washed with phosphate buffer (0.1 M, pH 7.2) and resuspended in 500 µL TE buffer (25 mM Tris-HCl [pH 8.0], 25 mM ethylenediaminetetraacetic acid). After that, it was successively treated with lysozyme and sodium dodecyl sulfate (10%) for proper cell lysis. DNA was extracted with phenol–chloroform reagent, and aqueous layers were separated carefully. Genomic DNA was precipitated with isopropanol and washed with ice-cold 70% ethanol. It was dissolved in 100 µL TE buffer (pH 8) and directly used as template for 16s rDNA amplification.18 For this purpose, the primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) were used.19 Reactions were performed in a polymerase chain reaction (PCR) machine (Mastercycler® nexus, Eppendorf). Reaction parameters were as follows: initial denaturation at 94°C for 5 minutes followed by 30 cycles of denaturation at 95°C for 60 seconds, annealing at 55°C for 60 seconds, and primer extension at 72°C for 90 seconds. A final extension was done at 72°C for 10 minutes. PCR products were purified with Hi-PurA™ PCR product purification spin kit (Himedia).20 Forward and reverse DNA-sequencing reactions of purified PCR amplicon was carried out with the same primers using BigDye Terminator v3.1 Cycle Sequencing Kit on ABI 3730xl Genetic Analyzer.
Phylogeny construction
Consensus sequence of 16s rDNA was generated from forward and reverse sequence data using aligner software. The sequence was used to carry out BLAST with the “nr” database of NCBI GenBank. Based on the maximum identity, sequences were selected and aligned using the multiple alignment software program, ClustalW. The phylogenetic tree was constructed using MEGA5.21 The evolutionary history was inferred using the neighbor-joining method.22 The evolutionary distances were computed using the Kimura’s two-parameter method.23
Production and purification of anti-infective metabolites
Production of active compound by strain KL1 was carried out in liquid TYG media at 30°C for three days under shaking conditions (150 rpm). A total of 1.5 L culture media was centrifuged (10,000 rpm—15 minutes) to separate cell mass. Culture filtrate was then extracted twice with an equal volume of ethyl acetate (Merck—analytical grade). Organic phase was separated and made water free by adding anhydrous Na2SO4. It was brought to dryness with in vacuo evaporation (rotary evaporator; HS-2005S, Hanshin). The crude metabolites were dissolved in a minimum amount of methanol and subjected to activity-guided thin-layer chromatography (TLC) for purification of an anti-infective compound. About 15 µL crude materials was loaded on silica-coated alumina TLC plate (Silica gel 60 F254, Merck), and separation was done with solvent mixture (chloroform–methanol; 10:1). Separated compounds were detected upon ultraviolet (UV) rays (longer wave length), and Rf values were determined. Bioautogram was studied24 by overlaying the TLC plates with molten MHA media (45°C) containing B. cereus (50 µL of 105 cfu mL−1). The TLC plates were kept inside a sterile Petri plate under moist conditions and incubated at 37°C for 24 hours. Cell viability was checked by spraying methylthiazoltetrazolium (5 mg/mL). The position of active compound was located by comparing pathogen inhibition zone and band position on TLC plates. The most active compound was scrapped from TLC plates, dissolved in methanol, and ~5 µL was subjected for further separation with a different solvent mixture (chloroform–methanol; 10:0.5). Separation was detected by exposition of TLC plates on UV, and active fraction was located by the same bioautogram study. Rf value was determined for the active compound and considered spectral characterization.
Determination of IC50 of active compound
It is an essential parameter to determine the effective concentration of any purified antimicrobial agent. Slightly modified microbroth dilution assays25 were employed to determine the IC50 value of the most active purified metabolite produced by endophytic strain KL1. For this purpose, one Gram-positive strain B. cereus and one Gram-negative strain P. vugaris were selected. Stock solution was prepared by dissolving 17 mg (final TLC purified) of active metabolite in 5 mL of methanol (high-performance liquid chromatography grade). It was then serially diluted (200:1–10:1) in Müller-Hinton broth (1 mL) and inoculated separately with test pathogens (25 µL of OD 0.6). Positive and negative controls were prepared without an antimicrobial agent and without inocula, respectively. The IC50 value was determined by plotting OD values of test samples against the concentration of the active compound administered. The activity of purified substance was also determined against the same pathogen by disk diffusion assay,26 where 25 µL methanolic solutions were soaked in sterile disks and placed on individual plates seeded with pathogens separately.
Spectroscopic characterization of purified active compound
The purified active compound produced by endophytic bacterium KL1 was dissolved in chloroform, and absorption spectrum was recorded with ultraviolet-visible (UV-Vis) spectrophotometer (UV-1800; Shimadzu) from 200 to 600 nm with respect to chloroform as control. The active compound was also analyzed for its spectrum in infrared (IR) region. Dried samples were mixed well with KBr and sample—KBr pellet was analyzed from 600 to 4,000 nm in Fourier transform infrared spectrophotometer (Spectrum T; PerkinElmer).
Results
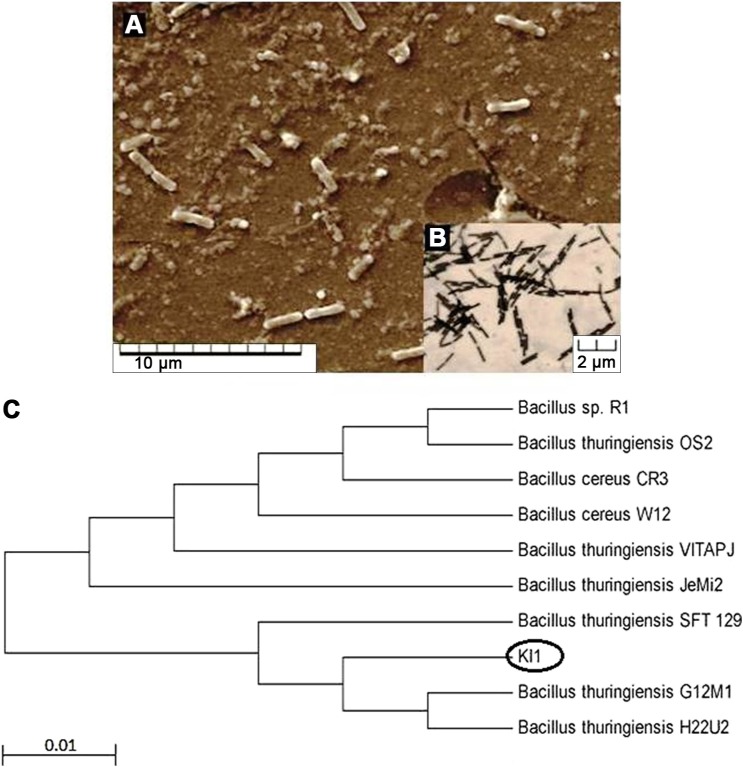
Endophytic bacteria appeared within a 48-hour incubation of surface-sterilized leaves of Andrographis on the isolation media. A total of 23 endophytic bacteria were isolated in this study, and most of the strains were found to inhibit at least one of the test pathogens. However, the rational of selecting strain KL1 was due to its broad-spectrum anti-infective property. It inhibited all of the test pathogenic bacteria except Pseudomonas (Table 1). This strain was our choice of interest due to its anti-infectivity against both Gram-positive and -negative bacteria. The strain was found to be Gram-positive, rod-shaped (Fig. 1), and with endospore. It produced characteristics of various extracellular enzymes. Sugar utilization patterns and other detailed characterization parameters are summarized in Table 2. The strain utilized most of test sugars though could not utilize fructose, xylose, and raffinose at all. Genomic DNA was isolated from 24-hour grown culture of KL1 from which 16s rDNA (~1.5 kb) was amplified. Approximately 900 bases of the amplified product were sequenced, and homology was determined in NCBI database. The constructed phylogenetic tree revealed that the strain showed close sequence similarity (99% similarity with 100% query coverage and E value 0) with different strains of B. thuringiensis (Fig. 1). The isolated endophtyic strain KL1 was proposed to be identified as B. thuringiensis KL1. It showed a regular growth fashion in batch culture (Fig. 2) with ~5 hours as lag phase in TYG medium and other mentioned growth conditions though it reached the maximum cell density at 22 hours.
Table 1.
Antimicrobial activities of strain KL1 against several bacterial pathogens.
| PATHOGENS | ZONE OF INHIBITION* (mm) BY KL1 CULTURE FILTRATE | ZONE OF INHIBITION* (mm) OF PURIFIED SUBSTANCE | IC50 (µg/mL) |
|---|---|---|---|
| Bacillus subtilis (ATCC 11774) | 12.5 ± 0.311 | 27.3 ± 0.033 | 119.00 |
| Bacillus cereus (ATCC 14579) | 12 ± 0.613 | NT | NT |
| Vibrio parahaemolyticus ATCC 1782 | 10.5 ± 0.133 | NT | NT |
| Aeromonas caviae (ATCC 15468) | 10 ± 0.235 | NT | NT |
| Proteus vulgaris (ATCC 12453) | 11.5 ± 0.073 | 24.6 ± 0.133 | 153.00 |
| Pseudomonas aeruginosa (ATCC 9027) | – | NT | NT |
Notes:
Values are represented as mean ± standard deviation of triplicate experiments.
Abbreviations: NT, not tested; –, no inhibition.
Figure 1.
Endophytic strain B. thuringiensis KL1; (A) scanning electron microscopy, (B) optical microscopy (100×), and (C) position of the strain in phylogenetic tree showing similarities with related strains.
Table 2.
Cultural and physiological and biochemical characteristics of endophytic B. thuringiensis KL1.
| CHARACTERIZATION PARAMETERS | APPEARANCE |
|---|---|
| Colony morphology | White to off white color, slightly raised elevation and regular margin |
| Cell morphology | Rod shaped, slightly swollen centrally, 1.5–1.8 µm length, 0.25 µm diameter |
| Gram character | Positive |
| Endospore | Positive |
| Extracellular enzymes | |
| Catalase | + |
| Amylase | + |
| Cellulase | + |
| Lipase | + |
| Caseinase | − |
| Gelatinase | + |
| Urease | − |
| Phenyl alanine hydrolase | + |
| Sugar utilization | |
| Dextrose | + |
| Fructose | − |
| Galactose | + |
| Rhamnose | + |
| Xylose | − |
| Raffinose | − |
| Maltose | + |
| Sucrose | + |
| Lactose | + |
| Starch | + |
| Cellulose | + |
Figure 2.
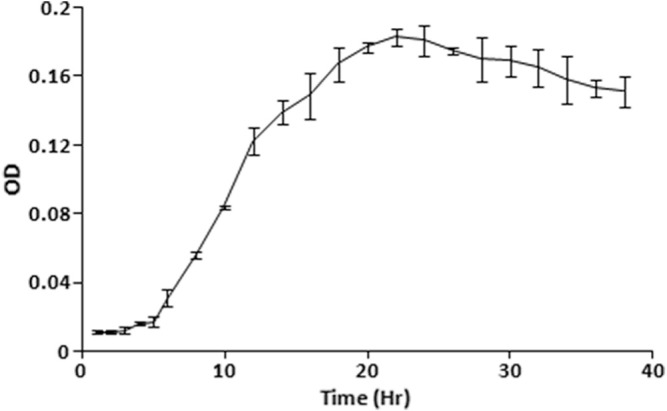
Growth pattern of strain KL1 in batch culture.
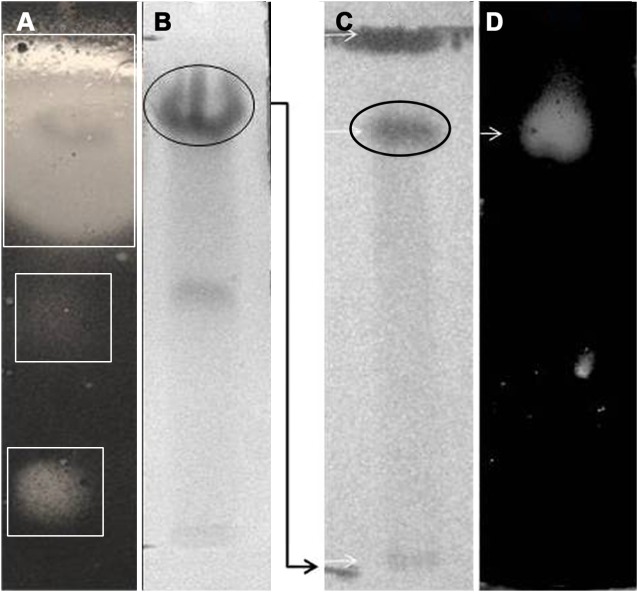
The solvent-extracted crude metabolites appeared as greenish amorphous substances with a sharp smell. This mixture was separated through TLC, and several fractions were obtained among which three compounds were detected (Rf—0.813, 0.480, and 0.053 in chlororform–methanol; 10:1) to inhibit the growth of B. subtilis (Fig. 3A). The compound with an Rf value of 0.813 was found as the major anti-infective compound with the highest activity. However, a further purification of this compound indicated that the compound with an Rf value of 0.784 (chlororform–methanol; 10:0.5) was the most active anti-infective compound produced by endophytic B. thuringiensis KL1 (Fig. 3B). The pure active material was found to absorb UVmax at 259 and 371 nm (Fig. 4), while IRmax was found with (KBr) cm−1: 2,924, 2,854, 1,634–1,632, 1,592, 1,508–1,494, 1,372, and 758 (Fig. 5). These spectroscopic data are characteristic features of different structural properties and indicate the presence of a few functional groups within the active compound. Antimicrobial activity determination revealed that the purified substance was active against both types of microorganisms with varying IC50 values. It was found that the IC50 values are 119.0 and 153.0 µg/mL, respectively, against B. cereus and P. vulgaris (Table 1).
Figure 3.
Activity-guided TLC-based purification of the most active anti-infective metabolite produced by KL1; (B, C) represent thin layer chromatogram in which (B) indicates three active metabolites and (C) represents the most active band indicated inside the oval shape. (A, D) Demonstrate bioautogram analysis indicating activity among the separated compounds.
Figure 4.
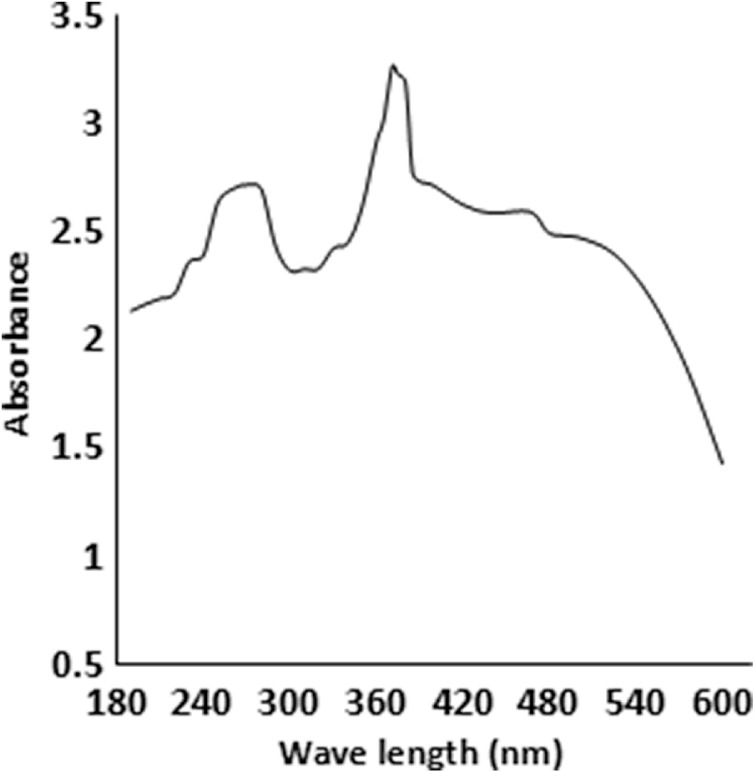
UV-Vis spectrum of most active metabolites by strain KL1.
Figure 5.
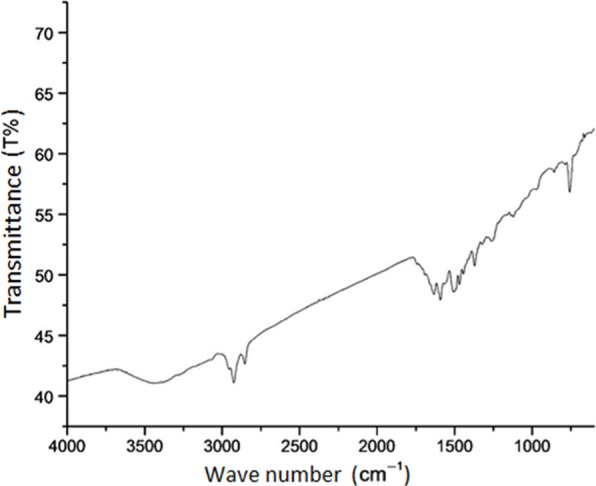
IR spectrum of the most active anti-infective metabolite of strain KL1.
Discussion
The large number of secondary metabolites so far isolated from microbial cultures and plant extracts have no doubt significant industrial values. Like many other microbial species, Bacillus also produces many antibacterial secondary metabolites.27 Bacillus, traditionally isolated from the soil, is renowned for the production of various antibiotics such as bacitracin, polymyxin, zwittermicin A, and several others.28 Unfortunately, reisolation of the same microorganism from the same source and the throttled success of combinatorial chemistry in providing new compounds indicate uncertainty in future antimicrobial therapy. Thus, finding new groups of microbes from unexplored or less-explored habitats became mandatory by pursuing them as sources of novel bioactive compounds. Different bacteria are already reported for endophytic origin such as Azorhizobium, Bacillus, Bradyrhizobium, Gluconacetobacter, Klebsiella, Burkholderia, Enterobacter, Pseudomonas, and Streptomyces.29 B. thuringiensis is an aerobic, Gram-positive endospore-forming bacterium and well known for crystalline inclusions or δ-endotoxins. These proteins are selectively insecticidal, but the strain also secretes zwittermicin A, a linear aminopolyol antibiotic effective against Gram-negative bacteria mostly.30 A number of lipopeptides with anti-infectivity were also purified from different species of Bacillus. A fengycin-like substance was purified from a soil-dwelling B. thuringiensis CMB26, which exhibited fungicidal, bactericidal, and insecticidal activities.31 Present findings report the isolation of the endophytic isolate KL1, which is identified as B. thuringiensis KL1. The strain was isolated on ISP2 agar media from surface-sterilized leaves of A. paniculata Nees. ISP2 agar media is an effective isolation media for endophytic bacteria as it contains minimum nutritional requirements for growth. Endophytes are able to emerge from plant parts and grow using plant extracts and nutrients provided by media, whereas other microorganisms are unable to access plant extracts and their growth is not supported so much. Sugar utilization pattern of this strain indicates that it can use some polysaccharides such as cellulose and starch as carbon source, which are very usual in plants. Besides, its utilization of disaccharides and few monosaccharides explores its wide carbon utilization property that might help it to grow in the specialized microenvironment. Presence of different extracellular enzymes also helps such group of microbes to exist in such an association as was found in this endophytic strain. A report states that its leaves contain about 103–104 cfu mL−1 when 1 g of surface-sterilized leaves were crushed in 10 mL distilled water and subjected for isolation of endophytic bacteria,32 though this study reports of 23 bacterial isolates from the same. Interestingly, B. thuringiensis var israelensis (Bti), a nonendophytic isolate, and ethanolic extracts of A. paniculata were found for their larvicidal as well as pupicidal activity against Anopheles stephensi Liston in a synergistic fashion.33 Here, we report that the endophytic strain B. thuringiensis KL1 was able to inhibit both Gram-positive and Gram-negative pathogens employed though it could not inhibit Pseudomonas at all. TLC along with bioautogram analysis revealed that the compound is slightly nonpolar in nature and the strain produced at least three different compounds that were inhibitory to test pathogen. Spectroscopic characterization of most active compounds showed strong UV absorption at 259 and 361 nm. UVmax at 259 nm suggests the presence of conjugated diene in benzene ring, while at 361 nm sharply, it indicates the anthracene nature of the active compound. IR absorbance values at 2,854 and 2,924 cm−1 specify some alkane stretch, whereas the presence of monosubstituted alkene was indicated by 1,634–1,632 cm−1. –COOH attached with the aromatic ring was indicated at 1,592 cm−1, and the presence of aromatic ring was confirmed by IR absorption at 1,508–1,494 cm−1. The active compound consisted –NO2 as the functional group that was suggested by absorption at 1,372 cm−1. Therefore, the spectroscopic characterization recommends the active compound as anthracene derivative consisting poly alkane or alkene, where phenyl group may be substituted with COOH and NO2 groups. Anthracenes are reported to inhibit at different steps in DNA/RNA biosynthesis,34 which is considered a potent target site for antibiotics. The active principal here shows marked effectivity to both Gram-positive and -negative pathogens indicating the probability of similar inhibition mechanisms. However, this compound does not bear any similarity with either compound isolated from B. thuringiensis previously reported.30,31 Internal plant tissue is a specialized ecosystem that may be responsible of such an alteration in gene sequence during coevolution.35 Here is the opportunity of getting variations in metabolites from similar microorganisms isolated from other common habitats. The isolated endophytic strain may be a good source for the antimicrobial compound due to its broad-spectrum antibacterial property.
Conclusion
Endophytic bacteria from medicinal plants are good sources of new class anti-infective compounds. This study showed potential existence of endophytic bacteria in the studied plant. Moreover, the strain, identified as B. thuringiensis KL1, is found to possess broad-spectrum anti-infective property. The ethyl acetate extract of this isolate yielded three different anti-infective metabolites among which the major and most active anti-infective metabolite was anthracenic in nature. Detailed structural elucidation and toxicity determination are necessary for exploiting the active metabolites as future drug.
Footnotes
ACADEMIC EDITOR: Raúl Rivas, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 613 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the experiments: DB and SR. Analyzed the data: DB, SR, SG. Wrote the first draft of the manuscript: SR, SG, SY, SB. Contributed to the writing of the manuscript: SR, SG, SY, SB, DB. Agree with manuscript results and conclusions: SR, SG, SY, SB, DB. Jointly developed the structure and arguments for the paper: SR, DB, SG. Made critical revisions and approved final version: DB, SR. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Panghal M, Arya V, Yadav S, Kumar S, Yadav JP. Indigenous knowledge of medicinal plants used by Saperas community of Khetawas, Jhajjar District, Haryana, India. J Ethnobiol Ethnomed. 2010;6:4. doi: 10.1186/1746-4269-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao RR. Traditional knowledge and sustainable development key role of ethnobiologists. Ethnobotany. 1996;8:14–24. [Google Scholar]
- 3.Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;1:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishra SK, Sangwan NS, Sangwan RS. Andrographis paniculata (Kalmegh): a review. Phcog Rev. 2007;1:283–298. [Google Scholar]
- 5.Saxena S, Jain DC, Bhakuni RS, Sharma RP. Chemistry and pharmacology of Andrographis species. Indian Drugs. 1998;35:458–467. [Google Scholar]
- 6.Strobel GA. Endophytes as sources of bioactive products. Microbes Infect. 2003;5:535–544. doi: 10.1016/s1286-4579(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 7.Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev. 2003;67:491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee D. Endophytic fungal diversity in tropical and subtropical plants. Res J Microbiol. 2011;6:54–62. [Google Scholar]
- 9.Ghadin N, Zin NM, Sabaratnam V, et al. Isolation and characterization of a novel endophytic Streptomyces SUK06 with antimicrobial activity from Malaysian plant. Asian J Plant Sci. 2008;7:189–194. [Google Scholar]
- 10.Zhang B, Salituro D, Li Z, et al. Discovery of small molecule insulin mimetic with antidiabetic activity in mice. Science. 1999;284:974–981. doi: 10.1126/science.284.5416.974. [DOI] [PubMed] [Google Scholar]
- 11.Demain AL. Microbial natural products: a past with a future. In: Wringley SK, Hayes MA, Thomas R, Chrystal EJT, Nicholson N, editors. Biodiversity: New Leads for Pharmaceutical and Agrochemical Industries. Cambridge: The Royal Society of Chemistry; 2000. pp. 3–16. [Google Scholar]
- 12.Guo B, Dai J, Huang NY, Leong C, Ong W, Carte BK. Cytonic acids A, and B: novel tridepside inhibitors of hCMV protease from the endophytic fungus Cytonema sp. J Nat Prod. 2000;63:602–604. doi: 10.1021/np990467r. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Lobkovsky E, Pliam NB, Strobel GA, Clardy J. Subglutinols A and B: immunosuppressive compounds from the endophytic fungus Fusarium subglutinans. J Org Chem. 1995;61:7076–7077. [Google Scholar]
- 14.Zin NM, Loi CS, Sarmin MN, Rosli AN. Cultivation-dependent characterization of endophytic actinomycetes. Res J Microbiol. 2010;5:717–724. doi: 10.3923/jm.2010.717.724. [DOI] [Google Scholar]
- 15.Roy S, Banerjee D. Bioactive endophytic actinomycetes of Cinnamomum sp.; isolation, identification, activity guided purification and process optimization of active metabolite. Am J Microbiol. 2015;6:4–13. [Google Scholar]
- 16.Perez C, Paul M, Bazerque P. Antibiotic assay by agar-well diffusion method. Act Biol Med Exp. 1990;15:113–115. [Google Scholar]
- 17.Franson TR, Sheth NK, Rose HD, Sohnle PG. Scanning electron microscopy of bacteria adherent to intravascular catheters. J Clin Microbiol. 1984;20:500–505. doi: 10.1128/jcm.20.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee O, Choi GJ, Choi YH, et al. Isolation and characterization of endophytic actinomycetes from Chinese cabbage roots as antagonists to Plasmodiophora brassicae. J Microbiol Biotechnol. 2008;18:1741–1746. doi: 10.4014/jmb.0800.108. [DOI] [PubMed] [Google Scholar]
- 19.Santhi VS, Solomon RDJ. Phylogenetic analysis and antimicrobial activities of Streptomyces isolates from mangrove sediment. J Basic Microbiol. 2011;51:71–79. doi: 10.1002/jobm.201000107. [DOI] [PubMed] [Google Scholar]
- 20.Roy S, Banerjee D. Broad spectrum antibacterial activity of granaticinic acid, isolated from Streptomyces thermoviolaceus NT1; an endophyte in Catharanthus roseus (L.) G. Don. J Appl Pharm Sci. 2015;5:6–11. [Google Scholar]
- 21.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 23.Kimura MA. Simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 24.Choma IM, Grzelak EM. Bioautography detection in thin-layer chromatography. J Chromatogr A. 2011;1218:2684–2691. doi: 10.1016/j.chroma.2010.12.069. [DOI] [PubMed] [Google Scholar]
- 25.Castillo UF, Strobel GA, Ford EJ, et al. Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigriscans. Microbiology. 2002;148:2675–2685. doi: 10.1099/00221287-148-9-2675. [DOI] [PubMed] [Google Scholar]
- 26.Bauer AW, Kirby WM, Sheriss JC, Turc M. Antibiotic susceptibility testing by standardized single disc method. Am J Clin Pathol. 1996;45:493–496. [PubMed] [Google Scholar]
- 27.Sansinenea E, Ortiz A. Secondary metabolites of soil Bacillus spp. Biotechnol Lett. 2011;33:1523–1538. doi: 10.1007/s10529-011-0617-5. [DOI] [PubMed] [Google Scholar]
- 28.Elizabeth ABE, Amy KK, Michael GT, Jo H. Genetics of zwittermicin A production by Bacillus cereus. Appl Environ Microbiol. 2004;70:104–113. doi: 10.1128/AEM.70.1.104-113.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollman J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW. Bacterial endophytes in agricultural crops. Can J Microbiol. 1997;43:895–914. [Google Scholar]
- 30.Sansinenea E, Ortiz A. An antibiotic from Bacillus thuringiensis against Gram-Negative Bacteria. Biochem Pharmacol (Los Angel) 2013;2:e142. doi: 10.4172/2167-0501.1000e142. [DOI] [Google Scholar]
- 31.Kim PI, Bai H, Bai D, et al. Purification and characterization of a lipopeptide produced by Bacillus thuringiensis CMB26. J Appl Microbiol. 2004;97:942–949. doi: 10.1111/j.1365-2672.2004.02356.x. [DOI] [PubMed] [Google Scholar]
- 32.Gusmani, Aziz SA, Munif A, Sopandie D, Bermawie N. Isolation and selection of endophytic bacteri consortia from medicinal plant (Andrographis paniculata) as plant growth promoting agent. J Agron. 2013;12:113–121. [Google Scholar]
- 33.Chennippan K, Chellamuthu V, Dhandapani A, Kadarkarai M. Synergistic activity of Andrographis paniculata nees with Bacillus thuringiensis var israelensis against malarial vector, Anopheles stephensi Liston (Diptera: Culicidae) J Entomol Res Soc. 2011;13:71–86. [Google Scholar]
- 34.Pommier Y, Leo E, Zhang H, Marchand C. DNA to poisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Germaine K, Keogh E, Garcia-Cabellos G, et al. Colonisation of poplar trees by gfp expressing bacterial endophytes. FEMS Microbiol Ecol. 2004;48:109–118. doi: 10.1016/j.femsec.2003.12.009. [DOI] [PubMed] [Google Scholar]