Abstract
Vesicular transport involving soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) proteins is known to be responsible for many major cellular activities. In steroidogenic tissues, chronic hormone stimulation results in increased expression of proteins involved in the steroidogenic pathway, whereas acute hormone stimulation prompts the rapid transfer of cholesterol to the inner mitochondrial membrane to be utilized as substrate for steroid hormone production. Several different pathways are involved in supplying cholesterol to mitochondria, but mobilization of stored cholesteryl esters appears to initially constitute the preferred source; however, the mechanisms mediating this cholesterol transfer are not fully understood. To study the potential contribution of SNARE proteins in steroidogenesis, we examined the expression levels of various SNARE proteins in response to hormone stimulation in steroidogenic tissues and cells and established an in vitro mitochondria reconstitution assay system to assess the contribution of various SNARE proteins on cholesterol delivery for steroidogenesis. Our results from reconstitution experiments along with knockdown studies in rat primary granulosa cells and in a Leydig cell line show that soluble N-ethylmaleimide sensitive factor attachment protein-α, synaptosomal-associated protein of 25 kDa, syntaxin-5, and syntaxin-17 facilitate the transport of cholesterol to mitochondria. Thus, although StAR is required for efficient cholesterol movement into mitochondria for steroidogenesis, specific SNAREs participate and are necessary to mediate cholesterol movement to mitochondria.
Steroidogenesis is largely confined to the adrenal cortex, testicular Leydig cells, ovarian granulosa cells, and placental syncytiotrophoblast cells (1). Unlike cells that produce polypeptide hormones, which can store large amounts of mature hormones for rapid release, there is very little steroid hormone storage in steroidogenic cells. Therefore, upon stimulation, there is a rapid response from the steroidogenic cells to synthesize new steroids (2, 3), a process that requires a constant supply of cholesterol as a precursor for conversion to steroid hormones. The cholesterol utilized for steroidogenesis is derived from a combination of sources (4–6): 1) de novo cellular cholesterol synthesis, 2) the mobilization of cholesteryl esters (CEs) stored in lipid droplets (LDs), and 3) lipoprotein-derived CEs obtained by either low-density lipoprotein (LDL) receptor-mediated endocytic uptake or selective cellular uptake via the scavenger receptor, class B type 1 (SR-B1). Whereas CEs delivered via LDL receptor-mediated lipoprotein uptake are hydrolyzed by lysosomal acid lipase, releasing unesterified free cholesterol (FC) from lysosomes that traffics to the endoplasmic reticulum (ER) and plasma membrane and is then available to traffic to mitochondria (1, 7), CEs delivered via SR-B1 appear to be incorporated directly into LDs (8, 9). CEs in LDs must be hydrolyzed to FC before being used in steroidogenesis. Adrenal CE stores within LDs are rapidly depleted following ACTH treatment (10), supporting the notion that mobilization of stored CEs via the actions of hormone-sensitive lipase (HSL), the major neutral cholesteryl ester hydrolase in the adrenal (11), initially constitutes the preferred source for cholesterol. After LD depletion, lipoprotein-derived CEs delivered via SR-B1 constitute the dominant source of cholesterol for steroidogenesis in rodents (12–14).
Hormonal stimulation of steroidogenesis involves three separate but equally important processes including the following: 1) mobilization of FC from LDs, 2) transport of newly released FC to the outer mitochondrial membrane (OMM), and 3) transfer of this cholesterol from the OMM to the inner mitochondrial membrane (IMM) (15–17). The trafficking of cholesterol for steroid hormone production has been the subject of intense investigation for the last 50 years (2, 3, 18–23). Upon stimulation by trophic hormones, ACTH in adrenals, and LH in the gonads, there is an activation of the cAMP-protein kinase A pathway that exerts regulation of steroid hormone production. The acute response is characterized by a rapid increase in the rate of steroid hormone biosynthesis as a result of the induction of steroidogenic acute regulatory protein (StAR) (18, 19, 24, 25) and delivery of cholesterol to mitochondrial CYP11A1 located on the IMM (20, 21, 25). This final step in cholesterol trafficking, the StAR-mediated movement of cholesterol from the OMM to the IMM, is the critical, rate-limiting step in steroid production (26, 27). However, because mitochondria are relatively cholesterol poor (18, 21), it is necessary for cholesterol to be transported from intracellular sites to replenish the cholesterol in the OMM for subsequent transfer to the IMM and conversion to steroids.
The factors and processes responsible for the transport of LD-derived FC to the OMM are thought to involve protein-protein interactions between LDs and mitochondria, cytoskeletal elements, and putative cholesterol transport proteins, although exact mechanisms are not fully understood. Early electron microscopic observations suggested that LDs and mitochondria become juxtaposed during stimulation by tropic hormone (28). Additional evidence has shown potential interactions between LDs and cellular organelles, including mitochondria, in several nonsteroidogenic cell systems (29, 30). Recently some constituent proteins of soluble NSF attachment protein receptor (SNARE) complexes (31), including N-ethylmaleimide-sensitive factor (NSF), alpha-soluble N-ethylmaleimide-sensitive factor attachment protein (α-SNAP), and SNAREs, synaptosomal-associated protein of 23 kDa (SNAP23), syntaxin-5, and vesicle-associated membrane protein-4 (VAMP-4), were reported to be associated with LDs, and it was suggested that VAMP-4, syntaxin-5 and SNAP23 are required for triacylglycerol (TAG)-rich LD fusion (32). More recently, another report provided evidence that SNAP23 promotes interaction between TAG-rich LDs and mitochondria (33). Steroidogenic cells express high levels of some SNARE proteins such as syntaxin-17 (34), SNAP23 (35), and synaptosomal-associated protein of 25 kDa (SNAP25) (36), and expression of SNAP25 has been reported to be hormonally regulated in granulosa cells (37). These various observations strongly suggest that SNARE proteins are likely involved in LD-derived cholesterol transport to steroidogenic mitochondria.
In the current paper, we have surveyed the expression levels of various SNARE proteins in response to hormone stimulation in steroidogenic tissues and cells and established an in vitro mitochondria reconstitution assay system to examine the contribution of various SNARE proteins on cholesterol delivery for steroidogenesis. Our results show that α-SNAP, SNAP25, and syntaxin-17 facilitate the transport of cholesterol to mitochondria in this system. Using rat primary granulosa cells and a mouse Leydig tumor cell line, we present data demonstrating that α-SNAP, SNAP25, syntaxin-5, and syntaxin-17 are required for effective cellular cholesterol movement to mitochondria for steroidogenesis. Thus, although StAR is required for efficient cholesterol movement into mitochondria for steroidogenesis, specific SNAREs participate and are necessary to mediate cholesterol movement to mitochondria.
Materials and Methods
Chemicals and reagents
Reagents were obtained from the following sources: pregnenolone and progesterone ELISA kit from Alpco Diagnostics; cholesterol LiquiColor test from Stanbio; bicinchoninic assay protein assay kit from Pierce Biotechnology, Inc; DL-aminoglutethimide, soybean L-1α-phosphatidylcholine, 22(R)-hydroxycholesterol, 25-hydroxycholesterol, and trilostane from Sigma; abiraterone from Axon Biochemicals BV; MitoTracker Red FM from Invitrogen; and organic solvents from J.T. Baker; all other reagents were from Sigma unless otherwise noted. Antibodies against syntaxin-5 (SC-365124) and syntaxin-17 (SC-107095) were obtained from Santa Cruz Biotechnology, Inc. Antibodies against SNAP25 (ab53723), SNAP23 (ab4114), α-SNAP (ab133673), and syntaxin-7 (ab68982) were obtained from Abcam Inc.
Animals
Sixteen-week-old control C57BL/6J mice were purchased from Jackson Laboratory. Three-week-old Sprague Dawley female rats and adult male Sprague Dawley rats were purchased from Harlan Laboratories. Mice and rats were housed in the animal facility at the Veterans Affairs Palo Alto Health Care System on a 12-hour light, 12-hour dark cycle. All procedures were in accordance with institution guidelines and approved by the Institutional Animal Care and Use Committee of the Veterans Affairs Palo Alto Health Care System. Before the experiments, these animals were fed a standard diet for 1 week to allow them to acclimatize to a controlled new environment (25°C ± 2°C, 55% ± 5% relative humidity with a 12-hour light, 12-hour dark cycle). For the detection of SNARE mRNA expression in mouse adrenals, C57BL/6J mice were injected with ACTH (Cortrosyn, 5 IU) sc every 24 hours for 4 days, with the ACTH injection on day 4 given 1 hour prior to the time the animals were sacrificed. For the isolation of rat primary granulosa cells, 20 3-week-old Sprague Dawley female rats were injected with 1 mg/rat 17β-estradiol sc for 4 consecutive days. Rats were sacrificed on the fifth day and the ovaries were removed for granulosa cell isolation.
Cell culture
Mouse Leydig tumor cell (MLTC) cells (obtained from American Type Culture Collection; catalog number ATTC-CRL-2065) were cultured with RPMI 1640 and supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Before RNA isolation, MLTC cells were treated with or without 2.5 mM dibutyryl cAMP (Bt2cAMP) for 24 hours. For the isolation and culture of rat primary granulosa cells, the granulosa cells were isolated by puncturing the ovaries with a 25-gauge needle, pooled, and collected by centrifugation. Granulosa cells were cultured for 72 hours (DMEM/F12 and 15 mM HEPES and supplemented with 2 μg/mL insulin, 5 μg/mL transferrin, 40 ng/mL hydrocortisone, 150 ng/mL dihydrotestosterone, and 100 U/mL penicillin and 100 μg/mL streptomycin) and then treated with or without 2.5 mM Bt2cAMP for 24 hours.
RNA isolation and quantitative real-time PCR analysis
For RNA isolation, rat ovary granulosa cells and MLTC cells were incubated with or without 2.5 mM Bt2cAMP for 24 hours and collected in 500 μL of TRIzol reagent. For mouse adrenals, after hormone treatment, adrenal samples were homogenized in 500 μL of TRIzol reagent using a power homogenizer (Ultra-Turrax T25; Labortechnik). After the ethanol precipitation step, total RNA was dissolved in 30 μL ribonuclease-free water, reamplified and converted to cDNA for real-time PCR analysis. Real-time PCR was performed with the cDNA prepared as above using an ABI Prism 8500 system using SYBR green master mix reagent (Applied Biosystems). The relative mass of specific RNA was calculated by the comparative cycle of threshold detection method according to the manufacturer's instruction. Genes examined included the following: VDAC1, VDAC3, StAR, SNAP23, SNAP25, NSF, α-SNAP, STX2, STX3, STX4, STX5, STX6, STX7, STX8, STX11, STX12, STX16, STX17, and STX18. Supplemental Table 1 shows the primer sets used for each gene.
Cloning, expression, and purification of recombinant proteins
SNARE proteins, including NSF, α-SNAP, SNAP23, SNAP25, syntaxin (STX)-5, STX7, and STX17 as well as StAR (N-62), were amplified by PCR from American Type Culture Collection cDNA clones using a forward primer and a reverse primer, both with BamH I restriction sites except NSF, which used NdeI restriction sites. The primers used and species (the species selected were based on the availability of the clones from American Type Culture Collection) are shown in Supplemental Table 2. The purified PCR fragments were digested with BamH I or NdeI and subcloned into pET15b. The expression vectors were then individually transformed into BL21DE3, and protein expression induced by 1 mM isopropyl-1-thio-β-D-galactopyranoside for 3 hours as previously described (38). The cells were collected by centrifugation at 2500 × g for 5 minutes, dissolved in lysis buffer (10 mM Tris; 130 mM NaCl; 1% Triton X-100; 10 mM NaF, pH 7.4) on ice for 45 minutes and sonicated three times with 10 bursts each. The cell lysates were centrifuged at 40000 × g for 45 minutes. Each of the proteins, except STX5 and STX7, was soluble and recovered in the supernatant. For protein purification, the supernatant was incubated with Ni-NTA agarose overnight at 4°C. The agarose beads were eluted with 6XHis-wash buffer and the purified individual proteins were stored at −20°C. The proteins were separated by SDS-PAGE to document the identity and purity of the individual proteins.
Isolation of mitochondria from rat adrenals
For isolation of mitochondria from rat adrenals, age-matched male rats were killed and adrenals removed and cleaned free of fat. Adrenals from six rats were pooled and finely minced with scissors and homogenized in ice-cold HEPES/sucrose buffer (10 mM HEPES; 250 mM sucrose, pH 7.4). The homogenate was centrifuged at 700 × g, 4°C, for 10 minutes, and then the supernatant was centrifuged at 12 000 × g, 4°C, for 15 minutes. The resultant pellet enriched in mitochondria was washed twice and resuspended in 400 μL HEPES/sucrose buffer. Mitochondria protein concentration was measured with a bicinchoninic assay protein assay kit, and mitochondria morphology was detected by electron microscopy (38). Mitochondrial purification and integrity of the OMM were determined by measurement of cytochrome C oxidase activity (39), whereas ER contamination was determined by dihydro nicotinamide adenine dinucleotide phosphate (NADPH)-dependent cytochrome C reductase activity (40). Both cytochrome C oxidase and NADPH-cytochrome C reductase assay kits were purchased from TriBiosciences Inc and carried out according to the manufacturer's instructions. To evaluate the mitochondrial integrity, aliquots of mitochondria were incubated with and without n-dodecyl β-D-maltoside prior to assay for cytochrome C oxidase activity. One unit of cytochrome C oxidase activity oxidizes 1.0 μmol of ferrocytochrome C per minute at pH 7.0 at 25°C. One unit of NADPH-cytochrome C-reductase activity reduces 1.0 μmol of oxidized cytochrome C in the presence of 100 μM of NADPH per minute at pH 7.8 at 25°C.
Cholesterol-phosphatidylcholine vesicles (lipid emulsion)
Cholesterol-phosphatidylcholine lipid emulsion was prepared by a slight modification of the procedure of Bloj and Zilversmit (41) as described previously (42). Briefly, 2.7 mg cholesterol was dissolved with 307 μL soybean L-α-phosphatidylcholine and dried under N2. The dried cholesterol was dissolved with 500 μL tauro buffer (10 mM K3PO4 and 5 mM taurocholic acid) and sonicated three times with 10 bursts each.
Mitochondria reconstitution assay
The reconstitution assay system was conducted as previously described (43) with modifications in a final volume of 100 μL. Each substrate was added into a glass tube as follows: HEPES/sucrose buffer, 2 μL 5 mM succinic acid, 2 μL lipid emulsion (10.8 μg cholesterol), 1 μg each SNARE protein, 10 μg GTP or GTPγs, ±100 μg cytosol, and 200 μg mitochondria. For pregnenolone detection, mitochondrial preparations were treated with trilostane (10 μM) or trilostane (5 μM) and abiraterone (10 μM) for 2.5 minutes at room temperature and then added into the reconstitution assay system. After 10 minutes, the reconstitution assay system was centrifuged at 1500 × g, 4°C, for 15 minutes, and the supernatant was collected for pregnenolone assay by ELISA using a kit from Alpco.
For cholesterol detection, mitochondrial preparations were treated with aminoglutethemide (100 μM) for 10 minutes at room temperature and then added into the reconstitution assay system. After a 1-hour reaction, mitochondria were collected by centrifugation and washed two times with HEPES/sucrose buffer. Mitochondria were laid on top of 2 mL of 0.4 M sucrose in centrifuge tubes and centrifuged at 10 000 × g, 4°C, for 15 minutes. After that, mitochondria were washed two times with HEPES/sucrose buffer. Lipid extraction from mitochondria was performed as described previously (44). Briefly, mitochondria were mixed with 20 times volume of chloroform/methanol (2:1) and vortexed. After that, 0.2 vol of 0.05% CaCl2 was added to the mixture. The organic phase was transferred to a new tube and dried under N2. The lipids were dissolved with 20 μL ethanol, and cholesterol was measured using a cholesterol LiquiColor test kit.
For detection of cholesterol movement to mitochondria by confocal microscopy, bodipy-CE was added to the lipid emulsion to label cholesterol and MitoTracker Red FM (Invitrogen) was added to mitochondria for 10 minutes. Then the reconstitution assay was conducted accordingly. After 10 minutes of reaction, the tubes was centrifuged at 1500 × g, 4°C, for 15 minutes, and mitochondria were collected for imaging. The mitochondria fluorescence images were obtained with a Zeiss LSM 7100 confocal microscope.
Transfections
SNAP23, SNAP25, STX-7, and α-SNAP silencer predesigned small interfering RNAs (siRNAs) were purchased from Ambion Life Technologies, Inc. STX-5 (sc-153994) and STX-17 siRNA (sc-153992) and scrambled siRNA were purchased from Santa Cruz Biotechnology. Scrambled siRNA was used as a negative control. Rat primary granulosa cells were used for transfection with SNAP25 α-SNAP, SNAP23, STX-7, STX-17 siRNA, and scrambled siRNA. MLTC cells were used for transfection with α-SNAP siRNA and scrambled siRNA. Cells were plated in 12-well culture plates and transfection was performed with PolyJet™ reagent (SignaGen Laboratories) following the manufacturer's protocol as described previously (45). Cells were cultured at 37°C, 5% CO2 for 6.5 hours, and then the medium was replaced with fresh medium with 2.5 mM Bt2cAMP and 500 μg/mL high-density lipoprotein. For a positive control, 5 μM 22α-hydroxycholesterol or 25-hydroxycholesterol was added to the media 5 hours before the media collection. After 48 hours, the media were collected for progesterone assay by an ELISA, and the cells were collected for Western blotting.
Statistics
Data are expressed as means ± SEM. Statistical analyses were performed by a one-way ANOVA using Prism 6.02 for Mac OS X (GraphPad Software, Inc). Differences between groups were considered statistically significant at P < .05.
Results
SNARE mRNA expression in steroidogenic cells and tissues
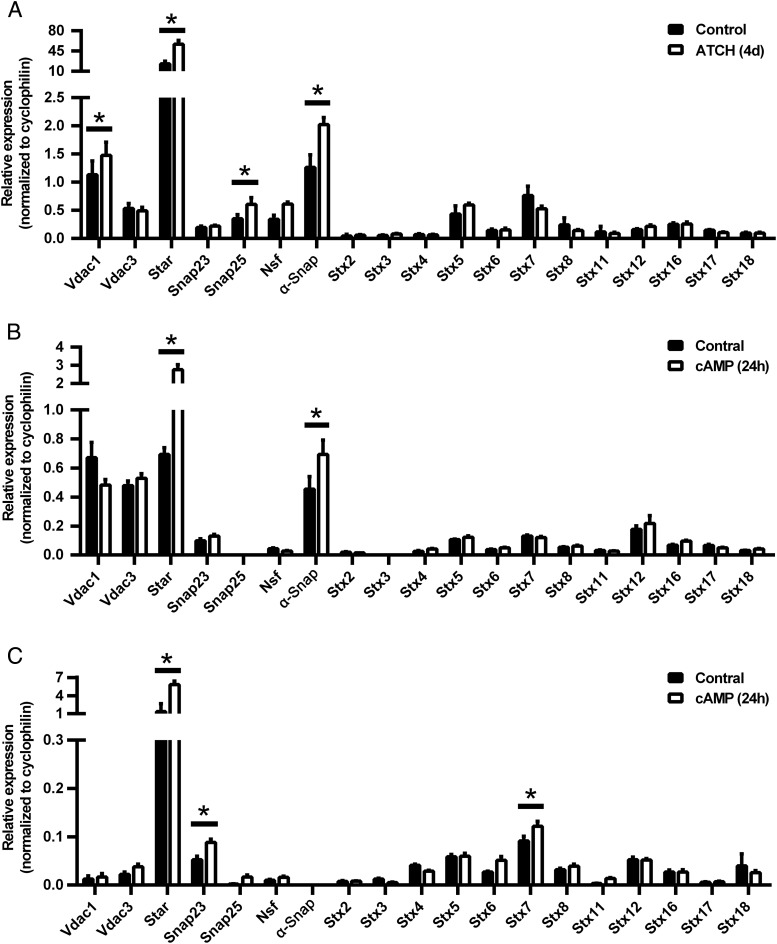
To analyze SNARE mRNA expression in steroidogenic tissues, mice were injected with ACTH (5 IU) or saline (control) daily for 4 days, adrenals were collected for analysis of mRNA expression 1 hour after the last dose, and each of the SNARE proteins examined was detected by quantitative RT-PCR. Compared with control, SNAP25 and α-SNAP mRNA expression was increased significantly (P < .05) along with StAR and voltage-dependent anion channel 1 (VDAC1), which functions with StAR (46), mRNA (Figure 1A). ACTH did not alter the expression of any of the other SNAREs examined. The control saline injections would be expected to increase endogenous ACTH secretion due to stress, leading to the potential attenuation of observed changes. To analyze SNARE mRNA expression in other steroidogenic cells, the MLTC line and rat primary granulosa cells were treated with or without 2.5 mM Bt2cAMP for 24 hours, and cells were collected for analysis of mRNA expression. The MLTC cell line expressed all of the SNAREs except SNAP25 and STX-3. α-SNAP mRNA, along with StAR, expression was increased significantly by Bt2cAMP treatment compared with control (Figure 1B, P < .05), without any changes in other SNAREs. Each of the SNARE proteins examined was detected by quantitative RT-PCR in rat primary granulosa cells, except α-SNAP (Figure 1C). SNAP23 and STX-7 mRNA expression, as well as StAR, was increased significantly with Bt2cAMP treatment compared with control (Figure 1C, P < .05). Thus, SNARE proteins are expressed in steroidogenic tissues and cells, albeit at generally low levels; however, there appears to be some cell-specific expression because granulosa cells do not express α-SNAP and MLTC cells do not express either SNAP25 or syntaxin-3. Moreover, the expression of some SNAREs appears to be responsive to hormone stimulation in a tissue-specific manner.
Figure 1.
SNARE mRNA expression in steroidogenic cells and tissues. A, Levels of SNARE mRNA in mouse adrenals. Mice were injected with ACTH or carrier daily for 4 days before mRNA analysis (n = 3). B, Levels of SNARE mRNA in MLTC cells. Results are representative of three independent experiments, each with an n = 5. C, Levels of SNARE mRNA in primary rat ovary granulosa cells. Results are representative of three independent experiments, each with an n = 5. In panels B and C, MLTC and rat ovary granulosa cells were incubated with or without 2.5 mM Bt2cAMP for 24 hours before mRNA analysis. *, P < .05; **, P < .01; ***, P < .001.
Features of the mitochondria reconstitution assay system
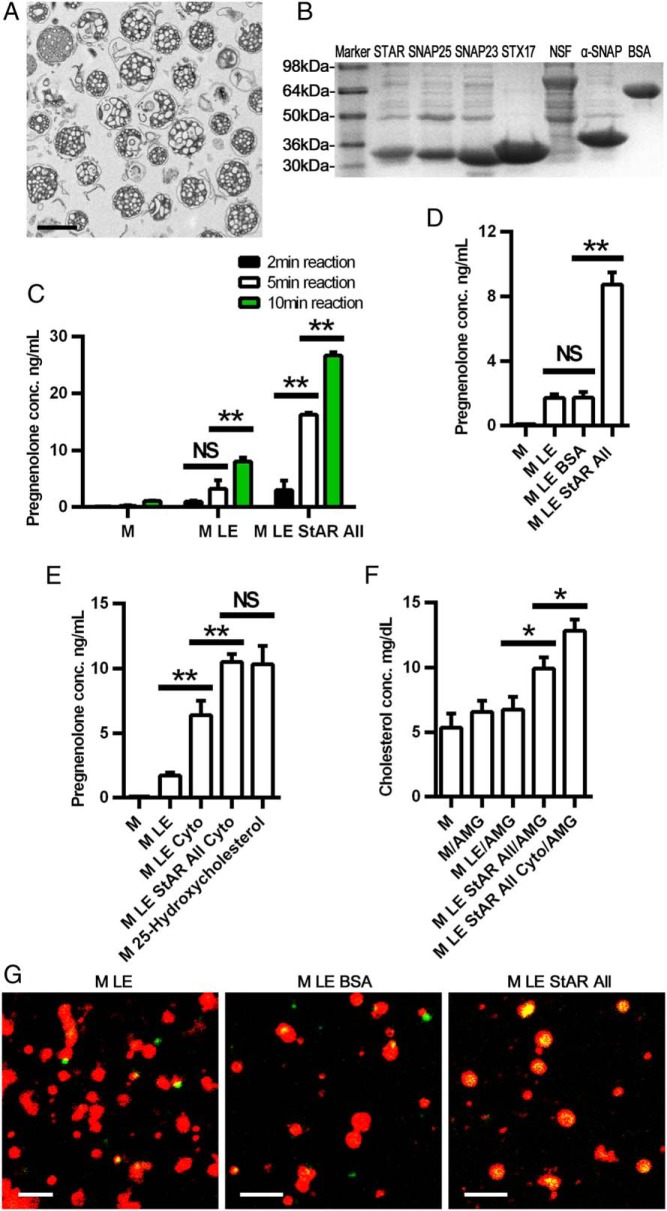
To establish a reconstitution assay system in vitro to analyze SNARE function on cholesterol movement to mitochondria, we modified a system previously described by Toaff et al (43). For these studies, mitochondria were first isolated from normal rat adrenals. Morphological examination of the isolated mitochondria revealed that the organelles were intact and relatively pure (Figure 2A), although some scattered membranous segments probably derived from smooth endoplasmic reticulum and plasma membranes were present. Analysis of the purification of the preparations based on the activities of cytochrome C oxidase and NADPH-dependent cytochrome C reductase showed substantial mitochondrial enrichment (6.6-fold) along with only a small contamination of ER (3%). Moreover, 92% of mitochondria in the preparations were intact, based on the activity of cytochrome C oxidase activity distribution. Second, recombinant StAR (N-62) and seven SNARE proteins, which are present in steroidogenic tissues including SNAP23, SNAP25, NSF, α-SNAP, STX-5, STX-7, and STX-17, were generated with an N-terminal His-tag and purified by Ni-NTA agarose chromatography. The species of origin of the recombinant proteins were selected as a matter of convenience based on the availability of clones from American Type Culture Collection. Additionally, it should be noted that the recombinant StAR used in these studies lacked the first 62 N-terminal amino acids, which mediate mitochondrial processing (47, 48); however, N-62 StAR retains cholesterol transport activity (49–52). Due to their insolubility in the bacterial expression system, STX-5 and STX-7 could not be further studied. Separation by SDS-PAGE (Figure 2B) documented the relative purity of each recombinant protein, with the size of each product slightly larger than the native protein due to the N-terminal tag.
Figure 2.
Establishment of a mitochondrial reconstitution assay system. A, Electron micrograph of isolated rat adrenal mitochondria. Scale bar, 1 μm. B, Purity of recombinant SNARE proteins after Ni-NTA agarose chromatography. C, Short time course of pregnenolone production. Mitochondria were pretreated with trilostane (5 μM) and abiraterone (10 μM) for 2.5 minutes at room temperature before being added to the reconstitution assay for the indicated times. D, Effects of BSA on pregnenolone production. The incubation was conducted for 10 minutes. E, Effects of recombinant SNARE proteins and adrenal cytosol (cyto) on pregnenolone production compared with 25-hydroxycholesterol. The incubation was conducted for 10 minutes. F, Effect of recombinant SNARE proteins and cytosol on cholesterol movement to mitochondria. Mitochondria were pretreated with 100 μM aminoglutethimide (AMG) for 10 minutes at room temperature before being added to the reconstitution assay and incubated for 1 hour. G, Confocal image of the effects of SNARE proteins and BSA on cholesterol movement to mitochondria (100 times). Mitochondria (red) were labeled with MitoTracker Red FM (Invitrogen), and cholesterol (green) was labeled with bodipy-CE. Scale bar, 2 μm. In panels D, E, and G, mitochondria were pretreated with trilostane (10 μM) for 2.5 minutes at room temperature before being added to the reconstitution assay. Pregnenolone was measured by an ELISA in each group. *, P < .05; **, P < .01, ***; P < .001. All, cocktail containing all recombinant SNARE proteins; LE, lipid emulsion; M, mitochondria. Results are representative of three independent experiments, each with an n = 3..
The characteristics of the reconstitution assay to detect cholesterol movement to mitochondria were then examined by incubating isolated mitochondria with a cholesterol emulsion, which functioned as model LDs, along with succinate for energy generation because ATP, a mitochondrial electrochemical gradient and temperature, had previously been shown to be needed for activity (20, 53, 54), and the recombinant proteins. As a measure of cholesterol movement into the interior of mitochondria, pregnenolone production was assayed (43) because mitochondrial CYP11A1 located on the IMM mediates the initial step in the conversion of cholesterol to steroids through the production of pregnenolone. First, we examined the time course of pregnenolone production with components of the reconstitution system (Figure 2C). Incubation of mitochondria alone resulted in a small increase in pregnenolone production with time, presumably due to the use of cholesterol present in mitochondrial membranes or in the small amounts of contaminating ER and plasma membranes. Addition of a cholesterol emulsion to the mitochondria significantly increased pregnenolone production at 10 minutes, reflecting greater delivery of cholesterol to mitochondria; however, the addition of recombinant StAR and SNAREs increased pregnenolone production further (P < .01). In preliminary experiments we noted that pregnenolone levels in the reconstitution assay diminished over time, presumably due to further metabolism of pregnenolone by 3β-hydroxysteroid dehydrogenase (3βHSD) and any 17α hydroxylase present in ER, contaminating the mitochondrial preparations. Addition of trilostane and/or abiraterone, inhibitors of 3βHSD and 17α hydroxylase, respectively, prevented further conversion of pregnenolone and were included in the reconstitution assay. To ensure that the increase in pregnenolone production reflects a specific action of the recombinant proteins and not simply the addition of protein to the system, the recombinant proteins were replaced by BSA in the reconstitution assay (Figure 2D); BSA had no effect on cholesterol movement to mitochondria when added along with mitochondria and cholesterol emulsion. However, when the recombinant StAR and SNAREs were added to the reconstitution assay system, pregnenolone production was robust (P < .01).
To evaluate the components of the reconstitution system further, the ability of adrenal cytosol, presumably containing all of the factors necessary to mediate cholesterol movement into mitochondria, was compared with the recombinant StAR and SNAREs (Figure 2E). As expected, the addition of adrenal cytosol to the mitochondria and cholesterol emulsion in the reconstitution assay significantly increased pregnenolone production (P < .01). Interestingly, the addition of recombinant StAR and SNAREs to the adrenal cytosol increased pregnenolone production further to levels observed with incubation with 25(R)-hydroxycholesterol alone, an oxysterol that can freely diffuse within cells and move into mitochondria without StAR. This observation might reflect the fact that the stoichiometry and concentrations of the recombinant StAR and SNAREs used in the in vitro assay likely do not accurately match those that exist in vivo.
As another means of documenting the movement of cholesterol into mitochondria in the reconstitution assay, we determined the effects of the different components of the assay on mitochondrial cholesterol content in the presence of aminoglutethimide, an inhibitor of CYP11A1. Mitochondria were pretreated with 100 μM aminoglutethimide for 10 minutes before added to the reconstitution assay system. After 1 hour of reaction, total lipids were extracted from mitochondria and cholesterol level was measured (Figure 2F). Mitochondrial cholesterol content did not change when mitochondria alone or mitochondria and cholesterol emulsion were included in the incubation; however, the addition of recombinant StAR and SNAREs significantly increased mitochondrial cholesterol levels (P < .05). Moreover, the combination of cytosol and the recombinant StAR and SNARE proteins further increased mitochondrial cholesterol content (P < .05). Confocal images visualizing cholesterol movement into the mitochondria showed similar results (Figure 2G). In this experiment mitochondria were labeled with MitoTracker (Invitrogen; red) and cholesterol was labeled with bodipy-CE (green). When BSA was included in the reconstitution system, there was little yellow signal (mitochondria and cholesterol signals merged) observed inside mitochondria. However, when recombinant StAR and SNARE proteins were included, substantial yellow signals were observed inside mitochondria, thus documenting the movement of cholesterol into mitochondria. It is noteworthy that StAR is normally phosphorylated during hormonal stimulation (55, 56), which modulates its activity (57); however, the bacterially produced StAR used in these studies was not phosphorylated, which might have contributed to the relatively small effect observed with addition of StAR in the reconstitution assay.
Effects of SNARE proteins on cholesterol movement to mitochondria
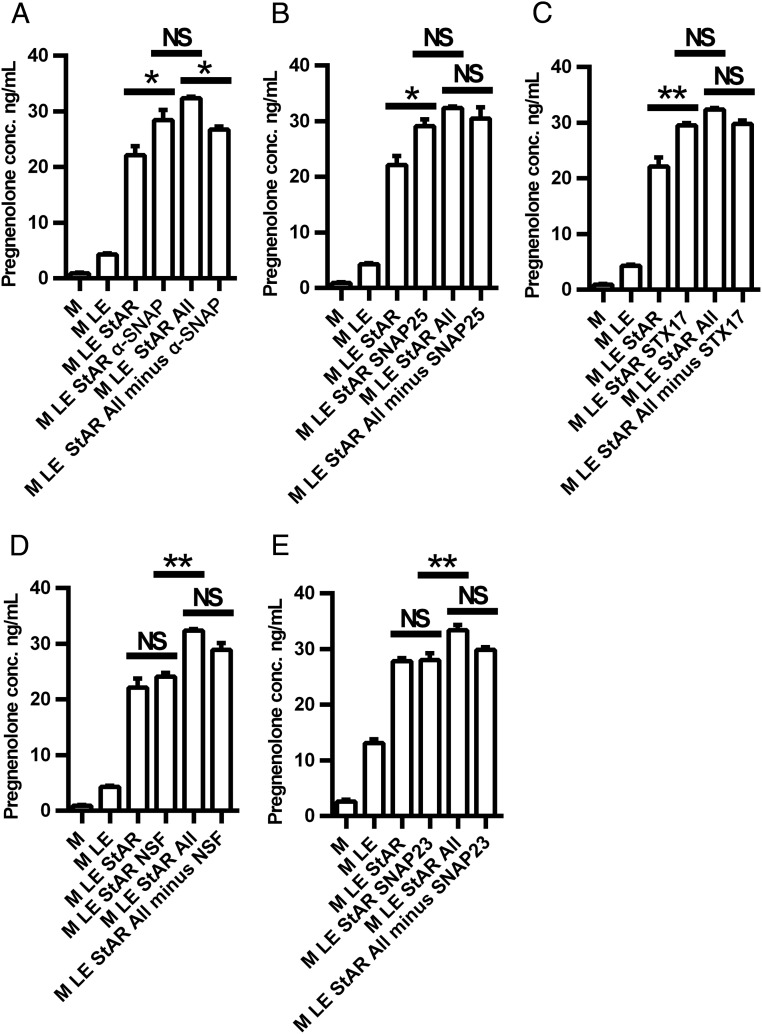
To characterize the function of the individual components studied in the reconstitution assay further, we next studied the impact of recombinant StAR in the reconstitution assay. Consistent with its known function to stimulate the movement of cholesterol from the OMM to the IMM, the addition of recombinant StAR to the reconstitution system containing mitochondria and cholesterol emulsion significantly (P < .01) increased pregnenolone production (Figure 3A). Interestingly, when the recombinant SNARE proteins were included in the reconstitution assay in the absence of StAR (Figure 3B), pregnenolone production was increased to a similar extent as observed with StAR alone (P < .01). The addition of StAR to the recombinant SNARE proteins increased pregnenolone production still further (P < .01). Confocal images of mitochondrial cholesterol movement confirmed these findings (Figure 3C). Inclusion of the recombinant SNARE proteins in the reconstitution assay increased mitochondrial cholesterol, as evidenced by the merged yellow signal; whereas the addition of StAR to the assay system along with the SNARE proteins increased mitochondrial cholesterol still further.
Figure 3.
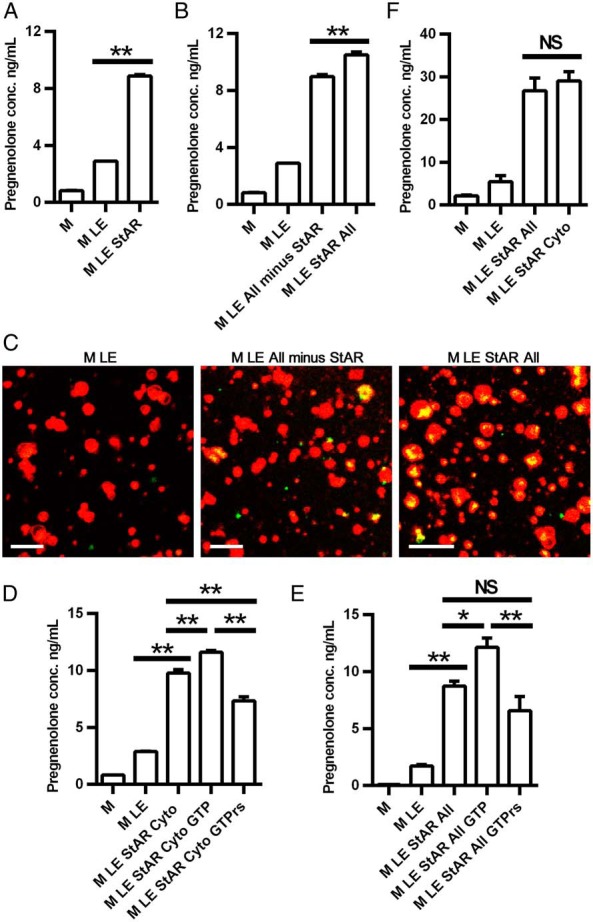
Effects of recombinant proteins on cholesterol movement to mitochondria. A and B, Effect of StAR on cholesterol movement to mitochondria. C, Confocal image of StAR-mediated cholesterol movement to mitochondria (100 times). Mitochondria (red) were labeled with MitoTracker Red FM (Invitrogen), and cholesterol (green) was labeled with bodipy-CE. Scale bar, 2 μm. D and E, Effects of GTP and GTPγs on SNARE function. F, Comparison of recombinant SNARE proteins and cytosol on cholesterol movement to mitochondria. In panels A, B, D, and E, mitochondria were pretreated with trilostane (10 μM) for 2.5 minutes at room temperature before being added to the reconstitution assay. In panel D, mitochondria were pretreated with trilostane (5 μM) and abiraterone (10 μM) for 2.5 minutes at room temperature before being added to the reconstitution assay. Pregnenolone was measured by an ELISA. *, P < .05; **, P < .01; ***, P < .001. All, cocktail containing all recombinant SNARE proteins; cyto, cytosol; LE, lipid emulsion; M, mitochondria. Results are representative of three to five independent experiments, each with an n = 3.
In view of the fact that SNARE proteins are GTP-binding proteins and binding and hydrolysis of GTP is implicated in the organization of membrane domains, membrane trafficking and the specific tethering of transport vesicles to target membranes, we tested the effects of GTP and GTPγs (a nonhydrolyzable GTP analog) on pregnenolone production in the reconstitution system. As shown in Figure 3D, when StAR and cytosol were present in the assay system, the addition of GTP increased pregnenolone production significantly (P < .01), whereas the addition of GTPγs decreased pregnenolone production significantly (P < .01), consistent with previous observations that GTP stimulates cholesterol metabolism by adrenal mitochondria (58). Because cytosol contains a number of components (SNAREs, GTP, etc) that might be required for cholesterol movement to and into mitochondria, we then examined the effects of GTP and GTPγs on pregnenolone production in the reconstitution system containing only mitochondria, cholesterol emulsion, and recombinant StAR and SNARE proteins. Under these conditions, GTP again increased pregnenolone production significantly (P < .01), whereas this was not observed with GTPγs (Figure 3E). Of note, the ability of the recombinant StAR and SNARE proteins to stimulate pregnenolone production in the reconstitution system was similar to that observed with the addition of cytosol along with recombinant StAR (Figure 3F). This suggests either that the five SNARE proteins constitute sufficient machinery required for cholesterol movement to and into mitochondria or that the concentrations of the SNARE proteins used in the reconstitution assay obscure the impact of other components present in cytosol.
To determine the relative contribution of the SNARE proteins to the movement of cholesterol to and into mitochondria for steroid production, we next tested the impact of each of the selected recombinant SNARE proteins individually and in combination (Figure 4, A–E). Thus, each of the recombinant SNAREs, NSF, α-SNAP, SNAP23, SNAP25, and STX-17, was added to the reconstitution assay system containing mitochondria, cholesterol emulsion, and recombinant StAR and pregnenolone production measured. As shown in Figure 4A, α-SNAP increased pregnenolone production significantly and omitting α-SNAP from the cocktail of SNARE proteins decreased pregnenolone production. Interestingly, the addition of either SNAP25 (Figure 4B) or STX-17 (Figure 4C) alone also increased pregnenolone production above that observed with recombinant StAR alone; however, omitting either SNAP25 or STX-17 from the cocktail of SNARE proteins had no effect. Neither the addition nor omission of NSF (Figure 4D) or SNAP23 (Figure 4E) affected pregnenolone production. Thus, α-SNAP appears to be a vital component contributing to the cholesterol movement to the mitochondria, whereas SNAP25 and STX-17 also appear to contribute to the cholesterol movement; however, other SNARE proteins can compensate for their actions.
Figure 4.
Effects of individual recombinant SNARE proteins on cholesterol movement to mitochondria. A, Effect of α-SNAP on cholesterol movement to mitochondria. B, Effect of SNAP25 on cholesterol movement to mitochondria. C, Effect of Stx17 on cholesterol movement to mitochondria. D, Effect of NSF on cholesterol movement to mitochondria. E, Effect of SNAP23 on cholesterol movement to mitochondria. Mitochondria were pretreated with trilostane (5 μM) and abiraterone (10 μM) for 2.5 minutes at room temperature before being added to the reconstitution assay. Pregnenolone was measured by ELISA. *, P < .05; **, P < .01; ***, P < .001. All, cocktail containing all recombinant SNARE proteins; LE, lipid emulsion; M, mitochondria. Results are representative of three independent experiments, each with an n = 3–4.
Effects of SNAREs knockdown on cholesterol movement to mitochondria
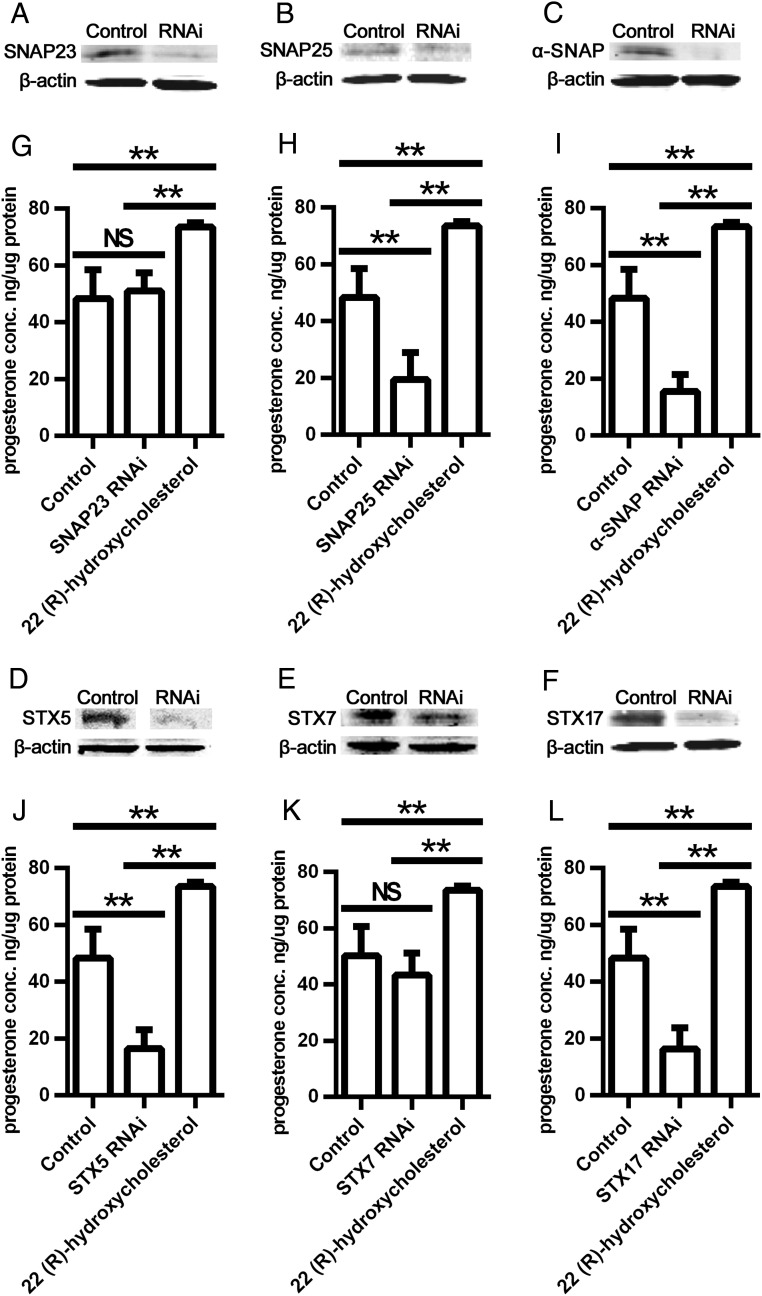
To further document the function of SNARE proteins on cholesterol movement to mitochondria for steroid production in steroidogenic cells, rat granulosa cells were isolated and used for transfection of siRNAs for SNAP23, SNAP25, STX-5, STX-7, and STX-17. Because α-SNAP was not detectable in rat granulosa cells, siRNA knockdown of α-SNAP was performed in cultured MLTC cells. The expression levels of SNARE proteins in transfected and control (scrambled siRNA) cells were detected by Western blot, and progesterone production and secretion into the media were assayed by ELISA. Compared with the control, SNAP23 protein expression was decreased significantly in granulosa cells transfected with SNAP23 siRNA (Figure 5A); however, there was no impact on progesterone production (Figure 5G), although 22-hydroxycholesterol increased progesterone. Compared with control, SNAP25 protein expression was decreased significantly in granulosa cells transfected with SNAP25 siRNA (Figure 5B), and the production of progesterone decreased significantly upon knockdown of SNAP25 (P < .01) but not with the negative control siRNA (Figure 5H). Similarly, expression levels of α-SNAP, STX-5, STX-7, and STX-17 proteins were all decreased significantly by transfection with each respective SNARE siRNA (Figure 5, C–F). Progesterone production was decreased in α-SNAP, STX-5, and STX-17 siRNA-transfected cells (P < .05); however, STX-7 siRNA did not affect progesterone production (Figure 5, I–L). Relatively similar results were obtained when siRNA knockdown experiments were performed in cultured MLTC cells, in which the knockdown of α-SNAP resulted in a decreased production of progesterone, whereas the knockdown of the other SNAREs (SNAP23, STX-7, and STX-17) did not affect progesterone production in the MLTC cells (data not shown).
Figure 5.
Effect of SNARE knockdown on cholesterol movement to mitochondria. A–F, Western blot of SNAP23, SNAP25, α-SNAP, STX-5, STX-7, and syntaxin-17 in cells transfected with scrambled (control) or the respective siRNA. G–L, Progesterone production in cells transfected with scrambled (control) or SNAP23, SNAP25, α-SNAP, STX-5, STX-7, or STX-17 siRNA. Primary rat granulosa cells were used in panels A, B, D–H, and J–L. MLTC cells were used in panels C and I. *, P < .05; **, P < .01; ***, P < .001. Results are representative of three independent experiments, each with an n = 3–4.
Discussion
Vesicular transport involving SNARE proteins is responsible for directing intracellular molecular trafficking to ensure a variety of cellular functions. Interactions between specific pairs of SNARE proteins located on different cellular compartments facilitate/target vesicular transfer. Upon acute hormone stimulation of steroidogenesis, there is a rapid transfer of cholesterol to the IMM to be used as substrate for CYP11A1, the initial enzymatic step in steroid biosynthesis that converts cholesterol to pregnenolone. In fact, the transfer of cholesterol from the OMM to the IMM, mediated primarily by StAR and its partners (25, 46), is the rate-limiting step in steroid biosynthesis. There are several different pathways that supply cholesterol for steroid production (4–6), including endogenous cholesterol biosynthesis in the ER, uptake of lipoprotein-derived cholesterol via LDL receptor-mediated endocytosis or via SR-B1-mediated selective uptake, and hydrolysis of CEs stored within LDs (6). Although the mechanisms responsible for cholesterol trafficking to mitochondria are not fully understood and would appear to differ, depending on the pathway involved in delivery of cholesterol (59), hydrolysis of CEs stored within LDs seems to be the preferred source for cholesterol, at least in the adrenal, after acute hormonal stimulation (10).
FC is produced by the hydrolysis of CEs derived from SR-B1-mediated uptake and from CEs stored within cytosolic LDs through the actions of HSL (8, 9). While generating FC that can traffic to mitochondria for steroidogenesis, HSL hydrolysis also leads to increased oxysterol production and activation of liver X receptors that up-regulate StAR transcription in a feed-forward fashion for steroidogenesis (60). Previous studies have observed that LDs and mitochondria traffic to be in close apposition after hormonal stimulation (28), suggesting that their close proximity could facilitate cholesterol transfer from LDs to mitochondria. Additional evidence supporting the concept that LD-mitochondrial interactions could be important for steroidogenesis have been obtained from studies examining TAG-rich LDs, in which research has indicated that protein-protein interactions between LDs and mitochondria could be involved in the process of fatty acid transfer to mitochondria for oxidation (32) and observations that components of SNARE complexes have been documented on lipid droplets (33). Although the association of LDs and ER (61), as well as LDs and mitochondria, has been previously reported, the involvement of specific SNARE proteins has not been documented. Our observations that ACTH increases the expression of SNAP25 and α-SNAP in the adrenals, whereas cAMP increases α-SNAP in Leydig cells and SNAP23 and syntaxin-7 in granulosa cells suggest the potential involvement of these SNARE members, as well as the various intracellular organelles associated with these SNARE proteins, in the steroidogenic pathway. Among the increased SNARE proteins, many of them have been shown to be involved in trafficking between Golgi, ER, and mitochondria. STX-17 has been shown to be abundant in steroidogenic cells and functions in a vesicle-trafficking step to the smooth ER (34). STX-17 has also been shown to function along with VDAC1 for autophagosome formation at ER-mitochondria contact sites (62). SNAP23 has been shown to form transient interactions with vesicle-associated membrane protein-2 in response to isoproterenol stimulation, to facilitate cAMP-mediated exocytosis (63) and to facilitate the interaction of TAG-rich LDs with mitochondria. The short form of STX-5 has been shown to reside in the Golgi complex and to be involved in mediating transport to the Golgi (64), and the longer form has also been identified in the ER and shown to contribute to the organization of the ER structure by interacting with both cytoskeleton-linking membrane protein (CLIMP)-63 and microtubules (65). NSF is associated with Golgi (66) and reported to be important for facilitating LD fusion (32).
In vitro reconstitution for study of vesicular transport in cell free systems has been used as an important tool for elucidating the molecular mechanisms of intracellular protein trafficking (67, 68). In our effort to further examine the role of some of the SNARE proteins in the process of transferring cholesterol to mitochondria, we refined a system in which production of pregnenolone in isolated mitochondria was used as the measure of successful transfer of cholesterol to the mitochondria. In this system, cholesterol in the form of an emulsion, ie, model LDs, was provided as substrate for CYP11A1, and succinate was used as a source for energy because ATP, a mitochondrial electrochemical gradient and temperature had previously been shown to be needed for activity (20, 53, 54). Trilostane and abiraterone were added as inhibitors of 3βHSD and 17α hydroxylase, respectively, to prevent the further conversion of pregnenolone. Therefore, even though SNAREs might be involved in multiple steps along the steroidogenic pathway, the in vitro system that we used exclusively targeted early steps in steroidogenesis of cholesterol movement to OMM and then to the IMM and CYP11A1. Because StAR is required for steroidogenesis by facilitating the movement of cholesterol from the OMM to the IMM (1, 25), it was included in the system. Our results reconfirmed that StAR was essential for the transfer of cholesterol for steroidogenesis, further validating our in vitro reconstitution system. It is noteworthy that StAR is normally phosphorylated during hormonal stimulation (55, 56), which modulates its activity (57). Whereas the bacterially produced StAR used in these studies was not phosphorylated, which might have contributed to the relatively small effect observed with addition of StAR in the reconstitution assay, there are several reports showing that recombinant nonphosphorylated StAR is highly effective in stimulating pregnenolone production in reconstituted mitochondrial systems (50, 69, 70). When the contribution of each of the SNARE proteins to the transfer of cholesterol to mitochondria was evaluated by either the addition of individual purified recombinant SNARE proteins or the elimination of each of the proteins from a cocktail containing all of the recombinant proteins, α-SNAP, SNAP25, and STX-17 all appeared to facilitate cholesterol movement and pregnenolone production. These SNARE proteins are all GTP-binding proteins, and they responded to GTP treatment in the reconstitution assay system with increased pregnenolone production, whereas a nonhydrolyzable GTP analog, GTPβs, was ineffective. These findings were consistent with previous observations that GTP stimulates cholesterol metabolism by adrenal mitochondria (58).
Even though StAR is needed for the movement of cholesterol from the OMM to the IMM for steroid production, it is remarkable that a cocktail containing all of the recombinant SNARE proteins examined in our studies was capable of stimulating mitochondrial cholesterol trafficking and pregnenolone production in the absence of StAR to a similar degree as observed when StAR was present. This observation implies that, when pathways are able to provide ample mitochondrial cholesterol enrichment of the OMM, induction of StAR is not required for maximum steroid synthesis. Nonetheless, in vivo data have confirmed the essential requirement for StAR for steroidogenesis (26, 27). Thus, it is possible that our observation that the ability of the cocktail containing all of the recombinant SNARE proteins was capable of stimulating mitochondrial cholesterol trafficking and pregnenolone production in the absence of StAR to a similar degree as observed when StAR was present might be a result of the artificial conditions of the in vitro reconstitution system in which the concentrations and stoichiometry of the SNAREs likely exceed those occurring in vivo. Hence, the in vitro conditions might have enabled the SNAREs to traffic sufficiently large amounts of cholesterol to the OMM that then moved to the IMM by mass diffusion or possibly impacted the integrity of the OMM to stimulate cholesterol movement to the IMM. The fact that a subset of SNAREs, including STX-5, display cholesterol binding properties (71) could provide a possible mechanism for their involvement in LD-cholesterol trafficking by their directly mediating unesterified cholesterol transfer to the OMM.
Knockdown of each of the individual SNARE proteins in primary granulosa cells and in the MLTC cell line confirmed the importance of α-SNAP, SNAP25 and STX-17 in steroid production. Although we were not able to assess the role of STX-5 in our in vitro reconstitution assay due to difficulties in expressing recombinant STX-5 in a soluble form, knockdown of STX-5 in primary granulosa cells revealed its importance for steroidogenesis. Whereas these knockdown experiments documented the involvement of these SNAREs in steroidogenesis and, based on our in vitro reconstitution experiments, suggest a role for these SNAREs in mediating cholesterol trafficking to the mitochondria, it is possible that the SNAREs could impact additional steps in steroidogenesis in the cell knockdown studies. It is noteworthy that SNAP23, which has previously been shown to facilitate the interaction of TAG-rich LDs with mitochondria (33), had no apparent effects on cholesterol movement to mitochondria or pregnenolone production. Thus, CE-rich and TAG-rich LDs not only differ in their lipid and proteomic compositions (72–74) but also display differences in the metabolic pathways and cell machinery involved in their metabolism, signifying the necessity for caution when drawing inferences from observations in TAG-rich LDs applied to CE-rich LDs.
In conclusion, using a mitochondria reconstitution assay system to examine cholesterol movement to mitochondria in vitro, we demonstrated that SNARE proteins promote functional interactions between model LDs and mitochondria, facilitating the transport of cholesterol from LDs to mitochondria. In combination with knockdown experiments, α-SNAP, SNAP25, and STX-5 and STX-17 were shown to be vital components contributing to cholesterol movement to mitochondria in support of the action of StAR and steroid production.
Additional material
Supplementary data supplied by authors.
Acknowledgments
Current address for Y.L. and X.H.: College of Life Science, Northeast Agricultural University, Harbin, China.
Current address for R.H.: Munich Medical School, Munich, Germany.
Current address for V.K.K.: Genentech, Inc, South San Francisco, CA, USA.
This work was supported by grants from the Department of Veterans Affairs, Office of Research and Development, Medical Research Service (to S.A. and F.B.K.) and Grant 2R01HL33881 (to S.A.) from the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Bt2cAMP
- dibutyryl cAMP
- CE
- cholesteryl ester
- ER
- endoplasmic reticulum
- FC
- free cholesterol
- 3βHSD
- 3β-hydroxysteroid dehydrogenase
- HSL
- hormone-sensitive lipase
- IMM
- inner mitochondrial membrane
- LD
- lipid droplet
- LDL
- low-density lipoprotein
- MLTC
- mouse Leydig tumor cell
- NADPH
- dihydro nicotinamide adenine dinucleotide phosphate
- NSF
- N-ethylmaleimide-sensitive factor
- OMM
- outer mitochondrial membrane
- siRNA
- small interfering RNA
- SNARE
- soluble NSF attachment protein receptor
- SNAP
- soluble NSF attachment protein
- SNAP23
- synaptosomal-associated protein of 23 kDa
- SNAP25
- synaptosomal-associated protein of 25 kDa
- SR-B1
- scavenger receptor, class B type 1
- StAR
- steroidogenic acute regulatory protein
- STX
- syntaxin
- TAG
- triacylglycerol
- VAMP-4
- vesicle-associated membrane protein-4
- VDAC1
- voltage-dependent anion channel 1.
References
- 1. Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res. 2011;52:2111–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davis WW, Garren LD. On the mechanism of action of adrenocorticotropic hormone. The inhibitory site of cycloheximide in the pathway of steroid biosynthesis. J Biol Chem. 1968;243:5153–5157. [PubMed] [Google Scholar]
- 3. Garren LD, Ney RL, Davis WW. Studies on the role of protein synthesis in the regulation of corticosterone production by adrenocorticotropic hormone in vivo. Proc Natl Acad Sci USA. 1965;53:1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andersen JM, Dietschy JM. Relative importance of high and low density lipoproteins in the regulation of cholesterol synthesis in the adrenal gland, ovary, and testis of the rat. J Biol Chem. 1978;253:9024–9032. [PubMed] [Google Scholar]
- 5. Gwynne JT, Strauss JF., III The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev. 1982;3:299–329. [DOI] [PubMed] [Google Scholar]
- 6. Kraemer FB. Adrenal cholesterol utilization. Mol Cell Endocrinol. 2007;265–266:42–45. [DOI] [PubMed] [Google Scholar]
- 7. Faust JR, Goldstein JL, Brown MS. Receptor-mediated uptake of low density lipoprotein and utilization of its cholesterol for steroid synthesis in cultured mouse adrenal cells. J Biol Chem. 1977;252:4861–4871. [PubMed] [Google Scholar]
- 8. Connelly MA, Kellner-Weibel G, Rothblat GH, Williams DL. SR-BI-directed HDL-cholesteryl ester hydrolysis. J Lipid Res. 2003;44:331–341. [DOI] [PubMed] [Google Scholar]
- 9. Kraemer FB, Shen WJ, Harada K, et al. Hormone-sensitive lipase is required for high-density lipoprotein cholesteryl ester-supported adrenal steroidogenesis. Mol Endocrinol. 2004;18:549–557. [DOI] [PubMed] [Google Scholar]
- 10. Vahouny GV, Chanderbhan R, Hinds R, Hodges VA, Treadwell CR. ACTH-induced hydrolysis of cholesteryl esters in rat adrenal cells. J Lipid Res. 1978;19:570–577. [PubMed] [Google Scholar]
- 11. Kraemer FB, Shen W-J, Natu V, et al. Adrenal neutral cholesteryl hydrolase: identification, subcellular distribution and sex differences. Endocrinology. 2002;143:801–806. [DOI] [PubMed] [Google Scholar]
- 12. Kraemer FB, Shen WJ, Patel S, Osuga J, Ishibashi S, Azhar S. The LDL receptor is not necessary for acute adrenal steroidogenesis in mouse adrenocortical cells. Am J Physiol Endocrinol Metab. 2007;292:E408–E412. [DOI] [PubMed] [Google Scholar]
- 13. Verschoor-Klootwyk AH, Verchoor L, Azhar S, Reaven GM. Role of exogenous cholesterol in regulation of adrenal steroidogenesis in the rat. J Biol Chem. 1982;257:7666–7671. [PubMed] [Google Scholar]
- 14. Xie C, Richardson JA, Turley SD, Dietschy JM. Cholesterol substrate pools and steroid hormone levels are normal in the face of mutational inactivation of NPC1 protein. J Lipid Res. 2006;47:953–963. [DOI] [PubMed] [Google Scholar]
- 15. Jefcoate CR, McNamara BC, Artemenko I, Yamazaki T. Regulation of cholesterol movement to mitochondrial cytochrome P450scc in steroid hormone synthesis. J Steroid Biochem Mol Biol. 1992;43:751–767. [DOI] [PubMed] [Google Scholar]
- 16. Miller WL. StAR search—what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol Endocrinol. 2007;21:589–601. [DOI] [PubMed] [Google Scholar]
- 17. Rone MB, Fan J, Papadopoulos V. Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochim Biophys Acta. 2009;1791:646–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crivello JF, Jefcoate CR. Intracellular movement of cholesterol in rat adrenal cells. Kinetics and effects of inhibitors. J Biol Chem. 1980;255:8144–8151. [PubMed] [Google Scholar]
- 19. Krueger RJ, Orme-Johnson NR. Acute adrenocorticotropic hormone stimulation of adrenal corticosteroidogenesis. Discovery of a rapidly induced protein. J Biol Chem. 1983;258:10159–10167. [PubMed] [Google Scholar]
- 20. Paul DP, Gallant S, Orme-Johnson NR, Orme-Johnson WH, Brownie AC. Temperature dependence of cholesterol binding to cytochrome P-450scc of the rat adrenal. Effect of adrenocorticotropic hormone and cycloheximide. J Biol Chem. 1976;251:7120–7126. [PubMed] [Google Scholar]
- 21. Privalle CT, Crivello JF, Jefcoate CR. Regulation of intramitochondrial cholesterol transfer to side-chain cleavage cytochrome P-450 in rat adrenal gland. Proc Natl Acad Sci USA. 1983;80:702–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller WL. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim Biophys Acta. 2007;1771:663–676. [DOI] [PubMed] [Google Scholar]
- 23. Stocco DM. Intramitochondrial cholesterol transfer. Biochim Biophys Acta. 2000;1486:184–197. [DOI] [PubMed] [Google Scholar]
- 24. Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem. 1994;269:28314–28322. [PubMed] [Google Scholar]
- 25. Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol. 2001;63:193–213. [DOI] [PubMed] [Google Scholar]
- 26. Bose HS, Sugawara T, Strauss JF, III, Miller WL. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. N Engl J Med. 1996;335:1870–1878. [DOI] [PubMed] [Google Scholar]
- 27. Caron KM, Soo SC, Wetsel WC, Stocco DM, Clark BJ, Parker KL. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci USA. 1997;94:11540–11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Merry BJ. Mitochondrial structure in the rat adrenal cortex. J Anat. 1975;119:611–618. [PMC free article] [PubMed] [Google Scholar]
- 29. Murphy S, Martin S, Parton RG. Lipid droplet-organelle interactions; sharing the fats. Biochim Biophys Acta. 2009;1791:441–447. [DOI] [PubMed] [Google Scholar]
- 30. Walther TC, Farese RV., Jr The life of lipid droplets. Biochim Biophys Acta 2009;1791:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boström P, Andersson L, Rutberg M, et al. SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nat Cell Biol. 2007;9:1286–1293. [DOI] [PubMed] [Google Scholar]
- 33. Jägerström S, Polesie S, Wickström Y, et al. Lipid droplets interact with mitochondria using SNAP23. Cell Biol Int. 2009;33:934–940. [DOI] [PubMed] [Google Scholar]
- 34. Steegmaier M, Oorschot V, Klumperman J, Scheller RH. Syntaxin 17 is abundant in steroidogenic cells and implicated in smooth endoplasmic reticulum membrane dynamics. Mol Biol Cell. 2000;11:2719–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grant NJ, Hepp R, Krause W, Aunis D, Oehme P, Langley K. Differential expression of SNAP-25 isoforms and SNAP-23 in the adrenal gland. J Neurochem. 1999;72:363–372. [DOI] [PubMed] [Google Scholar]
- 36. Jo M, Gieske MC, Payne CE, et al. Development and application of a rat ovarian gene expression database. Endocrinology. 2004;145:5384–5396. [DOI] [PubMed] [Google Scholar]
- 37. Grosse J, Bulling A, Brucker C, Berg U, Amsterdam A, Mayerhofer A, Gratzl M. Synaptosome-associated protein of 25 kilodaltons in oocytes and steroid-producing cells of rat and human ovary: molecular analysis and regulation by gonadotropins. Biol Reprod. 2000;63:643–650. [DOI] [PubMed] [Google Scholar]
- 38. Solinas P, Fujioka H, Tandler B, Hoppel CL. Isolation of rat adrenocortical mitochondria. Biochem Biophys Res Commun. 2012;427:96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thompson DA, Ferguson-Miller S. Lipid and subunit III depleted cytochrome c oxidase purified by horse cytochrome c affinity chromatography in lauryl maltoside. Biochemistry. 1983;22:3178–3187. [DOI] [PubMed] [Google Scholar]
- 40. Vermilion JL, Coon MJ. Highly purified detergent-solubilized NADPH-cytochrome P-450 reductase from phenobarbital-induced rat liver microsomes. Biochem Biophys Res Commun. 1974;60:1315–1322. [DOI] [PubMed] [Google Scholar]
- 41. Bloj B, Zilversmit DB. Complete exchangeability of cholesterol in phosphatidylcholine/cholesterol vesicles of different degrees of unsaturation. Biochemistry. 1977;16:3943–3948. [DOI] [PubMed] [Google Scholar]
- 42. Prokocimer PG, Maze M, Vickery RG, Kraemer FB, Gandjei R, Hoffman BB. Mechanism of halothane-induced inhibition of isoproterenol-stimulated lipolysis in isolated rat adipocytes. Mol Pharmacol. 1988;33:338–343. [PubMed] [Google Scholar]
- 43. Toaff ME, Strauss JF, 3rd, Flickinger GL, Shattil SJ. Relationship of cholesterol supply to luteal mitochondrial steroid synthesis. J Biol Chem. 1979;254:3977–3982. [PubMed] [Google Scholar]
- 44. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 45. Hu Z, Hu J, Zhang Z, et al. Regulation of expression and function of scavenger receptor class B, type I (SR-BI) by Na+/H+ exchanger regulatory factors (NHERFs). J Biol Chem. 2013;288:11416–11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bose M, Whittal RM, Miller WL, Bose HS. Steroidogenic activity of StAR requires contact with mitochondrial VDAC1 and phosphate carrier protein. J Biol Chem. 2008;283:8837–8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Artemenko IP, Zhao D, Hales DB, Hales KH, Jefcoate CR. Mitochondrial processing of newly synthesized steroidogenic acute regulatory protein (StAR), but not total StAR, mediates cholesterol transfer to cytochrome P450 side chain cleavage enzyme in adrenal cells. J Biol Chem. 2001;276:46583–46596. [DOI] [PubMed] [Google Scholar]
- 48. Epstein LF, Orme-Johnson NR. Regulation of steroid hormone biosynthesis. Identification of precursors of a phosphoprotein targeted to the mitochondrion in stimulated rat adrenal cortex cells. J Biol Chem. 1991;266:19739–19745. [PubMed] [Google Scholar]
- 49. Tuckey RC, Headlam MJ, Bose HS, Miller WL. Transfer of cholesterol between phospholipid vesicles mediated by the steroidogenic acute regulatory protein (StAR). J Biol Chem. 2002;277:47123–47128. [DOI] [PubMed] [Google Scholar]
- 50. Arakane F, Kallen CB, Watari H, et al. The mechanism of action of steroidogenic acute regulatory protein (StAR). StAR acts on the outside of mitochondria to stimulate steroidogenesis. J Biol Chem. 1998;273:16339–16345. [DOI] [PubMed] [Google Scholar]
- 51. Arakane F, Sugawara T, Strauss JF., III Steroidogenic acute regulatory protein (StAR) retains activity in the absence of its mitochondrial import sequence: Implications for the mechanism of StAR action. Proc Natl Acad Sci USA. 1996;93:13731–13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bose HS, Lingappa VR, Miller WL. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature. 2002;417:87–91. [DOI] [PubMed] [Google Scholar]
- 53. Hanukoglu I, Privalle CT, Jefcoate CR. Mechanisms of ionic activation of adrenal mitochondrial cytochromes P-450scc and P-45011β. J Biol Chem. 1981;256:4329–4335. [DOI] [PubMed] [Google Scholar]
- 54. King SR, Stocco DM. ATP and a mitochondrial electrochemical gradient are required for functional activity of the steroidogenic acute regulatory (StAR) protein in isolated mitochondria. Endocr Res. 1996;22:505–514. [DOI] [PubMed] [Google Scholar]
- 55. Pon LA, Hartigan JA, Orme-Johnson NR. Acute ACTH regulation of adrenal corticosteroid biosynthesis. Rapid accumulation of a phosphoprotein. J Biol Chem. 1986;261:13309–13316. [PubMed] [Google Scholar]
- 56. Pon LA, Orme-Johnson NR. Acute stimulation of corpus luteum cells by gonadotrophin or adenosine 3′,5′-monophosphate causes accumulation of a phosphoprotein concurrent with acceleration of steroid synthesis. Endocrinology. 1988;123:1942–1948. [DOI] [PubMed] [Google Scholar]
- 57. Arakane F, King SR, Du Y, et al. Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J Biol Chem. 1997;272:32656–32662. [DOI] [PubMed] [Google Scholar]
- 58. Kowluru R, Yamazaki T, McNamara BC, Jefcoate CR. Metabolism of exogenous cholesterol by rat adrenal mitochondria is stimulated equally by physiological levels of free Ca2+ and by GTP. Mol Cell Endocrinol. 1995;107:181–188. [DOI] [PubMed] [Google Scholar]
- 59. Prasad M, Kaur J, Pawlak KJ, Bose M, Whittal RM, Bose HS. Mitochondria-associated endoplasmic reticulum membrane (MAM) regulates steroidogenic activity via steroidogenic acute regulatory protein (StAR)-voltage-dependent anion channel 2 (VDAC2) interaction. J Biol Chem. 2015;290:2604–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Manna PR, Cohen-Tannoudji J, Counis R, et al. Mechanisms of action of hormone-sensitive lipase in mouse Leydig cells: its role in the regulation of the steroidogenic acute regulatory protein. J Biol Chem. 2013;288:8505–8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Robenek H, Hofnagel O, Buers I, Robenek MJ, Troyer D, Severs NJ. Adipophilin-enriched domains in the ER membrane are sites of lipid droplet biogenesis. J Cell Sci. 2006;119:4215–4224. [DOI] [PubMed] [Google Scholar]
- 62. Hamasaki M, Furuta N, Matsuda A. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013;495:389–393. [DOI] [PubMed] [Google Scholar]
- 63. Takuma T, Shitara A, Arakawa T, Okayama M, Mizoguchi I, Tajima Y. Isoproterenol stimulates transient SNAP23-VAMP2 interaction in rat parotid glands. FEBS Lett. 2013;587:583–589. [DOI] [PubMed] [Google Scholar]
- 64. Nonnenmacher ME, Cintrat JC, Gillet D, Weber T. Syntaxin 5-dependent retrograde transport to the trans-Golgi network is required for adeno-associated virus transduction. J Virol. 2015;89:1673–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Miyazaki K, Wakana Y, Noda C, Arasaki K, Furuno A, Tagaya M. Contribution of the long form of syntaxin 5 to the organization of the endoplasmic reticulum. J Cell Sci. 2012;125:5658–5666. [DOI] [PubMed] [Google Scholar]
- 66. Glick BS, Rothman JE. Possible role for fatty acyl-coenzyme A in intracellular protein transport. Nature. 1987;326:309–312. [DOI] [PubMed] [Google Scholar]
- 67. Balch WE, Dunphy WG, Braell WA, Rothman JE. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. [DOI] [PubMed] [Google Scholar]
- 68. Rothman JE, Urbani LJ, Brands R. Transport of protein between cytoplasmic membranes of fused cells: correspondence to processes reconstituted in a cell-free system. J Cell Biol. 1984;99:248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Huang MC, Miller WL. Creation and activity of COS-1 cells stably expressing the F2 fusion of the human cholesterol side-chain cleavage enzyme system. Endocrinology. 2001;142:2569–2576. [DOI] [PubMed] [Google Scholar]
- 70. Tuckey RC, Bose HS, Czerwionka I, Miller WL. Molten globule structure and steroidogenic activity of N-218 MLN64 in human placental mitochondria. Endocrinology. 2004;145:1700–1707. [DOI] [PubMed] [Google Scholar]
- 71. Enrich C, Rentero C, Hierro A, Grewal T. Role of cholesterol in SNARE-mediated trafficking on intracellular membranes. J Cell Sci. 2015;128:1071–1081. [DOI] [PubMed] [Google Scholar]
- 72. Bartz R, Li WH, Venables B, et al. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J Lipid Res. 2007;48:837–847. [DOI] [PubMed] [Google Scholar]
- 73. Khor VK, Ahrends R, Lin Y, et al. The proteome of cholesteryl-ester-enriched versus triacylglycerol-enriched lipid droplets. PLoS One. 2014;9:e105047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yang L, Ding Y, Chen Y, et al. The proteomics of lipid droplets: structure, dynamics, and functions of the organelle conserved from bacteria to humans. J Lipid Res. 2012;53:1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.