Abstract
A miniaturized neuraminidase inhibition (NI) assay using HA-mismatched H6 reassortant viruses was performed to examine the neuraminidase (NA)-specific antibody response in ferrets immunized with live-attenuated influenza vaccine (LAIV) strains. The strains tested possessed different NAs derived from seasonal H1N1 and H3N2, 2009 pandemic H1N1, and the highly pathogenic influenza H5N1 virus. The anti-NA antibodies from the 2009 pandemic strain (A/California/7/2009) immunized ferrets cross-reacted with the NA of H5N1 but not with the NA of seasonal H1N1 viruses. The plaque size reduction assay confirmed the cross-reactivity between the NAs of A/California/7/2009 and the H5N1 virus. Sequence and structural analyses of these N1 NA proteins showed that the NA of the 2009 pandemic H1N1 strain shared at least 22 more amino acids in the head domain with the NAs of the avian H5N1 strains than with the NAs of seasonal human H1N1 viruses. Our data demonstrated LAIV-induced NA antibody responses in ferrets and cross-reactive NA antibodies induced by 2009 pandemic H1N1 and H5N1 LAIV viruses.
Keywords: influenza virus, live-attenuated influenza vaccine, ferret, anti-neuraminidase antibody response, neuraminidase inhibition assay, cross-reactivity
Introduction
The hemagglutinin (HA) and neuraminidase (NA) are the two major surface integral membrane glycoproteins of influenza viruses. HA binds to sialic acid-containing receptors on target cells and initiates viral entry. NA catalyzes the removal of terminal sialic acid residues from oligosaccharide chains, thereby facilitating virus release from infected cells and spread in a host [1, 2]. In addition, NA may also be important in the early stage of infection by promoting virus entry as demonstrated in human airway epithelial cells [3]. The functional balance between the receptor binding of the HA and the receptor destroying property of NA is critical for efficient viral replication [4, 5].
Although the HA protein is known to be the most important protective antigen of the virus and HA antibodies can prevent viral infection, NA antibodies that inhibit NA activity are also considered to play an important role in inhibiting viral release and cell-to-cell spread thereby limiting viral replication [6–8]. NA-specific immunity was reported to reduce the severity of clinical illness and viral shedding among infected persons [9–12]. Recombinant NA proteins produced in different expression systems were also shown to induce immune responses that protect against influenza virus infection in animal models [13–15]. Immunization with both the HA and NA proteins elicited a broader protective immunity than HA protein alone, supporting the inclusion of NA as a vaccine component [16–19].
Live attenuated influenza vaccines (LAIV) and some inactivated influenza vaccines contain both the HA and NA antigens. However, immune responses following vaccination have been most commonly assessed by the hemagglutination inhibition (HAI) assay that only detects antibodies to HA. The contribution of NA antibodies in vaccine-mediated immune responses has been largely ignored due to the lack of a reliable NI assay. Recently, Sandbulte et al. [20] reported a 96-well plate format NI assay miniaturized from the conventional colorimetric NI assay using fetuin as a substrate. To eliminate any inhibition caused by HA antibody, virus reassortants were generated by reverse genetics that contain the HA from a different HA subtype and the NA from the testing virus. The assay was able to detect NA antibodies in vaccinated ferrets and clinical samples [20, 21]. In this study, an antigenically distinct H6 HA was chosen to produce reassortant viruses that contain different NA segments from various viruses to detect NA antibodies in LAIV immunized ferrets using the miniaturized NI assay. The antigenic relatedness of these NAs and cross-reactivity between the H1N1 and H5N1 viruses were analyzed.
Materials and Methods
Ethics Statement
All ferret studies were conducted in an AAALAC certified facility in accordance with protocols approved by MedImmune’s Institutional Animal Care and Use Committee (IACUC). The detailed ferret study protocol in this study was approved by IACUC with a protocol ID ACF-081.
Influenza viruses and post-infection ferret serum
The HA and NA cDNAs of influenza A viruses were amplified by reverse transcription-polymerase chain reaction (RT-PCR) using vRNA as the template and cloned between the two BsmB I sites in the pAD3000 vector [22]. The HA and NA gene plasmids were cloned from seasonal wild-type (wt) influenza A viruses obtained from the Centers for Disease Control and Prevention (CDC): A/New Caledonia/20/99 (NC99, H1N1), A/South Dakota/6/2007 (SD07, H1N1), A/Panama/2007/99 (Pan99, H3N2), A/California/07/04 (CA04, H3N2), A/Uruguay/716/07 (Uru07, H3N2). The HA and NA plasmids of H5N1 viruses A/Hong Kong/213/2003 (HK03) and A/Vietnam/1203/2004 (VN04) were generated as described previously [23, 24]. The HA and NA plasmids from A/California/7/2009 (CA09, pH1N1) LAIV were used in this study [25]. The 6:2 reassortant LAIV viruses containing the HA and NA genes from a specific influenza virus and the six internal protein gene segments from cold-adapted (ca) A/Ann Arbor/6/60 were generated by the eight plasmid transfection system [22, 23]. For the NI assay, H6 reassortant viruses bearing the HA gene from A/teal/Hong Kong/W312/97 (H6N1)[26], the NA gene from a different influenza virus and the six internal protein gene segments from ca A/Ann Arbor/6/60 were generated. Viruses were propagated in 10- to 11-day old embryonated chicken eggs (Charles River SPAFAS, Franklin, CT). The HA and NA genes of the rescued viruses were confirmed by sequencing.
Eight- to 10-week-old male or female ferrets (Simonsen Laboratories, Gilroy, CA) were inoculated intranasally with 7.0 log10FFU of LAIV vaccine viruses in 0.2ml. Serum samples were collected before infection and after two to three weeks post-infection.
Neuraminidase inhibition (NI) assay
A miniaturized format of the conventional NI assay was applied to detect the NA specific antibody titers [20]. To eliminate the interference by HA-specific antibodies, H6 reassortants containing the HA from A/teal/Hong Kong/W312/97 (H6N1) and the relevant NA gene were used as the testing viruses. The NA activities of each virus were analyzed by quantification of the free sialic acid released from the fetuin substrate using the 96-well plate format. Each virus was 2-fold serially diluted in PBS with 0.1% BSA and 8 µl of the diluted virus was transferred to duplicate wells in a 96-well PCR plate. Eight µl of fetuin (25mg/ml in PBS) was added to each well, mixed and incubated at 37°C for 15 hours. To detect the released sialic acid, 8 µl of periodate reagent (200mM NaIO4 in 53% H3PO4) was added and incubated at room temperature for 15 min. Forty µl of arsenite reagent (1M AsNaO2, 700mM Na2SO4 in 0.3% H2SO4) was then added and mixed well until the yellow color disappeared. Next, 80 µl of thiobarbituric acid reagent (50mM TBA, 625mM Na2SO4) was added and the PCR plate was heated at 99°C for 15 min. After the plate was cooled down, 120 µl of Warrenoff solution (95% 1-butanol, 5% HCl) was added and mixed by vortex to extract the pink chromogen into the upper phase. After a brief centrifugation at 800 rpm for 5 min, 50 µl of the upper phase was transferred to a 96-well half area flat bottom plate and absorbance at 550nm was measured. The final OD550 value was corrected by subtraction of fetuin-only background and standardizing to a 1.0cm cuvette value. All chemical compounds used were from Sigma-Aldrich (St. Louis, MO). The above procedures involving the handling of the chemicals were conducted in a chemical hood. The NA activity of each virus was defined as the reciprocal of the virus dilution that produced a mean OD550 of 1.0 as analyzed by GraphPad Prizm non-linear curve fitting. For the NI assay, test viruses were diluted to an NA activity that gave the OD550 value of 1.0 and 8 µl of 2-fold serially diluted serum samples were mixed with an equal volume of the diluted virus, followed by the NA activity assay as described above. The OD550 value of virus-antiserum mixtures and virus only control were determined. NI antibody titers were defined as the reciprocal of the highest serum dilution at which the mean OD550 was ≤50% of the mean OD550 of the virus control. Geometric mean NI titers were calculated from the titers of three ferrets.
Hemagglutination inhibition (HAI) assay
The standard hemagglutination inhibition (HAI) assay was used to measure the HA-specific antibody levels against homologous or heterologous viruses. Briefly, ferret serum was treated with receptor-destroying enzyme (RDE, Denka Seiken Co., Tokyo, Japan) at 37°C overnight and 25 µl of 2-fold serially diluted serum samples were mixed with 4 HA units of the indicated viruses (25 µl) in 96-well V-bottom microplates. After incubating at room temperature for 30 min, 50 µl of 0.5% turkey erythrocytes (tRBC) were added to each well and incubated for an additional 45 min. The HAI titer was defined as the reciprocal of the highest serum dilution that inhibited virus hemagglutination. Geometric mean HAI titers were calculated from the titers of three ferrets.
Plaque size reduction assay
Plaque assay was performed in MDCK cells at 33°C as described previously [23]. To test the effect of post-infection ferret serum on virus plaque size, MDCK cells in 6-well plates were infected with approximately 50 PFU (plaque-forming units) of virus. After 60 min of adsorption, the virus inocula were removed and replaced with 1% agarose overlay containing serially diluted serum [27]. The plaques were immunostained with a polyclonal influenza A virus antiserum as described before [23].
Results
Detection of NA antibodies in LAIV vaccinated ferrets
We applied the miniaturized neuraminidase inhibition (NI) assay to detect the NA antibody responses in ferrets intranasally immunized with live-attenuated influenza vaccine viruses (LAIV), including seasonal H1N1 vaccine strains A/New Caledonia/20/99 (NC99) and A/South Dakota/6/2007 (SD07), H5N1 A/Hong Kong/213/2003 (HK03) candidate vaccine, 2009 pandemic H1N1 vaccine A/California/7/09 (CA09), and seasonal H3N2 vaccine strains A/Panama/2007/99 (Pan99, H3N2), A/California/07/04 (CA04, H3N2) and A/Uruguay/716/07 (Uru07, H3N2). H6 reassortants bearing the HA gene from A/teal/Hong Kong/W312/97 (H6N1) [26], the NA gene from these N1 and N2 viruses were produced and used as the testing antigens. The H6 reassortants showed comparable NA activities within subtypes (Table 1).
Table 1.
The NA activities of H6 reassortant viruses containing different NA
| H6 reassortants | ||||
|---|---|---|---|---|
| Source of the NA gene (subtype) | Abbrev. | HA titer | Virus titer (log10PFU/ml) |
NA activity1 |
| A/New Caledonia/20/99 (H1N1) | NC99 | 1024 | 9.1 | 149 |
| A/South Dakota/6/07 (H1N1) | SD07 | 1024 | 9.0 | 126 |
| A/California/7/09 (pH1N1) | CA09 | 1024 | 8.6 | 263 |
| A/HongKong/213/03 (H5N1) | HK03 | 1024 | 8.7 | 296 |
| A/Panama/2007/99 (H3N2) | Pan99 | 1024 | 9.0 | 79 |
| A/California/07/04 (H3N2) | CA04 | 1024 | 9.1 | 97 |
| A/Uruguay/716/07 (H3N2) | Uru07 | 1024 | 8.9 | 75 |
Each virus was adjusted to HA titer of 1024 and virus titer was determined by plaque assay. NA activity was determined by the miniaturized colorimetric assay using fetuin substrate.
NA activity was defined as the reciprocal of virus dilution at which the OD550 value is 1.0. The values are the average of two independent experiments.
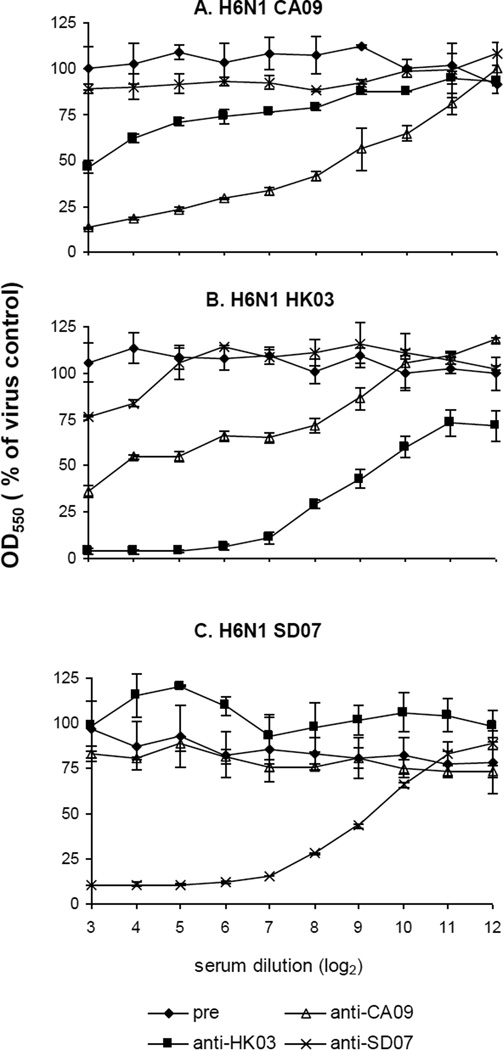
To assess the sensitivity and specificity of the NI assay, one ferret serum sample from each of the pH1N1 CA09, H5N1 HK03 and H1N1 SD07 vaccinated animals with a HAI titer of 512 were examined for NI activity against H6 reassortants containing the NA from CA09, HK03 or SD07 (Fig. 1). Serial dilutions of each post-infection ferret serum were incubated with the respective test viruses and NA activities were tested. The OD550 value compared to that of the virus control was determined. As shown in Figure 1, pre-immune normal ferret serum did not inhibit the NA activity of the three test viruses. However, CA09, HK03 and SD07 post-infection serum specifically inhibited the NA activity of the virus containing the homologous NA. The homologous 50% inhibition NI titers of the three serum samples all reached 9 log2 (512), indicating that the sensitivity of the NI assay is comparable to that of the HAI assay. CA09 and HK03 post-infection sera cross-inhibited the NA activity of each other with a 50% inhibition titer of approximately 8 and 16 respectively, whereas SD07 post-infection serum did not show any significant inhibition of activity of the NA from CA09 and HK03 viruses (Fig. 1A and 1B) and conversely, CA09 and HK03 post-infection sera did not inhibit the NA of SD07 (Fig. 1C). These data indicated that the NI assay could be used for quantitating the NA antibody levels against different NA antigens.
Figure 1.
Detection of ferret anti-NA antibodies against different NA antigens by the miniaturized colorimetric NI assay. One serum sample from each of the pre-immune (pre) and post-immune ferret groups (anti-CA09, anti-HK03, or anti-SD07) was serially diluted and incubated with H6 reassortants bearing NA antigens from CA09 (A), HK03 (B) or SD07(C). Fetuin substrate was added and NA activity was tested. The OD550 value compared to that of the virus control (%) at each serum dilution was determined and plotted. The data represent the average of duplicate experiments and the standard error is indicated for each data point.
NA antibody response and cross-reactivity among different N1 and N2 strains
To systematically determine the NA antibody responses of ferrets vaccinated with LAIVs, groups of three ferrets were intranasally inoculated with the seasonal H1N1 NC99 and SD07 LAIV, H5N1 HK03 LAIV strain, or the pH1N1 CA09 LAIV strain. Ferret sera were collected 2 to 3 weeks post-infection and examined by both HAI and NI assays, expressed as geometric mean titers of the data from three ferrets (Table 2). Ferret serum against NC99 had a homologous HAI titer of 203, but did not cross-react well with the more recent SD07 strain (HAI titer of 25); similarly, SD07 post-infection sera had a homologous HAI titer of 256 and a low titer of 32 to NC99 because of the antigenic difference between the HA genes of these two strains. NC99 and SD07 post-infection sera had homologous NI titers of 32 and 128 respectively, demonstrating that NA antibodies were elicited in LAIV vaccinated ferrets. Both NC99 and SD07 viruses elicited NA antibody with limited cross-reactivity (>4 fold lower NI titers than the homologous titers), indicating that antigenic drift of the NA accompanied antigenic drift of the HA from NC99 to SD07.
Table 2.
HA and NA antibody response and cross-reactivity in LAIV immunized ferrets
|
Geometric mean serum NI titers of ferrets immunized with 1 dose of LAIV |
||||
| Test antigen* | NC99 H1N1 |
SD07 H1N1 |
HK03 H5N1 |
CA09 pH1N1 |
| NC99 | 32 | 20 | <8 | <8 |
| SD07 | 8 | 128 | <8 | <8 |
| HK03 | <8 | <8 | 161 | 13 |
| CA09 | <8 | <8 | 40 | 512 |
|
Geometric mean serum HAI titers of ferrets immunized with 1 dose of LAIV |
||||
| Test antigen | NC99 H1N1 |
SD07 H1N1 |
HK03 H5N1 |
CA09 pH1N1 |
| NC99 | 203 | 32 | <8 | <8 |
| SD07 | 25 | 256 | <8 | <8 |
| HK03 | <8 | <8 | 102 | <8 |
| CA09 | <8 | <8 | <8 | 645 |
Reassortant H6 viruses bearing the NA from the indicated virus.
Groups of three ferrets were inoculated intranasally with 107 FFU/ml of the indicated LAIV viruses. Serum was collected 2 to 3 weeks after immunization. The NI titers were determined by NI assay against the H6 reassortants containing the indicated NA test antigen. The HAI titers were determined by HAI assay using turkey RBC and the indicated viruses.
Ferret sera against H3N2 LAIV strains were also tested in both the HAI and NI assays (Table 3). Pan99, CA04 and Uru07 post-infection sera had homologous HAI titers of 40, 161 and 64 respectively but low cross-reactivity to each other, reflecting the antigenic drift of the H3N2 strains from 1999 to 2007. Pan99, CA04 and Uru07 had homologous NI titers of 81, 16 and 64, respectively. Consistent with the poor HAI cross-reactivity, no cross-reactive NA antibodies were detected between Pan99 and the other two strains. However, CA04 antisera had a good cross-reactive NI titer of 32 to Uru07; Uru07 antisera also cross-reacted with CA04, although the NI titer of 8 was 8-fold lower than the homologous titer, indicating that Pan99 is antigenically more distant from CA04 and Uru07.
Table 3.
HA and NA antibody response and cross-reactivity in H3N2 LAIV immunized ferrets
|
Geometric mean NI serum titers of ferrets immunized with 1 dose of LAIV |
|||
| Test antigen* | Pan99 H3N2 |
CA04 H3N2 |
Uru07 H3N2 |
| Pan99 | 81 | <8 | <8 |
| CA04 | <8 | 16 | 8 |
| Uru07 | <8 | 32 | 64 |
|
Geometric mean serum HAI titers of ferrets immunized with 1 dose of LAIV |
|||
| Test antigen | Pan99 H3N2 |
CA04 H3N2 |
Uru07 H3N2 |
| Pan99 | 40 | <8 | <8 |
| CA04 | 8 | 161 | 16 |
| Uru07 | <8 | 16 | 64 |
Reassortant H6 viruses bearing the NA from the indicated virus.
Groups of three ferrets were inoculated intranasally with 107 FFU/ml of the indicated LAIV viruses. Serum was collected 2 to 3 weeks after immunization. The NI titers were determined by NI assay against the H6 reassortants containing the indicated NA test antigen. The HAI titers were determined by HAI assay using turkey RBC and the indicated homologous viruses.
The HAI and NI data from LAIV vaccinated ferrets were also compared to assess the cross-reactivity between the seasonal H1N1, pH1N1 CA09, and H5N1 strains (Table 2). The H5N1 vaccine strain HK03 induced an HAI titer of 102 and an NI titer of 161 respectively. The NI titer (512) of CA09 also correlated with a high HAI titers (645). The HAI assay confirmed the lack of cross-reactivity in the HA antibodies among seasonal H1N1, pH1N1 and H5N1 viruses. However, some NI cross-reactivity was observed between the H5N1 HK03 and the pH1N1 CA09 strains, with NI titers of 40 and 13 respectively. The CA09 sera also cross-reacted with the NA from another H5N1 strain A/Vietnam/1203/04 (data not shown). In contrast, seasonal H1N1 NC99 and SD07 had no detectable NI cross-reactivity to either HK03 or CA09, and vice versa.
To examine whether the NA antibody cross-reactivity data reflected the phylogenetic distance of the N1 proteins, the amino acid sequences of the N1 proteins from the three groups of viruses were analyzed. The NA of pH1N1 CA09 is genetically closer to the NA of H5N1 (HK03 and VN04) than to the NA of seasonal H1N1 (NC99 and SD07) (Fig. 2A). The CA09 NA shared 89% and 88% amino acid sequence identity to the NA of HK03 and VN04 respectively; it had 82% and 81% sequence identity to the NA of NC99 and SD07 respectively. Excluding the identical residues among the five strains, the variable residues of the head domain of the N1 proteins were analyzed based on the crystal structure of the CA09 NA (Fig. 2B) [28]. Approximately 24 residues are conserved in the N1 proteins of CA09 and H5N1 strains, but differ from the NA proteins of seasonal H1N1 strains (highlighted in green). In contrast, only 2 amino acids are conserved in the N1 proteins of CA09 and seasonal H1N1 strains, but differ from the H5N1 strains (highlighted in red). Among the shared amino acids between the NA of pH1N1 CA09 and H5N1 strains, three regions on the surface of the NA structure, residues 219 to 220, residues 427 to 432, and residues 332 to 342, are near the catalytic site and may also contribute to the antigenicity of NA [1, 28].
Figure 2.
(A) A phylogenetic tree of the N1 proteins. The NA amino acid sequences of indicated viruses were aligned and plotted using the Mega software. The bar indicates the genetic distance. (B) Molecular surface structure of the NA of A/California/7/2009 (Protein Data Bank 5NSS, one monomer shown). The locations of the amino acids in the CA09 NA that are identical to both H5N1 strains (HK03 and VN04) but different from the two seasonal H1N1 strains (NC99 and SD07) are shown in green; the amino acids of the CA09 NA that are identical to the seasonal H1N1 strains but different from the H5N1 strains are shown in red. The amino acid positions are indicated by N2 numbering. The activity cavity (AC) is indicated and the catalytic sites are shown in wheat color. The picture was generated by using PyMOL software.
Cross-reactivity of NA antibodies by plaque size reduction assay
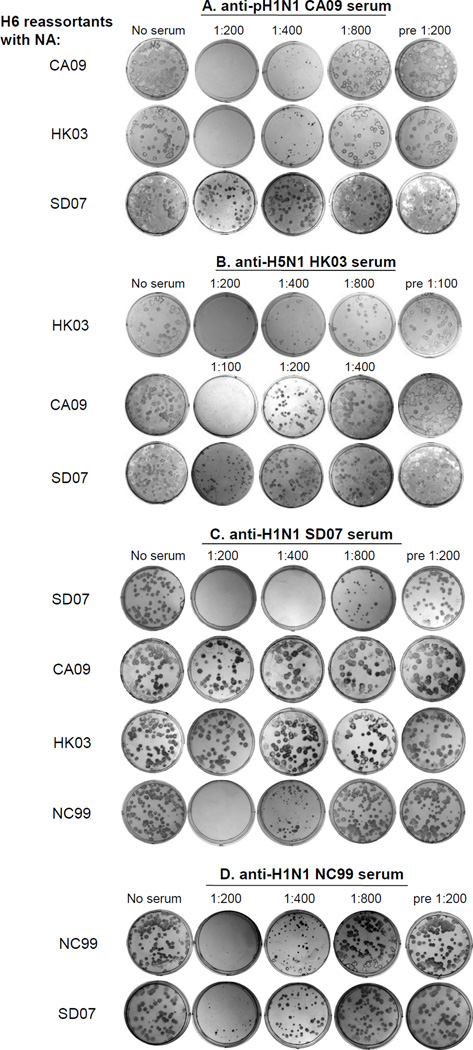
NA antibody has been previously shown to reduce plaque size [27]. To further confirm the cross-reactivity of NA antibodies between the CA09, SD07 and HK03 strains, a plaque size reduction assay was performed. H6 reassortants were plaqued in the presence and absence of serially diluted post-infection ferret antisera in an agarose overlay, and the plaque number and size were recorded. As shown in Fig. 3, CA09 serum inhibited the plaque size of H6 reassortants with NA from CA09 or HK03 (or VN04, data now shown) up to 1:400 serum dilution; no plaques were detected at a dilution of 1:200; however, CA09 post-infection serum did not inhibit the plaque size and number of H6 reassortants with SD07 NA even at a dilution of 1:200 (Fig. 3A). As a negative control, ferret pre-immune serum did not inhibit plaque size or number of three H6 reassortants containing NA from CA09, HK03 or SD07 at a dilution of 1:200. These results demonstrated the role of the NA antibody in inhibiting plaque size. CA09 post-infection serum was more potent in cross-reacting with the H5N1 strains than with seasonal H1N1 strains. Similarly, HK03 post-infection serum specifically inhibited the plaque size of the H6 reassortant containing the homologous HK03 NA up to a dilution of 1:400. At a lower dilution of 1:100, anti-HK03 serum greatly reduced the plaque size of the H6 reassortant containing the NA from CA09, but only caused a slight reduction in the plaque size of the H6 reassortant with the SD07 NA (Fig. 3B). SD07 post-infection serum inhibited the plaque size of the H6 reassortant containing homologous SD07 NA at a dilution of 1:800, and the previous seasonal H1N1 strain NC99 NA at a dilution of 1:400 (Fig. 3C). Similarly, NC99 post-infection serum inhibited the plaque size of the H6 reassortant with SD07 NA (Fig. 3D). In contrast, SD07 post-infection serum did not inhibit the plaque size of the H6 reassortants with CA09 or HK03 NA at a dilution of 1:200 (Fig. 3C). Thus, 2009 pandemic H1N1 and H5N1 LAIV induced NA-specific antisera that were able to cross-react with each other in the plaque size reduction assay. These data are consistent with the NA antibody cross-reactivity data detected by the NI assay.
Figure 3.
Plaque size reduction assay. A total of approximately 50 PFU of the indicated H6 reassortants with the indicated NA segment was inoculated in each well of a 6 well plate of MDCK cells. Serial dilutions of ferret preimmune serum, anti-pH1N1 CA09 serum, anti-H5N1 HK03, anti-H1N1 SD07, and anti-H1N1 NC99 serum were added to the agarose overlay. The cells were incubated at 33°C for 4 days. The plaques were visualized by immunostaining.
Discussion
HA antibody responses have been used as an indication of protective immune response from influenza vaccination. Although NA antibodies have been shown to contribute to protection against influenza in animal models and in humans, little has been done to evaluate the NA-mediated antibody responses to licensed vaccines due to the lack of a suitable and reliable assay to detect NA antibody [29–31]. In this study, we detected the NA antibody responses in LAIV vaccinated ferrets with the miniaturized NI assay using HA-mismatched viruses as test antigens and demonstrated antigenic relatedness and cross-reactivity of different NA proteins.
The conventional test tube NI assay described in the WHO manual was improved in a number of steps as described by Sandbulte et al.[20, 32]. First, the use of the PCR tubes and PCR thermocycler is more convenient and safer than using test tubes. Second, the use of H6 reassortant viruses eliminated the steric hindrance caused by antibodies to the matched HA. The lack of pre-existing anti-H6 antibodies in humans would allow the use of the H6 reassortants to detect the NA antibodies in human samples. Third, unlike some small molecule substrates such as MUNANA that can penetrate to the NA enzymatic center even in the presence of NA antibodies, fetuin is a larger glycoprotein substrate that is blocked by antibodies. Our results demonstrated that this improved NI assay could detect various NA antibody levels with good sensitivity and data consistency. We did not observe antigenic competition between HA and NA as reported previously [33]. We showed that the NI antibody levels generally correlate with the level of HAI antibodies in LAIV vaccinated ferrets. The CA09 strain elicited high HAI (645) and NI antibody titers (512). Ferret post-dose 1 serum is normally used to evaluate the HA antibody response and HA antigenic relatedness. The same post-dose 1 serum samples were also used in this study to examine NA antibody responses. A higher HAI antibody titer from post-dose 2 serum also correlated with a higher NI titer (data not shown). Thus, this NI assay can be used to semi-quantitatively evaluate vaccine-mediated NA antibody responses in both animal models and humans. In this study, we also tested the antigenic relatedness of N1 NAs. Similar to the conventional enzymatic NI assay, the NI assay described here also normalized different test viruses to equal NA activity. Although equal NA activity may not reflect equal amount of NA proteins in the test viruses, the NI results could show relative inhibition to the NA proteins of different test viruses. The correlation of cross-reactive NI titers to the functional plaque size reduction assay indicated that this assay could be useful to evaluate not only NA antibody responses but also NA cross-reactivity and antigenic relatedness. Due to the limited number of animals and data in this study, statistical analysis was not performed to define a cut-off NI titer for functional cross-reactivity. A cross-reactive NI titer of 8 could be still functional in plaque reduction, suggesting that a more comprehensive analysis of the NI data and functional inhibition is needed. One drawback of this assay is that it involves using small amount of hazardous chemicals. Alternative methods are being developed including an enzyme-linked lectin assay [34]. The sensitivity, specificity, consistency and functional correlation of these methods need to be carefully evaluated.
Consistent with a recent report showing that ferret antisera against recombinant N1 from 2009 H1N1 cross-reacted with N1 from avian H5N1 [17], our study demonstrated the cross-reactivity of LAIV-induced CA09 NA antisera to H5N1 strains. The cross-reactive NA antibody titers are modest but clearly seen in the dose-response curve (Fig. 1). The plaque size reduction assay demonstrated the cross-inhibition of NA antibodies between pH1N1 CA09 and H5N1 HK03 viruses. We also detected the cross-reactivity between pH1N1 CA09 and H5N1 VN04 virus in both the NI assay and plaque size reduction assay (data not shown). Due to the low antibody levels induced by LAIV VN04 in ferrets and the low NA activity of the short-stalk VN04 NA, the NI titers were not comparable and thus were not shown in this study [35–37]. Genetic analysis showed that pH1N1 CA09 NA is in the Eurasian swine lineage. The NA was reported to be originally derived from an avian influenza virus that entered the Eurasian swine population in 1979 [38]. Therefore, it is not surprising that CA09 NA is closer to the H5N1 NA that has an avian origin than to the seasonal human H1N1 NA. Analysis of the NA structure also showed that CA09 NA clustered together with the NA from the 1918 pandemic virus and the NA from the 2004 VN04 H5N1 virus [28]. Recently it was shown that inactivated seasonal influenza vaccines (TIV) induced serum antibodies to the NA of 2009 pandemic H1N1 virus mainly in populations older than 70 years, indicating that the 2009 H1N1 NA is more antigenically related to earlier human strains (e.g. pandemic 1918 H1N1) that are closer to the avian precursor viruses than to the contemporary seasonal human H1N1 viruses [32]. The NA antibodies against seasonal H1N1 were found to afford partial protection against H5N1 in mice [39]. The close relatedness of the NAs of H5N1 and pH1N1 indicated that antibodies to pH1N1 induced by vaccination may offer some protection against the highly pathogenic H5N1 viruses because their NA protein head region sequences are more conserved.
LAIV has been shown to be efficacious in humans [40]. The NA antibodies detected in this study indicated that an NA protein-mediated response is a component of LAIV-induced immune responses. In a pilot study of clinical samples from LAIV vaccine recipients, we detected anti-NA antibody responses that correlated with HAI antibody responses (data not shown). NA antibodies have been previously shown to correlate with immunity and protection in humans [9–12]. Cross-reactive NA antibodies within the same NA subtype may broaden the protection against influenza virus infection [41]. The development of this NI assay allows further evaluation of the contribution of the NA antibodies to the protective immune response elicited by vaccines or natural influenza infections.
Highlights.
We detected NA-specific antibodies in LAIV immunized ferrets by an NI assay.
NA antibodies against 2009 pandemic H1N1 cross-reacted with the NA of H5N1.
No cross-reactivity was detected between NAs of 2009 H1N1 and seasonal H1N1.
Plaque size reduction and sequence analysis confirmed the NA cross-reactivity.
Acknowledgments
We thank Dr. Maryna C. Eichelberger from CBER/FDA for providing the NI assay protocol. The H5N1 and H6N1 vaccine viruses and plasmids were produced under the Cooperative Research and Development Agreement (CRADA) between MedImmune and NIAID/NIH. This research was supported in part by the Intramural Research Program of NIAID, NIH. We thank MedImmune’s animal care facility for the ferret studies; cell culture group for providing tissue culture cells; lab service group for preparing reagents; Chin-fen Yang for helping with sequence analysis; Drs. Gary Van Nest, Amorsolo Suguitan Jr and Xing Cheng for reviewing the manuscript.
References
- 1.Colman PM. Neuraminidase Enzyme and Antigen. In: Krug R, editor. The Influenza Viruses. New York: Plenum Press; 1989. pp. 175–218. [Google Scholar]
- 2.Lamb RA, Krug RM. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PMea, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1487–1531. [Google Scholar]
- 3.Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J Virol. 2004;78(22):12665–12667. doi: 10.1128/JVI.78.22.12665-12667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitnaul LJ, Matrosovich MN, Castrucci MR, Tuzikov AB, Bovin NV, Kobasa D, et al. Balanced hemagglutinin and neuraminidase activities are critical for efficient replication of influenza A virus. J Virol. 2000;74(13):6015–6020. doi: 10.1128/jvi.74.13.6015-6020.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner R, Matrosovich MN, Klenk HD. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev Med Virol. 2002;12(3):159–166. doi: 10.1002/rmv.352. [DOI] [PubMed] [Google Scholar]
- 6.Kilbourne ED, Laver WG, Schulman JL, Webster RG. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J Virol. 1968;2(4):281–288. doi: 10.1128/jvi.2.4.281-288.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulman JL, Khakpour M, Kilbourne ED. Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J Virol. 1968;2(8):778–786. doi: 10.1128/jvi.2.8.778-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson BE, Bucher DJ, Kilbourne ED. Purified influenza virus hemagglutinin and neuraminidase are equivalent in stimulation of antibody response but induce contrasting types of immunity to infection. J Virol. 1989;63(3):1239–1246. doi: 10.1128/jvi.63.3.1239-1246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy BR, Kasel JA, Chanock RM. Association of Serum Anti-Neuraminidase Antibody with Resistance to Influenza in Man. N Engl J Med. 1972;286:1329–1332. doi: 10.1056/NEJM197206222862502. [DOI] [PubMed] [Google Scholar]
- 10.Couch RB, Kasel JA, Gerin JL, Schulman JL, Kilbourne ED. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J Infect Dis. 1974;129(4):411–420. doi: 10.1093/infdis/129.4.411. [DOI] [PubMed] [Google Scholar]
- 11.Ogra PL, Chow T, Beutner KR, Rubi E, Strussenberg J, DeMello S, et al. Clinical and immunologic evaluation of neuraminidase-specific influenza A virus vaccine in humans. J Infect Dis. 1977;135(4):499–506. doi: 10.1093/infdis/135.4.499. [DOI] [PubMed] [Google Scholar]
- 12.Beutner KR, Chow T, Rubi E, Strussenberg J, Clement J, Ogra PL. Evaluation of a neuraminidase-specific influenza A virus vaccine in children: antibody responses and effects on two successive outbreaks of natural infection. Infec Dis. 1979;140(6):844–850. doi: 10.1093/infdis/140.6.844. [DOI] [PubMed] [Google Scholar]
- 13.Deroo T, Jou WM, Fiers W. Recombinant neuraminidase vaccine protects against lethal influenza. Vaccine. 1996;14(6):561–569. doi: 10.1016/0264-410x(95)00157-v. [DOI] [PubMed] [Google Scholar]
- 14.Kilbourne ED, Pokorny BA, Johansson BE, Brett I, Milev Y, Matthews JT. Protection of mice with recombinant influenza virus neuraminidase. J Infect Dis. 2004;189(3):459–461. doi: 10.1086/381123. [DOI] [PubMed] [Google Scholar]
- 15.Martinet W, Saelens X, Deroo T, Neirynck S, Contreras R, Min Jou W, et al. Protection of mice against a lethal influenza challenge by immunization with yeast-derived recombinant influenza neuraminidase. Eur J Biochem. 1997;247(1):332–338. doi: 10.1111/j.1432-1033.1997.00332.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Matsuo K, Asanuma H, Takahashi H, Iwasaki T, Suzuki Y, et al. Enhanced protection against a lethal influenza virus challenge by immunization with both hemagglutinin- and neuraminidase-expressing DNAs. Vaccine. 1999;17(7–8):653–659. doi: 10.1016/s0264-410x(98)00247-3. [DOI] [PubMed] [Google Scholar]
- 17.Bosch BJ, Bodewes R, de Vries RP, Kreijtz JH, Bartelink W, van Amerongen G, et al. Recombinant soluble, multimeric HA and NA exhibit distinctive types of protection against pandemic swine-origin 2009 A(H1N1) influenza virus infection in ferrets. J Virol. 2010;84(19):10366–10374. doi: 10.1128/JVI.01035-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson BE. Immunization with influenza A virus hemagglutinin and neuraminidase produced in recombinant baculovirus results in a balanced and broadened immune response superior to conventional vaccine. Vaccine. 1999;17(15–16):2073–2080. doi: 10.1016/s0264-410x(98)00413-7. [DOI] [PubMed] [Google Scholar]
- 19.Sylte MJ, Suarez DL. Influenza neuraminidase as a vaccine antigen. Curr Top Microbiol Immunol. 2009;333:227–241. doi: 10.1007/978-3-540-92165-3_12. [DOI] [PubMed] [Google Scholar]
- 20.Sandbulte MR, Gao J, Straight TM, Eichelberger MC. A miniaturized assay for influenza neuraminidase-inhibiting antibodies utilizing reverse genetics-derived antigens. Influenza and Other Respiratory Viruses. 2009;3(5):233–240. doi: 10.1111/j.1750-2659.2009.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassantoufighi A, Zhang H, Sandbulte M, Gao J, Manischewitz J, King L, et al. A practical influenza neutralization assay to simultaneously quantify hemagglutinin and neuraminidase-inhibiting antibody responses. Vaccine. 2010;28(3):790–797. doi: 10.1016/j.vaccine.2009.10.066. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A. 2000;97(11):6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin H, Lu B, Zhou H, Ma C, Zhao J, Yang CF, et al. Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology. 2003;306(1):18–24. doi: 10.1016/s0042-6822(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 24.Suguitan ALJ, McAuliffe J, Mills KL, Jin H, Duke G, Lu B, Luke CJ, et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006;3(9):e360. doi: 10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Wang W, Zhou H, Suguitan ALJ, Shambaugh C, Kim L, et al. Generation of live attenuated novel influenza virus A/California/7/09 (H1N1) vaccines with high yield in embryonated chicken eggs. J Virol. 2010;84(1):44–51. doi: 10.1128/JVI.02106-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Santos C, Aspelund A, Gillim-Ross L, Jin H, Kemble G, et al. Evaluation of live attenuated influenza a virus H6 vaccines in mice and ferrets. J Virol. 2009;83(1):65–72. doi: 10.1128/JVI.01775-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jahiel RI, Kilbourne ED. Reduction in plaque size and reduction in plaque number as differing indices of influenza virus-antibody reactions. J Bacteriol. 1966;92(5):1521–1534. doi: 10.1128/jb.92.5.1521-1534.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q, Qi J, Zhang W, Vavricka CJ, Shi Y, Wei J, et al. The 2009 pandemic H1N1 neuraminidase N1 lacks the 150-cavity in its active site. Nat Struct Mol Biol. 2010;17(10):1266–1268. doi: 10.1038/nsmb.1909. [DOI] [PubMed] [Google Scholar]
- 29.Cate TR, Rayford Y, Niño D, Winokur P, Brady R, Belshe R, et al. A high dosage influenza vaccine induced significantly more neuraminidase antibody than standard vaccine among elderly subjects. Vaccine. 2010;28(9):2076–2079. doi: 10.1016/j.vaccine.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clements ML, Tierney EL, Murphy BR. Response of seronegative and seropositive adult volunteers to live attenuated cold-adapted reassortant influenza A virus vaccine. J Clin Microbiol. 1985;21(6):997–999. doi: 10.1128/jcm.21.6.997-999.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powers DC, Kilbourne ED, Johansson BE. Neuraminidase-specific antibody responses to inactivated influenza virus vaccine in young and elderly adults. Clin Diagn Lab Immunol. 1996;3(5):511–516. doi: 10.1128/cdli.3.5.511-516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcelin G, Bland HM, Negovetich NJ, Sandbulte MR, Ellebedy AH, Webb AD, et al. Inactivated seasonal influenza vaccines increase serum antibodies to the neuraminidase of pandemic influenza A(H1N1) 2009 virus in an age-dependent manner. J Infect Dis. 2010;202(11):1634–1638. doi: 10.1086/657084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson BE, Pokorny BA, Tiso VA. Supplementation of conventional trivalent influenza vaccine with purified viral N1 and N2 neuraminidases induces a balanced immune response without antigenic competition. Vaccine. 2002;20(11–12):1670–1674. doi: 10.1016/s0264-410x(01)00490-x. [DOI] [PubMed] [Google Scholar]
- 34.Lambré CR, Terzidis H, Greffard A, Webster RG. Measurement of anti-influenza neuraminidase antibody using a peroxidase-linked lectin and microtitre plates coated with natural substrates. J Immunol Methods. 1990;135(1–2):49–57. doi: 10.1016/0022-1759(90)90255-t. [DOI] [PubMed] [Google Scholar]
- 35.Matsuoka Y, Swayne DE, Thomas C, Rameix-Welti M, Naffakh N, Warnes C, et al. Neuraminidase stalk length and additional glycosylation of the hemagglutinin influence the virulence of influenza H5N1 viruses for mice. J Virol. 2009;83(9):4704–4708. doi: 10.1128/JVI.01987-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Lu B, Zhou H, Suguitan ALJ, Cheng X, Subbarao K, et al. Glycosylation at 158N of the hemagglutinin protein and receptor binding specificity synergistically affect the antigenicity and immunogenicity of a live attenuated H5N1 A/Vietnam/1203/2004 vaccine virus in ferrets. J Virol. 2010;84(13):6570–6577. doi: 10.1128/JVI.00221-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou H, Yu Z, Hu Y, Tu J, Zou W, Peng Y, et al. The special neuraminidase stalk-motif responsible for increased virulence and pathogenesis of H5N1 influenza A virus. PLoS One. 2009;4(7):e6277. doi: 10.1371/journal.pone.0006277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325(5937):197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandbulte MR, Jimenez GS, Boon AC, Smith LR, JJ T, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 2007;4(2):e59. doi: 10.1371/journal.pmed.0040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ambrose CS, Luke C, Coelingh K. Current status of live attenuated influenza vaccine in the United States for seasonal and pandemic influenza. Influenza Other Respi Viruses. 2008;2(6):193–202. doi: 10.1111/j.1750-2659.2008.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kilbourne ED, Johansson BE, Grajower B. Independent and disparate evolution in nature of influenza A virus hemagglutinin and neuraminidase glycoproteins. Proc Natl Acad Sci U S A. 1990;87(2):786–790. doi: 10.1073/pnas.87.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]