Summary
Background
To investigate the effect of gadoxetic acid disodium (Gd-EOB-DTPA) on T2 relaxation times and apparent diffusion coefficient (ADC) values of the liver and focal liver lesions on a 1.5-T system.
Material/Methods
Magnetic resonance (MR) studies of 50 patients with 35 liver lesions were retrospectively analyzed. All examinations were performed at 1.5T and included T2-weighted turbo spin-echo (TSE) and diffusion-weighted (DW) images acquired before and after intravenous administration of Gd-EOB-DTPA. To assess the effect of this hepatobiliary contrast agent on T2-weighted TSE images and DW images T2 relaxation times and ADC values of the liver and FLLs were calculated and compared pre- and post-injection.
Results
The mean T2 relaxation times of the liver and focal hepatic lesions were lower on enhanced than on unenhanced T2-weighted TSE images (decrease of 2.7% and 3.6% respectively), although these differences were not statistically significant. The mean ADC values of the liver showed statistically significant decrease (of 4.6%) on contrast-enhanced DW images, compared to unenhanced images (P>0.05). The mean ADC value of liver lesions was lower on enhanced than on unenhanced DW images, but this difference (of 2.9%) did not reach statistical significance.
Conclusions
The mean T2 relaxation times of the liver and focal liver lesions as well as the mean ADC values of liver lesions were not significantly different before and after administration of Gd-EOB-DTPA. Therefore, acquisition of T2-weighted and DW images between the dynamic contrast-enhanced examination and hepatobiliary phase is feasible and time-saving.
MeSH Keywords: Diffusion Magnetic Resonance Imaging, Liver Neoplasms, Magnetic Resonance Imaging
Background
Magnetic resonance imaging (MRI) is presently a leading modality for liver imaging. Gadoxetic acid disodium (Gd-EOB-DTPA)- enhanced MRI demonstrated improved diagnostic accuracy for detection and characterization of focal liver lesions (FLLs) [1–6]. GdEOB-DTPA displays properties of extracellular and hepatobiliary contrast agent and therefore can be used for both dynamic and hepatobiliary imaging [1–12]. Approximately 50% of this contrast material is taken up by hepatocytes and the maximum enhancement of the liver parenchyma during the hepatobiliary phase is perceived around 20 minutes after intravenous administration, resulting in roughly 15 minutes of interval between dynamic and hepatobiliary imaging [7,8]. Acquisition of diffusion-weighted (DW) and T2-weighted images during this interval could be cost-effective due to significant shortening of the overall imaging time.
However, as Gd-EOB-DTPA has almost two-fold higher T1 and T2 relaxivities than conventional paramagnetic contrast agents, it may cause higher magnetic susceptibility and T2 shortening, affecting both DW and T2-weighted images. Chiu et al. who investigated the effect of Gd-EOB-DTPA on DW imaging on a 3.0-T system noted that the apparent diffusion coefficient (ADC) values of the liver and liver lesions tended to decrease on contrast-enhanced images, although they did not reach statistical significance [13]. Other authors also observed a limited effect of Gd-EOB-DTPA on signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR) or ADC values, not precluding DW imaging after injection of this contrast agent [13–15].
To the best of our knowledge, there are no published studies investigating the effect of Gd-EOB-DTPA on T2 relaxation times of the liver and liver lesions. Saito et al., who performed a phantom study, observed that a concentration of Gd-EOB-DTPA higher than 0.4 mmol/L resulted in a decrease of signal intensity on T2-weighted images [15]. They also imaged 30 patients with hepatocellular carcinoma (HCC) noting that the SNR and CNR of HCCs showed no significant difference on T2-weighted images acquired before as well as 4 and 20 minutes after the administration of this contrast agent, while the SNR of the liver parenchyma on T2-weighted images was significantly different between prior and 4 minutes after injection [15].
Since the effect of Gd-EOB-DTPA on T2-relaxation times of the liver parenchyma and liver lesions has not been yet investigated the purpose of this study was to calculate and compare T2-relaxation times of the liver parenchyma and focal hepatic lesions before and after the administration of this contrast agent. A similar comparison was made for ADC values.
Material and Methods
Study population
Between January 2011 and October 2012 sixty-two MR studies utilizing hepatobiliary contrast agent Gd-EOB-DTPA were performed at our institution. For this retrospective analysis we excluded follow-up studies and studies of patients with missing or insufficient data to confirm the nature of detected hepatic lesions (n=12). Thus the evaluation was finally executed in 50 patients (24 male, 26 female) with a mean age of 54.6 years (age range: 25–80 years). In 26 of those patients 35 focal liver lesions were analyzed, including 8 benign and 27 malignant. Lesions smaller than 10 mm were excluded from the evaluation. The mean size of analyzed liver lesions was 39.7 mm (range: 10–132 mm). Benign lesions comprised 5 hemangiomas, 2 focal nodular hyperplasias (FNHs) and 1 abscesses. Among malignant lesions there were 10 metastases, 5 hepatocellular carcinomas (HCCs), 9 hemangioendotheliomas, 2 peripheral cholangiocarcinomas (CCAs), and 1 fibrolamellar carcinoma (FLC). The primary sites of metastatic lesions were colorectal carcinoma (n=4), gallbladder carcinoma (n=3), esophageal carcinoma (n=1), renal cell carcinoma (n=1) and melanoma (n=1). In 8 of 26 patients with focal liver lesions the reference standard for the diagnosis was histopathological proof. The samples were obtained intra-operatively in 5 patients and during biopsy in 3 patients. In the remaining 18 patients no histological proof was obtained and the final diagnosis was based on the results of previous imaging studies and follow-up with a minimum observation period of 6 months (US, CT, MRI), laboratory tests and clinical data.
Single liver lesions were evaluated in 22 patients, while 4 patients underwent analysis of more than one focal lesion (a maximum of 5 lesions per patient was assessed). In 24 patients who underwent Gd-EOB-DTPA-enhanced MR imaging due to biliary injury no focal hepatic lesion was identifiable on MR imaging and the evaluation was limited to the liver parenchyma. The study was approved by the institutional review board.
MR imaging
All MR studies were performed on a 1.5-T system (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany), with explorer gradients (maximum gradient of 40, 40, 45 mT/m along x, y and z axis respectively and slew rate of 200 mT/m/ms), using a phased-array multicoil system (12 elements).
After implementation of Gd-EOB-DTPA at our institution the imaging protocol of patients in whom this contrast agent was administered was modified so DWI imaging and T2-weighted imaging were performed before and after a contrast-enhanced dynamic study (before acquisition of hepatobiliary phase images). Intravenous administration of gadoxetic acid disodium (Gd-EOB-DTPA, Primovist, Bayer Schering Pharma AG, Berlin, Germany) was performed with a prefilled syringe (10 mL [0.25 mmol/mL]), followed by a 20-mL saline infusion. Then, acquisition of dynamic images and 5-minute delayed contrast-enhanced images using T1-weighted three-dimensional volumetric interpolated breath-hold examination (3D-VIBE) was executed, followed by acquisition of diffusion-weighted (DW) images and T2-weighted turbo spin-echo (TSE) images.
Diffusion-weighted images were acquired using SE single-shot echo-planar sequence in the axial plane with respiratory-triggering before and at approximately 6–10 minutes after injection of the contrast agent. Integrated parallel imaging technique (iPAT) with generalized autocalibrating partially parallel acquisition (GRAPPA) and acceleration factor of two was applied. The other parameters were as follows: repetition time (TR) 1700 ms, TE 90 ms, flip angle 90°, EPI factor 120, slice thickness 6 mm, 120×192 matrix, 2 acquisitions, field of view 344 mm, bandwidth 1736 Hz/pixel, spectral fat suppression. Diffusion gradients were applied in three orthogonal directions separately with eight increasing b-values of 0, 50, 100, 150, 200, 400, 800, 1200 s/mm2.
The breath-hold dual-echo TSE sequence was performed before and at approximately 11–13 minutes after the administration of the contrast agent, in the axial plane with repetition time (TR) of 1800 ms, 1st effective TE (TEeff) 84 ms, 2nd TEeff 228 ms, flip angle 150°, turbo factor 29, slice thickness 6 mm, 207×256 matrix, 1 acquisition, acceleration factor of 2, field of view 340 mm, bandwidth 260 Hz/pixel.
Image analysis
Quantitative analysis of all MR data was performed on commercial workstation (Leonardo, Siemens Medical Solutions, Erlangen, Germany).
To assess the effect of Gd-EOB-DTPA on T2 signal intensity (SI) of the liver and liver lesions, T2 relaxation times of the liver parenchyma and focal hepatic lesions were calculated before and after contrast material administration.
The signal intensities (SI) of the liver and liver lesions were measured on dual-echo T2-weighted TSE image signal, separately for each echo time (TE1=84 ms, TE2=228 ms). Two measurements were performed for the posterior and anterior segments of the right liver lobe as well as for the medial and lateral segments of the left liver lobe. Then all measurements were averaged and the mean values were used for calculation of T2 relaxation time of the liver. Two measurements were also performed for each echo time for all liver lesions and the mean values were used for T2 calculation. If the lesion included the solid and liquid part, regions of interest (ROIs) were confined to its solid component.
Assuming that TR is much higher than T1, the standard equation for SE signal intensity may be simplified to SI=K·e−(TE/T2), where K is a machine-dependent constant. Therefore, the natural logarithm of signal intensity on SE image is linearly related to TE with a slope of −1/T2.
T2 relaxation times of the liver and liver lesions were calculated according to the following formula:
where TE1 is the first echo time (84 ms), TE2 is the second echo time (228 ms), ln SI1 and ln SI2 are the natural logarithms of the measured SI for TE1 and TE2, respectively.
To assess the consequence of Gd-EOB-DTPA on diffusion-weighted imaging, the apparent diffusion coefficient (ADC) values of the liver and focal lesions were calculated before and after contrast material administration. The measurements of ADC values of the liver were made by drawing regions of interest (ROIs) in a way similar to measurement of T2 signal, including separately anterior and posterior segments of the right liver lobe as well as medial and lateral segments of the left liver lobe and then averaging those values. The measurements of ADC values of hepatic lesions were accomplished by drawing regions of interest (ROIs) on DWI images, which provided the best delineation of the analyzed liver lesions. ROI included the largest possible part of the lesion, avoiding partial volume effect, areas of necrosis, blood vessels and artifacts. Then, ROI was copied and pasted from a DWI image to a corresponding ADC map and the measurement on the ADC map was recorded. ADC was measured twice for each lesion and two measurements were averaged.
Apparent diffusion coefficient values (ADC) were calculated by mono-exponential regression with the following formula: S=S0·exp(−b·ADC), where S is the signal intensity after application of the diffusion gradient and S0 is the signal intensity at b=0 s/mm2. Eight b values (0, 50, 100, 150, 200, 400, 800, 1200 sec/mm2) were applied for ADC calculation.
Statistical analysis
The statistical analysis was performed using Statistica software (version 10.0).
The T2 relaxation times and ADC values of the liver and liver lesions before and after the administration of Gd-EOB-DTPA were compared by using the Wilcoxon signed-rank test. P-values of less than 0.05 were considered to represent statistically significant differences.
Results
The mean T2 relaxation times of the liver parenchyma and focal liver lesions as well as their ADC values before and after intravenous administration of Gd-EOB-DTPA are shown in Table 1.
Table 1.
The mean T2 relaxation times and ADC values of the liver parenchyma and focal liver lesions before and after intravenous administration of Gd-EOB-DTPA.
| Liver | Focal liver lesions | |
|---|---|---|
| Mean T2 time on unenhanced images (ms) | 107.3 | 148.3 |
| Mean T2 time on enhanced images (ms) | 104.4 | 143 |
| Mean T2 time decrease after Gd-EOB-DTPA | 2.7% | 3.6% |
| Mean ADC value on unenhanced images (mm2/s) | 0.931×10−3 | 1.226×10−3 |
| Mean ADC value on enhanced images (mm2/s) | 0.888×10−3 | 1.190×10−3 |
| Mean ADC value decrease after Gd-EOB-DTPA | 4.6% | 2.9% |
The mean T2 relaxation time of the liver showed 2.7% decrease on contrast-enhanced T2-weighted TSE images, compared to unenhanced images and this difference was not statistically significant (P=0.606) (Figure 1). The mean T2 relaxation time of focal liver lesions was lower on enhanced than on unenhanced T2-weighted images, but again this difference (of 3.6%) did not reach statistical significance (P=0.057) (Figure 1).
Figure 1.
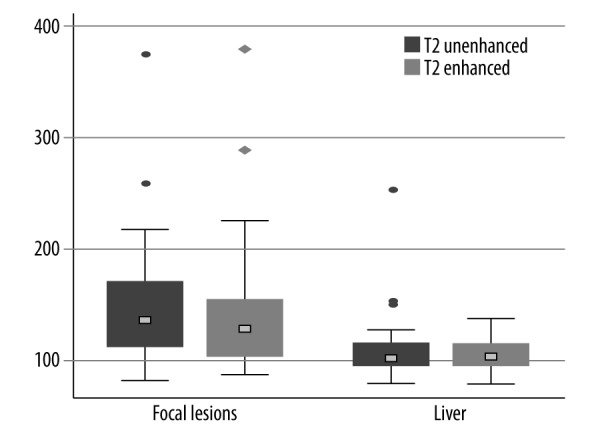
Box plots of T2 relaxation times of the liver and focal liver lesions before and after the administration of Gd-EOB-DTPA. No significant differences between the mean pre-contrast and post-contrast values were noted (P>0.05).
The mean ADC value of the liver was lower on enhanced than on unenhanced DW images and the difference (of 4.6%) was statistically significant (Figure 2). There was also a decrease of 2.9% of the mean ADC value of hepatic lesions on Gd-EOB-DTPA-enhanced images, compared to unenhanced images. However, that difference did not reach statistical significance (P=0.556) (Figure 2).
Figure 2.
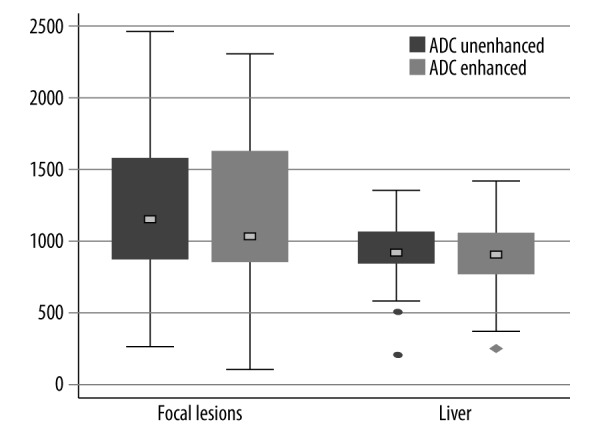
Box plots of the ADC values of the liver and focal liver lesions on unenhanced and Gd-EOB-DTPA-enhanced images. The difference between the mean pre-contrast and post-contrast ADC values of the liver was statistically significant (P<0.05), whereas no significant difference between the mean ADC values of FLLs before and after the administration of Gd-EOB-DTPA was demonstrated (P>0.05).
Discussion
The role of MR imaging enhanced with GD-EOB-DTPA for the diagnosis of focal liver lesions has progressively increased during recent years. This examination becomes increasingly important not only for the differentiation of hepatic lesions but also for their detection and for the evaluation of patients with liver cirrhosis suspected of having HCC [1–6,16].
As the hepatobiliary phase is usually acquired 20 minutes after the injection of this contrast agent, there is an approximately 15-minute interval between this phase and dynamic examination [7,8]. Initial MR protocols utilizing Gd-EOB-DTPA did not advocate imaging during this period, therefore overall imaging time was significantly longer than that of liver MRI study with extracellular contrast agent. Extended imaging time, along with a higher, compared to other paramagnetic contrast agents, cost of Gd-EOB-DTPA, decreased cost-benefit effects of such a procedure. For that reason some investigators proposed acquisition of T2-weighted TSE images and DW images between the dynamic examination and hepatobiliary phase, in order to reduce overall imaging time [13–15,17]. As Gd-EOB-DTPA has almost two-fold higher T1 and T2 relaxivities than Gd-DTPA on 1.5T and 3.0T systems, it has a higher potential to influence T2 relaxation times and, owing to increased magnetic susceptibility and T2 shortening, to alter ADC values, as compared with Gd-DTPA [14]. Consequently, it is of utmost importance to investigate and eventually exclude any significant effect of this contrast agent on post-contrast T2-weighted and DW images.
This study showed that both mean T2 relaxation times and mean ADC values of the liver parenchyma were moderately lower on Gd-EOB-DTPA-enhanced than on unenhanced images. The decrease of the mean T2 relaxation time of the liver parenchyma related to administration of Gd-EOB-DTPA was 2.7% (P>0.05), while the mean ADC value of the liver decreased by 4.6%, reaching statistical significance (P<0.05). The mean T2 relaxation time and the mean ADC value of liver tumors also decreased on Gd-EOB-DTPA-enhanced images (3.6% and 2.9%, respectively) compared to unenhanced images (Figure 3), yet those differences were not statistically significant.
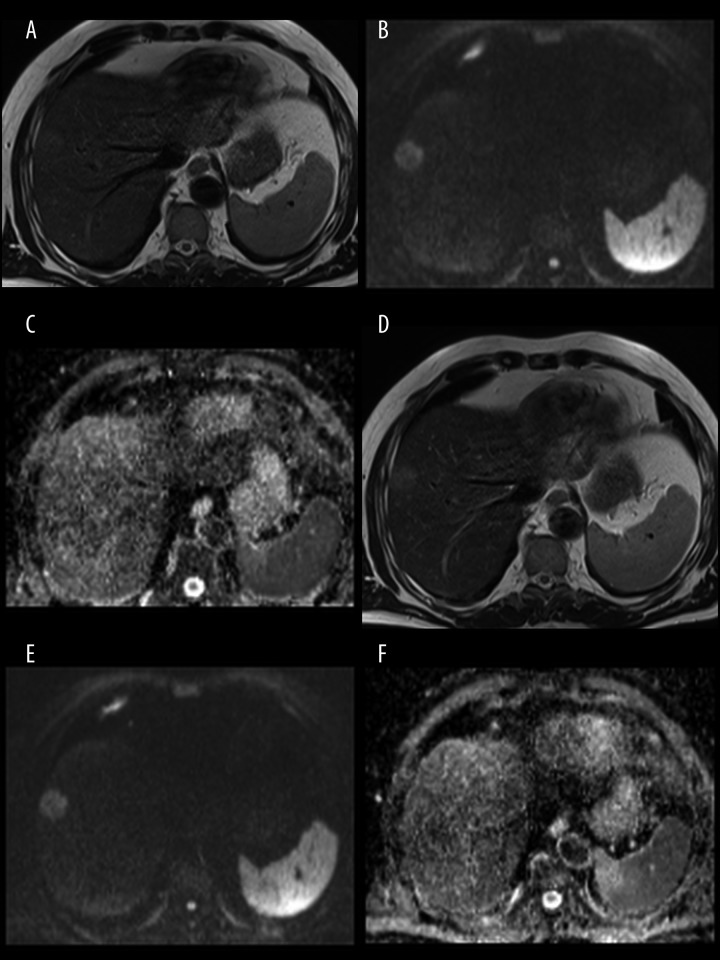
Figure 3.
A 64-year-old male patient with hepatic metastases from colorectal carcinoma. Pre-contrast images: T2-weighted TSE image (A), DWI at b=800 s/mm2 (B), ADC map (C) and post-contrast images: T2-weighted TSE image (D), DWI at b=800 s/mm2 (E), ADC map (F). The T2 relaxation times and ADC values were respectively: 111 ms and 0.873×10−3 mm2/s in pre-contrast images and 104 ms and 0.842×10−3 mm2/s in post-contrast images.
We believe that this is the first study assessing the effect of hepatobiliary contrast agent Gd-EOB-DTPA on T2 relaxation times of the liver and focal liver lesions. There was a single report exploring the effect of this contrast agent on T2-weighted images. However, only in terms of SNR and CNR of the liver parenchyma and detection of hepatocellular carcinoma [15]. They noted a significant difference in SNR of the liver parenchyma prior and 4 minutes after injection. However, since SNR and CNR of HCC showed no significant difference at any time, the authors concluded that it is acceptable to perform T2-weighted imaging after the administration of Gd-EOB-DTPA for the diagnosis of HCC. In the present study we chose to assess T2 relaxation times of the liver and focal hepatic lesions, as this parameter is less dependent on the calibration of the MR scanner and appears to be more objective and reproducible for the characterization of normal and pathologic tissues [18]. Our study results showed that there is no substantial impact of this contrast agent on T2 relaxation times of the liver and liver lesions, even though images were acquired 11–13 minutes post-injection, when the contrast agent had been already taken up by hepatocytes. Therefore, we presume that T2-weighted imaging of the liver after Gd-EOB-DTPA is feasible and should not influence the characterization of focal hepatic lesions.
This is the third analysis of the effect of GD-EOB-DTPA administration on ADC values of the liver and liver tumors on a 1.5-T system, comprising the second largest group of patients [15,17]. Two other, similar analyses were performed on 3.0-T scanners [13,14]. Chiu et al. who studied a small group of 20 patients on a 3.0-T scanner (using DW sequence with two b-values of 0 and 500 s/mm2) showed that the ADC values of the liver parenchyma and focal hepatic lesions tended to decrease 5 minutes after the administration of Gd-EOB-DTPA. However, the difference did not reach statistical significance. They concluded that it is feasible to perform post-contrast DWI in clinical practice [13].
Choi et al. who evaluated DW images of 34 patients with 50 liver lesions on a 3.0-T scanner (using 4 b-values of 0, 200, 400, and 800 s/mm2), demonstrated that SNRs and ADCs of the liver parenchyma were significantly lower on Gd-EOB-DTPA-enhanced images acquired between 7 and 10 minutes post-injection than on unenhanced images, whereas on DW images at b=200 s/mm2, CNRs of focal hepatic lesions were significantly higher on contrast-enhanced than on unenhanced images. However, as ADC values of focal liver lesions were not significantly different before and after the administration of the contrast agent, they concluded that at 3.0T, DWI after Gd-EOB-DTPA administration can be used as a substitute for unenhanced DWI [14].
Saito et al. analyzed 72 patients including 30 patients with HCC at 1.5T before as well as 4 and 20 minutes after the injection of Gd-EOB-DTPA utilizing DW sequence with two b-values, of 100 and 800 s/mm2. As SNR, CNR and ADC values of the liver parenchyma and HCC did not show any significant difference at any time, authors concluded that DWI can be performed soon after injecting Gd-EOB-DTPA [15].
In a recent report Benndorf et al. analyzed DW images of 31 patients with 50 malignant and 30 benign hepatic lesions on a 1.5-T unit (using two b-values, of 0 and 800 s/mm2). They did not find major differences between ADC values of liver lesions before and 10 minutes after the administration of Gd-EOB-DTPA, thus concluding that there is no significant influence of this contrast agent on DW imaging of liver lesions [17].
Our study results showing the significantly lower ADC values of the liver parenchyma on post-contrast DW images, are similar to those of Choi et al., who performed their analysis on a 3.0-T system using four b-values [14]. Other authors, using DW sequences with two b-values did not demonstrate statistically significant differences between the mean ADC values of the liver on pre-contrast and post-contrast images [13,15,17]. In our study we utilized the DW sequence with eight b-values (of 0, 50, 100, 150, 200, 400, 800 and 1200 s/mm2) – more than previous investigators. Such a protocol is supposed to incorporate the effect of both diffusion and perfusion. However, the use of higher b-values (400, 800, 1200 s/mm2) could, on the other hand, result in some decrease of the perfusion effect. Possibly, DW sequence with multiple b-values (ranging from 0 to 1200 s/mm2), increasingly used for liver imaging in academic centers, may be more sensitive in depicting the decrease of ADC values on Gd-EOB-DTPA-enhanced DW images. The liver parenchyma taking up more hepatobiliary contrast agent than the majority of liver lesions tends to be more prone to the susceptibility effects caused by Gd-EOB-DTPA. However, since no significant differences in ADC values of focal hepatic lesions were demonstrated between enhanced and unenhanced images, we presume that DW imaging of the liver may be performed after the administration of Gd-EOB-DTPA. Moreover, due to a relatively long time window of 15 minutes between dynamic imaging and hepatiobiliary phase, it would be possible to obtain high-spatial resolution DW images using respiratory-triggered techniques, which provide a better image quality and signal-to-noise ratio [19] as well as to apply non-breath hold TSE techniques.
There are several limitations of this study. First, the slice positioning of DW and T2-weighted sequences before and after the administration of Gd-EOB-DTPA could not have been perfectly matched, being a possible source of misregistration and bias. Second, the majority of liver lesions was confirmed by follow-up studies, laboratory and clinical data. Only 31% of hepatic lesions were confirmed histologically. Third, the number of analyzed focal liver lesions was relatively small. Thus, further studies, especially regarding the effect of Gd-EOB-DTPA on T2-weighted imaging, may be warranted.
Conclusion
There were minor decreases of the mean T2 relaxation times of the liver parenchyma and focal liver lesions as well as of the mean ADC values of liver lesions on Gd-EOB-DTPA-enhanced images, compared to unenhanced images. However, they did not reach statistical significance. Therefore, we conclude that T2-weighted TSE images and DW echo-planar images can be acquired after injection of Gd-EOB-DTPA, between the dynamic contrast-enhanced study and hepatobiliary phase, leading to shortening of overall imaging time and potentially improving cost-effectiveness of such a procedure.
References
- 1.Huppertz A, Balzer T, Blakeborough A, et al. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic cidenhanced MR images with intraoperative findings. Radiology. 2004;230:266–75. doi: 10.1148/radiol.2301020269. [DOI] [PubMed] [Google Scholar]
- 2.Halavaara J, Breuer J, Ayuso C, et al. Liver tumor characterization: comparison between liver-specific gadoxetic acid disodium-enhanced MRI and biphasic CT – a multicenter trial. J Comput Assist Tomogr. 2006;30:345–54. doi: 10.1097/00004728-200605000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Zech CJ, Herrmann KA, Reiser MF, Schoenberg SO. MR imaging in patients with suspected liver metastases: value of liver-specific contrast agent Gd-EOB-DTPA. Magn Reson Med Sci. 2007;6:43–52. doi: 10.2463/mrms.6.43. [DOI] [PubMed] [Google Scholar]
- 4.Hammerstingl R, Huppertz A, Breuer J, et al. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol. 2008;18:457–67. doi: 10.1007/s00330-007-0716-9. [DOI] [PubMed] [Google Scholar]
- 5.Sano K, Ichikawa T, Motosugi U, et al. Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid-enhanced MR imaging. Radiolog. 2011;261:834–44. doi: 10.1148/radiol.11101840. [DOI] [PubMed] [Google Scholar]
- 6.Ahn SS, Kim MJ, Lim JS, et al. Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology. 2010;255:459–66. doi: 10.1148/radiol.10091388. [DOI] [PubMed] [Google Scholar]
- 7.Reimer P, Schneider G, Schima W. Hepatobiliary contrast agents for contrast-enhanced MRI of the liver: properties, clinical development and applications. Eur Radiol. 2004;14:559–78. doi: 10.1007/s00330-004-2236-1. [DOI] [PubMed] [Google Scholar]
- 8.Ringe KI, Husarik DB, Sirlin CB, Merkle EM. Gadoxetate disodium-enhanced MRI of the liver: Part 1, protocol optimization and lesion appearance in the noncirrhotic liver. Am J Roentgenol. 2010;195:13–28. doi: 10.2214/AJR.10.4392. [DOI] [PubMed] [Google Scholar]
- 9.Filippone A, Blakeborough A, Breuer J, et al. Enhancement of liver parenchyma after injection of hepatocyte-specific MRI contrast media: a comparison of gadoxetic acid and gadobenate dimeglumine. J Magn Reson Imaging. 2010;31:356–64. doi: 10.1002/jmri.22054. [DOI] [PubMed] [Google Scholar]
- 10.Frydrychowicz A, Nagle SK, D’Souza SL, et al. Optimized high-resolution contrast-enhanced hepatobiliary imaging at 3 tesla: a cross-over comparison of gadobenate dimeglumine and gadoxetic acid. J Magn Reson Imaging. 2011;34:585–94. doi: 10.1002/jmri.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi S, Matsui O, Gabata T, et al. Relationship between signal intensity on hepatobiliary phase of gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA)-enhanced MR imaging and prognosis of borderline lesions of hepatocellular carcinoma. Eur J Radiol. 2012;81:3002–9. doi: 10.1016/j.ejrad.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Péporté AR, Sommer WH, Nikolaou K, et al. Imaging features of intrahepatic cholangiocarcinoma in Gd-EOB-DTPA-enhanced MRI. Eur J Radiol. 2013;82:e101–6. doi: 10.1016/j.ejrad.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Chiu FY, Jao JC, Chen CY, et al. Effect of intravenous gadolinium-DTPA on diffusion-weighted magnetic resonance images for evaluation of focal hepatic lesions. J Comput Assist Tomogr. 2005;29:176–80. doi: 10.1097/01.rct.0000157472.98277.5c. [DOI] [PubMed] [Google Scholar]
- 14.Choi JS, Kim MJ, Choi JY, et al. Diffusion-weighted MR imaging of liver on 3.0-Tesla system: effect of intravenous administration of gadoxetic acid disodium. Eur Radiol. 2010;20:1052–60. doi: 10.1007/s00330-009-1651-8. [DOI] [PubMed] [Google Scholar]
- 15.Saito K, Araki Y, Park J, et al. Effect of Gd-EOB-DTPA on T2-weighted and diffusion-weighted images for the diagnosis of hepatocellular carcinoma. J Magn Reson Imaging. 2010;32:229–34. doi: 10.1002/jmri.22219. [DOI] [PubMed] [Google Scholar]
- 16.Löwenthal D, Zeile M, Lim W, et al. Detection and characterization of focal liver lesions in colorectal carcinoma patients: comparison of diffusion-weighted and Gd-EOB-DTPA enhanced MR imaging. Eur Radiol. 2011;21:832–40. doi: 10.1007/s00330-010-1977-2. [DOI] [PubMed] [Google Scholar]
- 17.Benndorf M, Schelhorn J, Dietzel M, et al. Diffusion weighted imaging of liver lesions suspect for metastases: Apparent diffusion coefficient (ADC) values and lesion contrast are independent from Gd-EOB-DTPA administration. Eur J Radiol. 2012;81:e849–53. doi: 10.1016/j.ejrad.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Cieszanowski A, Anysz-Grodzicka A, Szeszkowski W, et al. Characterization of focal liver lesions using quantitative techniques: comparison of apparent diffusion coefficient values and T2 relaxation times. Eur Radiol. 2012;22:2514–24. doi: 10.1007/s00330-012-2519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kandpal H, Sharma R, Madhusudhan KS, Kapoor KS. Respiratory-triggered versus breath-hold diffusion-weighted MRI of liver lesions: comparison of image quality and apparent diffusion coefficient values. Am J Roentgenol. 2009;192:915–22. doi: 10.2214/AJR.08.1260. [DOI] [PubMed] [Google Scholar]