Abstract
The hemagglutinin of the 2009 pandemic H1N1 influenza virus is a derivative of and is antigenically related to classical swine but not to seasonal human H1N1 viruses. We compared the A/California/7/2009 (CA/7/09) virus recommended by the WHO as the reference virus for vaccine development, with two classical swine influenza viruses A/swine/Iowa/31 (sw/IA/31) and A/New Jersey/8/1976 (NJ/76) to establish the extent of immunologic cross-reactivity and cross-protection in animal models. Primary infection with 2009 pandemic or NJ/76 viruses elicited antibodies against the CA/7/09 virus and provided complete protection from challenge with this virus in ferrets; the response in mice was variable and conferred partial protection. Although ferrets infected with sw/IA/31 virus developed low titers of cross-neutralizing antibody, they were protected from pulmonary replication of the CA/7/09 virus. The data suggest that prior exposure to antigenically related H1N1 viruses of swine-origin provide some protective immunity against the 2009 pandemic H1N1 virus.
Keywords: 2009 pandemic influenza, classical swine H1N1 influenza, cross-protection
INTRODUCTION
In April 2009, the Centers for Disease Control and Prevention (CDC) reported the first two cases of human infection with a novel influenza H1N1 virus in the United States (2009a; 2009c) and over the course of the next several weeks, the virus spread around the world (WHO, http://www.who.int/csr/don/2009_07_06/en) prompting the World Health Organization (WHO) to declare a global influenza pandemic on June 11, 2009 (WHO, http://www.who.int/csr/disease/swineflu/en).
Antigenic and genetic characterization of the novel influenza H1N1 virus indicate that the virus contains a unique combination of gene segments from viruses that had been circulating in pigs in North American and Europe; 6 of the 8 gene segments (3 polymerase genes, HA, NP and NS) were derived from a triple reassortant North American H1N2 swine influenza virus and the NA and M gene segments were derived from a Eurasian lineage swine H1N1 virus (Garten et al., 2009). The 2009 pandemic H1N1 viruses isolated from human cases are antigenically equivalent to one another and are similar to North American swine H1N1 viruses, but distinct from seasonal human influenza H1N1 viruses (Garten et al., 2009).
The 2009 pandemic H1N1 virus is not the first swine-origin H1N1 virus to infect humans. In the past 35 years, more than 50 sporadic cases of swine influenza virus infection have occurred in humans, mostly caused by classical swine influenza viruses (Hay et al., 2001; Kendal et al., 1977; Myers, Olsen, and Gray, 2007; Shinde et al., 2009; Thompson et al., 1976; Webster et al., 1992). Signs and symptoms of infection with classic swine influenza virus in humans are often indistinguishable from those of infection with human influenza viruses (Myers, Olsen, and Gray, 2007). The most notable instance occurred in 1976, after the isolation of a swine H1N1 influenza virus (A/New Jersey/1976) from a military recruit at Fort Dix, NJ. There were 12 additional cases identified by virus isolation or serologic methods and one military recruit died of the disease (Gaydos et al., 1977; Hodder et al., 1977). Concerns of the pandemic potential of the A/New Jersey/1976 H1N1 influenza virus led to the implementation of a mass vaccination program, which resulted in the administration of ~45 million doses of inactivated vaccine in the U.S (Dowdle, 1997). The vaccine campaign was halted because the pandemic did not materialize and adverse events were reported in association with the vaccine. Since then, sporadic cases of swine influenza infection associated with clinical disease have occurred in people exposed to sick pigs (O'Brien et al., 1977) and were occasionally fatal (Rota et al., 1989); only limited, non-sustained human-to-human transmission of swine influenza viruses had been reported before April 2009 (Robinson et al., 2007; Top and Russell, 1977; Wells et al., 1991).
Because the hemagglutinin of the 2009 pandemic H1N1 virus is a derivative of and is antigenically related to classical swine influenza H1N1 viruses, we investigated the ability of swine influenza viruses to protect ferrets and mice from challenge with the 2009 pandemic H1N1 virus. We selected A/California/7/2009 (H1N1) and A/California/4/2009 (H1N1) viruses that were isolated from two unrelated cases of febrile respiratory illness in children who resided in adjacent counties in southern California and were the first swine-origin human 2009 pandemic H1N1 viruses identified in the United States (2009a; 2009c). Hemagglutination inhibition (HAI) assays reveal that the 2009 pandemic H1N1 viruses (A/California/7/2009 and A/California/4/2009 viruses) are antigenically similar and distinct from currently circulating seasonal H1N1 viruses (Garten et al., 2009). The two classical swine influenza viruses we included in the study were A/swine/Iowa/1931 (H1N1), a reference classical swine H1N1 virus closely related to the first isolate A/swine/Iowa/15/1930 and A/New Jersey/8/1976 (H1N1), an isolate from the limited outbreak in 1976 in Fort Dix, NJ. This comparison was made to gain insight into the degree of protective immunity that may be conferred by prior infection with influenza viruses that were derived from pigs or were antigenically related to swine influenza viruses.
MATERIALS AND METHODS
Viruses
The swine-origin 2009 H1N1 viruses, A/California/7/2009 (CA/7/09) and A/California/4/2009 (CA/4/09) used in this study were kindly provided by Drs. Ruben Donis and Alexander Klimov from the Influenza Division, Centers for Disease Control and Prevention, Atlanta, GA. The A/swine/Iowa/1931 (sw/IA/31) (H1N1) and A/New Jersey/8/1976 (NJ/76) (H1N1) viruses were kindly provided by Drs. Jeffery Taubenberger and Brian Murphy, respectively, from the National Institutes of Health, Bethesda, MD.
The CA/4/09 virus was amplified in Madin-Darby canine kidney (MDCK) cells and the other viruses were propagated in the allantoic cavity of 9 to 11-day old embryonated specific pathogen-free (SPF) hen's eggs (Charles River, Wilmington, MA), incubated at 37°C, harvested 48 h post-inoculation and tested for hemagglutinating activity. Infectious allantoic fluids were pooled, divided into aliquots and stored at −80°C until use. The fifty percent tissue culture infectious dose (TCID50) for each virus was determined by serial titration of virus in MDCK cells and calculated by the method developed by Reed and Muench (Reed and Muench, 1938).
Animal experiments were approved by the National Institutes of Health Animal Care and Use Committee.
Evaluation of viral replication in the respiratory tract of mice and antibody response to the H1N1 viruses in mice
Level of replication
To evaluate the level of replication of the viruses in the respiratory tract of mice, we administered 50μl containing 105 TCID50 of each of the H1N1 viruses or 50μl of Leibovitz-15 (L-15, Invitrogen-GIBCO) intranasally (i.n.) to four- to 6-week-old female BALB/c mice (Taconic Farms, Inc., Germantown, NY) lightly anesthetized with isoflurane. Based on previous data (Memoli et al., 2009) establishing the lethality of sw/IA/31 for mice, one group of mice also received 50μl of 10 TCID50 of sw/IA/31. On days 2, 3, 4 and 7 following inoculation, lungs, nasal turbinates and brains (including olfactory bulbs) were harvested from 4 mice in each group. In the mice that received 105 TCID50 of sw/IA/31, tissues were harvested on days 2, 3 and 4 following inoculation; in mice that received 10 TCID50 of sw/IA/31, tissues were harvested on days 2, 4 and 7 following inoculation. Organs were weighed and homogenized in L-15 medium (Invitrogen-GIBCO) containing antibiotic-antimycotic (penicillin, streptomycin, and amphotericin B) (Invitrogen-GIBCO) to make 5% (wt/vol) (nasal turbinates) or 10% (wt/vol) (lungs, brains) tissue homogenates. Tissue homogenates were clarified by centrifugation and titrated in 24- and 96-well tissue culture plates containing MDCK cell monolayers and TCID50 was calculated by the method developed by Reed and Munch (Reed and Muench, 1938). Titers are expressed as log10 TCID50/g of tissue.
Evaluation of antibody response
Neutralizing antibody titers in pre and post-infection (p.i.) sera were determined in a microneutralization (MN) assay as described earlier (Joseph et al., 2008). Serial two-fold dilutions of serum were prepared starting from a 1:10 or 1:20 dilution following receptor destroying enzyme (RDE) treatment and heat-inactivation. Equal volumes of serum and virus were mixed and incubated for 60 minutes at room temperature. The residual infectivity of the virus-serum mixture was determined in MDCK cells in four replicates for each dilution. Neutralizing antibody titers were defined as the reciprocal of the highest dilution of serum that completely neutralized the infectivity of 100 TCID50 of the virus as determined by the absence of CPE at day 4, as previously described (Joseph et al., 2008). The small volume of mouse sera precluded testing in a hemagglutination inhibition (HAI) assay.
Evaluation of viral replication and antibody response to H1N1 viruses in ferrets
Level of replication
The ability of H1N1 viruses to replicate in ferrets was compared in groups of 8-12 week-old ferrets (Triple F Farms, Sayre, PA) that were seronegative to seasonal H3N2, H1N1 and swine-origin 2009 H1N1 viruses. Each ferret was lightly anesthetized with isoflurane and inoculated i.n. with 106 TCID50 of each of the H1N1 viruses in a volume of 0.2 mL (0.1 mL per nostril). On days 1, 2, and 3 post-inoculation, groups of three ferrets were euthanized and nasal turbinates, left lower lobe of the lung, and portions from the anterior, middle and posterior part of the brain were harvested and 10% w/v tissue homogenates were titrated on MDCK monolayers, as previously described (Joseph et al., 2008). Titers are expressed as log10 TCID50/g of tissue.
Evaluation of clinical signs
In a separate study, groups of three ferrets were infected i.n with each of the H1N1 viruses to evaluate symptoms/clinical signs and antibody responses to the H1N1 viruses. The infected animals were monitored daily for 14 days for clinical signs of influenza infection, body temperature and weight. Ferrets were evaluated for nasal symptoms including nasal rattling, sneezing, nasal discharge, and mouth breathing and level of activity (Reuman, Keely, and Schiff, 1989).
Evaluation of antibody response
Serum samples collected prior to dosing and on days 14 and 28 p.i. were tested for HAI antibodies by standard methods using 4 HA units of virus in V-bottom 96-well microtiter plates with 0.5% turkey erythrocytes. Neutralizing antibody titers were also evaluated in a microneutralization assay, as described above.
Cross protection studies in mice
Groups of four 6-8 week-old BALB/c mice (Taconic Farms, Inc., Germantown, NY) were infected with 50μl containing 105 TCID50 of H1N1 viruses or L-15 medium (mock-infected) and were challenged with 50μl containing 105 TCID50 of the CA/7/09 virus i.n. 27 days later. The nasal turbinates, lungs, and brain tissue were harvested from these mice at days 2 and 4 following challenge and were homogenized and virus titers were determined in MDCK cells.
Cross protection studies in ferrets
Groups of three 8-12 week-old ferrets infected with 106 TCID50 of H1N1 viruses or L-15 medium (mock-infected) were challenged with 105 TCID50 of the CA/7/09 virus i.n. 28 days later. The nasal turbinates, lungs, and brain tissue were harvested from these ferrets 5 days following challenge and were homogenized and virus titers were determined in MDCK cells. We selected this time point for harvest of tissues because the difference in titer on days 3 and 5 was not statistically significant and day 5 was a later time point.
RESULTS
We first established the kinetics of replication and pathogenesis of the 2009 swine-origin pandemic H1N1 influenza viruses (CA/7/09 and CA/4/09) and two classical swine influenza viruses (sw/IW/31 and NJ/76) in mice and ferrets and then used these models to assess the extent of homologous and heterologous protection provided by these H1N1 viruses.
Level of viral replication in mice
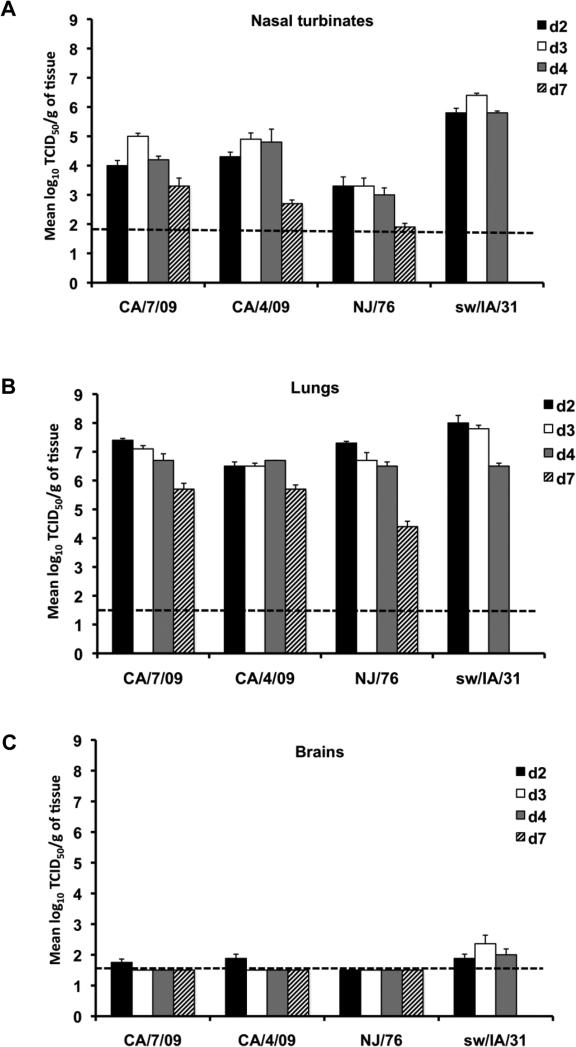
The kinetics of replication of the H1N1 influenza viruses are displayed in Figure 1. With the exception of the NJ/76 virus, all of the H1N1 viruses replicated to high titer in the upper respiratory tract with the sw/IA/31 being the highest (Figure 1A). In the upper respiratory tract, mean peak titers on day 3 p.i. of 105, 104.9, 103.3 and 106.4 TCID50/g were observed in mice inoculated with the CA/7/09, CA/4/09, NJ/76 and sw/IA/31 viruses, respectively. All of the H1N1 viruses replicated to high titer in the lower respiratory tract of mice (Figure 1B); peak titers achieved following inoculation of the viruses were as follows: 107.4 TCID50/g on day 2 p.i. in mice inoculated with CA/7/09; 106.7 TCID50/g on day 4 p.i. following inoculation of CA/4/09; 107.3 TCID50/g on day 2 p.i. following inoculation of NJ/76; and 108.0 TCID50/g on day 2 p.i. in mice inoculated with sw/IA/31virus. Virus was detected in the brain of mice infected with CA/7/09 and CA/4/09 on day 2 p.i. at mean titers of 101.75 and 101.9 TCID50/g, respectively (Figure 1C). We cannot conclude definitively that the 2009 swine-origin pandemic H1N1 influenza viruses (CA/7/09 and CA/4/09) spread to the brain of mice because olfactory bulbs were harvested along with the brain and it is possible that the detection of virus in these samples may represent direct infection from the nasal cavity rather than disseminated or systemic spread. Virus was not detected in the brain of mice that received the NJ/76 virus. Although virus was not detected in the brain of mice that were infected with the low dose (10 TCID50) of sw/IA/31 (data not shown), virus was detected in the brain of mice that received a higher dose (105 TCID50) of sw/IA/31, at mean titers of 101.9, 102.4 and 102 TCID50/g on days 2, 3 and 4 p.i., respectively (Figure 1C). The presence of virus at three time points following infection indicate that sw/IA/31 virus is capable of neuroinvasion and replication in the central nervous system.
Figure 1. Level of replication of H1N1 viruses in mice.
Groups of 4 mice were inoculated i.n. with 105 TCID50/50μl of CA/7/09, CA/4/09, NJ/76, and sw/IA/31 viruses. Virus titers in the nasal turbinates (A), lungs (B), and brains (C) of 4 mice per group sacrificed on days 2 (black bars), 3 (open bars), 4 (grey bars), and 7 (hatch bars) p.i., respectively are expressed as mean ± SE log10 TCID50/g of tissue. The dashed horizontal line indicates the lower limit of detection.
Clinical illness and level of viral replication in ferrets
None of the viruses caused lethargy, sneezing, ruffled fur, decreased interest in food, or nasal discharge in ferrets and weight loss was not observed (Figure 2A). A slight increase in body temperature was detected on day 3 especially with sw/IA/31 infection, however the temperatures remained within the normal range for ferrets (Figure 2B). Both the 2009 H1N1 viruses (CA/7/09 and CA/4/09) and the classical swine H1N1 viruses (sw/IA/31 and NJ/76) replicated to high titers in the upper respiratory tract of ferrets on 1, 3, and 5 days p.i. (Figure 2C). With the exception of the NJ/76 virus, all of the H1N1 viruses replicated to high titers in the lower respiratory tract (Figure 2D); peak replication of the CA/7/09 virus was detected on day 3 and CA/4/09 and sw/IA/31 viruses on day 5 p.i. The peak titer of the NJ/76 virus was statistically significantly lower than the other H1N1 viruses (p<0.05, Kruskal-Wallis). Virus was not detected in the brain of ferrets inoculated with these viruses (data not shown).
Figure 2. Evaluation of clinical signs and viral replication following inoculation of H1N1 viruses in ferrets.
Groups of 3 ferrets were inoculated i.n. with 106 TCID50/0.2ml of H1N1 CA/7/09 (●), CA/4/09 (×), NJ/76 (□), sw/IA/31(○) viruses. Mock-infected ferrets (◇) received L-15. Percentage change in body weight (A) and average body temperature (B) following i.n. inoculation of each indicated virus were monitored daily for 14 days in ferrets. Virus titers in the nasal turbinates (C) and lungs (D) of 3 ferrets per group sacrificed on days 1 (black bars), 3 (open bars), and 5 (grey bars) p.i., respectively are expressed as mean ± SE log10 TCID50/g of tissue. The dashed horizontal line indicates the lower limit of detection.
Effect of prior infection with H1N1 viruses on subsequent challenge with CA/7/09 virus in mice
In order to assess the extent of homologous and heterologous protection provided by these antigenically related viruses, we evaluated the effect of prior infection with 2009 pandemic H1N1 viruses (CA/7/09 and CA/4/09) or classical swine influenza viruses (NJ/76 and sw/IW/31) on subsequent challenge with the CA/7/09 virus. Following primary infection with H1N1 viruses, serum neutralizing antibody titers against the CA/7/09 virus and classical swine viruses (NJ/76 and sw/IW/31 viruses) were measured in sera collected on day 25 post-inoculation (Table 1) and the extent of replication of the CA/7/09 challenge virus is presented in Figure 3. Six of the eight mice inoculated with the CA/7/09 virus as the primary infection were euthanized between days 7-10 post-inoculation due to severe weight loss and only two mice in the group survived long enough for challenge; subsequent studies (data not shown) indicate that these mice received a dose that was approximately the LD50 of the CA/7/09 virus (104.7 TCID50). Serologic analysis was therefore limited to the sera from the two surviving mice. Because CA/7/09 and CA/4/09 viruses are antigenically similar, we used the CA/7/09 virus for serologic assays. Primary infection with each of the H1N1 viruses elicited a modest to robust homologous antibody response in 100% of the mice (Table 1, titers in bold). The NJ/76 virus induced a lower homologous neutralizing antibody response than the other H1N1 viruses, though the virus replicated to comparable titers in the lungs of mice. Primary infection with CA/7/09 and CA/4/09 viruses provided complete protection from subsequent homologous challenge in both the upper and lower respiratory tract of mice (Figure 3A and B). Primary infection with NJ/76 and sw/IA/31 viruses elicited low titers of cross-neutralizing antibody against the CA/7/09 virus in 7 of 8 mice. Consistent with the low homologous neutralizing antibody response, the NJ/76 virus induced a lower titer of cross-neutralizing antibody response than the sw/IA/31 virus. Although the level of cross-neutralizing antibody was low, primary infection with sw/IA/31 virus conferred significant protection from CA/7/09 virus challenge in the upper respiratory tract of mice on 2 days post-challenge (Figure 3A) (p<0.05 Kruskal-Wallis, compared with mock-infected animals) and NJ/76 virus infection resulted in a reduction in virus titer compared with mock-infected mice, though the difference did not achieve statistical significance. In the lower respiratory tract, primary infection with sw/IA/31 and NJ/76 viruses conferred partial protection; the reduction in titer was statistically significant for sw/IA/31 compared with mock-infected animals (p<0.05, Kruskal-Wallis) (Figure 3B).
TABLE 1.
Serum neutralizing antibodies elicited in mice following intranasal infection with H1N1 viruses
| Serum neutralizing antibody response against indicated virus |
||||||
|---|---|---|---|---|---|---|
| Primary infectiona | CA/7/09 | NJ/76 | sw/IA/31 | |||
| GMT (range)b | # with detectable antibody/totalc | GMT (range) | # with detectable antibody/total | GMT (range) | # with detectable antibody/total | |
| CA/7/09 | 1280d | 2/2 | NDe | NDe | ||
| CA/4/09 | 446 (320-806) | 8/8 | NDe | NDe | ||
| NJ/76 | 34 (10-63) | 7/8 | 58 (20-113) | 8/8 | 16 (10-25) | 3/8 |
| sw/IA/31 | 41 (10-101) | 7/8 | 29 (16-57) | 8/8 | 281 (202-403) | 8/8 |
Mice received 105 TCID50 of indicated virus i.n. and serum was obtained on day 25 p.i..
Values represent geometric mean antibody titers from 2-8 mice per group. Antibodies were not detectable in pre-infection sera. Homologous antibody titers are in bold. An undetectable serum neutralizing antibody titer was assigned a value of 10.
Numbers of mice elicited neutralizing antibody response from each group are indicated.
Serum neutralizing antibody titer in two of the eight mice in the group that survived following primary infection with CA/7/09.
ND: Not determined.
Figure 3. Effect of prior infection with H1N1 viruses on subsequent challenge with CA/7/09 virus in mice.
Groups of 4 mice were inoculated i.n. with 105 TCID50/50μl of CA/7/09 (horizontal hatch bars), CA/4/09 (grey bars), NJ/76 (diagonal hatch bars), and sw/IA/31 (open bars) viruses. Mock-infected mice (black bars) received L-15. On day 28 p.i., the mice were challenged with 105 TCID50/50μl of CA/7/09 virus. Virus titers in the nasal turbinates (A) and lungs (B) of 4 mice per group sacrificed on 2 and 4 days post-challenge are expressed as mean ± SE log10TCID50/g of tissue. The dashed horizontal line indicates the lower limit of detection.
Effect of prior infection with H1N1 viruses on subsequent challenge with the CA/7/09 virus in ferrets
The extent of homologous and heterologous protection provided by antigenically related H1N1 viruses was also evaluated in ferrets. Primary infection with the H1N1 viruses induced robust homologous HAI and neutralizing antibody responses (Table 2, titers in bold). The CA/7/09, CA/4/09 and NJ/76 viruses induced robust HAI and neutralizing antibodies against the CA/7/09 and NJ/76 viruses but none induced cross-reactive antibodies against sw/IA/31. The sw/IA/31 virus elicited low titers of cross-neutralizing antibodies against the CA/7/09 pandemic H1N1 virus (Table 2) and a cross-HAI antibody response was detected in 1 of 3 animals. These data indicated that sw/IA/31 was antigenically distinct from the 2009 pandemic H1N1 virus. The significance of cross-HAI and cross-neutralizing antibody titers that exceed homologous titers is unknown. Primary infection with each of the H1N1 viruses, including sw/IA/31 provided robust protection from replication of the CA/7/09 virus in the respiratory tract of ferrets; the titer of the challenge virus was below the limit of detection when assayed on day 5 post-challenge. The titer of the challenge virus was measured on day 5 post-challenge because virus titers in the lungs of ferrets on day 5 were similar to titers achieved on day 3 (Figure 2D). The titer of the challenge virus in the lungs and nasal turbinates of mock-infected animals was 107.5 TCID50/g and 107.8 TCID50/g, respectively and the absence of detectable virus in the other groups was statistically significant (p<0.05, Mann-Whitney U test).
TABLE 2.
Serum neutralizing and HAI antibodies elicited in ferrets following intranasal infection with H1N1 viruses
| Primary infectiona | Geometric mean (range) serum neutralizing antibody titers against indicated virusb |
Geometric mean (range) serum HAI antibody titers against indicated virusc |
||||
|---|---|---|---|---|---|---|
| CA/7/09 | NJ/76 | sw/IA/31 | CA/7/09 | NJ/76 | sw/IA/31 | |
| CA/7/09 | 1140 (254-5120) | 2560 (2032-3225) | 10 | 320 | 113 (80-160) | 5 |
| CA/4/09 | 2370 (1810-4064) | 941(508-1613) | 10 | 403 (320-640) | 80 (40-160) | 5 |
| NJ/76 | 436 (320-806) | 2463 (1613-3620) | 10 | 80 (40-160) | 127 (80-160) | 5 |
| sw/IA/31 | 37 (32-40) | 1881 (1016-4064) | 1280 (1016-2032) | 8 (5-20) | 64 (40-160) | 101 (40-320) |
Ferrets received 106 TCID50 of the virus i.n. and serum was obtained four weeks later. Values represent geometric mean antibody titers from three ferrets per group. Antibodies were not detectable in pre-infection sera. Homologous antibody titers are in bold. Ranges of of antibody titers are in parentheses.
An undetectable serum neutralizing antibody titer was assigned a value of 10.
An undetectable HAI antibody titer was assigned a value of 5.
DISCUSSION
In order to better understand the pathogenesis of the swine-origin pandemic 2009 H1N1 virus, several investigators have compared these viruses with seasonal influenza viruses. However, CA/7/09, the virus recommended by the WHO as the reference virus for vaccine development, has not been evaluated extensively in animal models. We evaluated the ability of this virus to cause disease in mice and ferrets and compared it with CA/4/09 (H1N1) as well as a progenitor classical swine H1N1 virus, sw/IA/31, and NJ/76, a swine influenza virus that caused a limited outbreak of human infections at a military camp in 1976. We used egg grown viruses, as have other investigators (Maines et al., 2009; Munster et al., 2009). Our goal was to establish the kinetics of replication in these models so that we could evaluate the extent of cross-protection conferred by classical swine H1N1 viruses against the 2009 pandemic H1N1 virus.
Consistent with findings of other investigators (Itoh et al., 2009; Maines et al., 2009), we observed that the pandemic 2009 H1N1 influenza viruses (CA/7/09 and CA/4/09) replicated efficiently in the respiratory tract of mice without prior host adaptation. Statistically significant differences were not observed in the replication kinetics of the 2009 pandemic and classical swine H1N1 viruses in either the upper or lower respiratory tract of mice. The replication kinetics and pathogenicity of the swine-origin pandemic 2009 H1N1 viruses in ferrets in our study were also consistent with findings of others (Itoh et al., 2009; Maines et al., 2009; Munster et al., 2009). However, the replication of the pandemic H1N1 influenza viruses in ferrets was not associated with significant signs or symptoms of illness in our experiments. In our study, female and male ferrets aged 8-12 weeks were inoculated intranasally with 106 TCID50 of virus in a volume of 0.2 mL while other investigators who infected older ferrets with a larger inoculum reported clinical illness (Maines et al., 2009). We used a smaller volume of inoculum because we have previously shown that intranasal administration of virus in larger volume (0.5 mL) can result in a pulmonary inflammatory response in the absence of significant local replication of the inoculated virus (Jin et al., 2007).
In both mice and ferrets, the pandemic 2009 H1N1 influenza viruses induced a robust homologous antibody response. Although NJ/76 and sw/IA/31 viruses are antigenically different from the CA/7/09 virus (Garten et al., 2009), primary infection with the classical swine H1N1 viruses provided partial protection from subsequent challenge with the CA/7/09 virus in mice. Despite the relatively low degree of amino acid sequence homology between the HAs of the pandemic 2009 H1N1 viruses and the classical swine viruses (CA/7/09 vs. NJ/76 and sw/IA/31 91% and 90%, respectively), primary infection with the classical swine H1N1 viruses elicited some cross-reactive neutralizing activity and provided robust protection from subsequent challenge with the CA/7/09 virus in ferrets. Primary infection with influenza viruses stimulates mucosal and systemic antibodies as well as cellular immune responses and these responses contribute to protection. However, our analysis was limited to serum antibody responses, which are the most well established correlate of protection. The difference in the level of cross-protection conferred by the swine influenza viruses in mice and ferrets in our study are consistent with the level of cross-reactive antibodies induced in the two species; higher titers of cross reactive antibodies were induced in ferrets associated with robust protection from challenge while lower titers of cross reactive antibodies in mice were associated with partial protection. Our findings are consistent with the observations of Manicassamy et al. and Kash et al. who found that antibodies elicited against 1918-like or classical swine H1N1 vaccines fully protect mice from lethal challenge with the 2009 pandemic H1N1 virus (Manicassamy et al.).
A proportion of people, especially older individuals, have antibodies that cross-react with the 2009 pandemic H1N1 virus (2009b; Greenberg et al., 2009; Hancock et al., 2009; Itoh et al., 2009; Kash et al.; McCullers et al.); these individuals were likely exposed to H1N1 viruses that were more closely related to classical swine H1N1 influenza viruses than recent seasonal H1N1 viruses are. The epidemiology of the 2009 H1N1 pandemic shows that the elderly are less affected by severe morbidity and mortality (Chowell et al., 2009), suggesting that they are protected from severe disease by cross-reactive immunity. Sera from a small sample of individuals who had received the swine flu vaccine in 1976 had cross-reactive antibodies to the 2009 H1N1 virus (Hancock et al., 2009). Our findings in animal models support these observations and suggest that the elderly and the ~45 million people who had prior infection with classical swine influenza virus and who received the swine flu vaccine in 1976 would be protected from severe disease caused by the pandemic 2009 H1N1 influenza viruses (CA/7/09).
ACKNOWLEDGEMENTS
We thank Jadon Jackson and the staff of the Comparative Medicine Branch, NIAID for technical support for animal studies performed at the NIH. We thank Dr. Catherine Luke for invaluable discussion throughout this work. This research was supported in part by the Intramural Research Program of the NIH, NIAID.
REFERENCES
- MMWR Morb Mortal Wkly Rep Outbreak of swine-origin influenza A (H1N1) virus infection - Mexico, March-April 2009. 2009a;58(17):467–70. [PubMed] [Google Scholar]
- MMWR Morb Mortal Wkly Rep Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. 2009b;58(19):521–4. [PubMed] [Google Scholar]
- MMWR Morb Mortal Wkly Rep Swine influenza A (H1N1) infection in two children--Southern California, March-April 2009. 2009c;58(15):400–2. [PubMed] [Google Scholar]
- Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M, Miller MA. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;361(7):674–9. doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
- Dowdle WR. Pandemic influenza: confronting a re-emergent threat. The 1976 experience. J Infect Dis 176 Suppl. 1997;1:S69–72. doi: 10.1086/514180. [DOI] [PubMed] [Google Scholar]
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, Lopez-Gatell H, Olivera H, Lopez I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD, Jr., Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325(5937):197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaydos JC, Hodder RA, Top FH, Jr., Soden VJ, Allen RG, Bartley JD, Zabkar JH, Nowosiwsky T, Russell PK. Swine influenza A at Fort Dix, New Jersey (January-February 1976). I. Case finding and clinical study of cases. J Infect Dis. 1977;136(Suppl):S356–62. doi: 10.1093/infdis/136.supplement_3.s356. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, Dawson G, Hu W, Leggio C, Washington D, Basser RL. Response after One Dose of a Monovalent Influenza A (H1N1) 2009 Vaccine -- Preliminary Report. N Engl J Med. 2009 doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, Liu F, Dong L, Devos JR, Gargiullo PM, Brammer TL, Cox NJ, Tumpey TM, Katz JM. Cross-Reactive Antibody Responses to the 2009 Pandemic H1N1 Influenza Virus. N Engl J Med. 2009 doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- Hay AJ, Gregory V, Douglas AR, Lin YP. The evolution of human influenza viruses. Philos Trans R Soc Lond B Biol Sci. 2001;356(1416):1861–70. doi: 10.1098/rstb.2001.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodder RA, Gaydos JC, Allen RG, Top FH, Jr., Nowosiwsky T, Russell PK. Swine influenza A at Fort Dix, New Jersey (January-February 1976). III. Extent of spread and duration of the outbreak. J Infect Dis. 1977;136(Suppl):S369–75. doi: 10.1093/infdis/136.supplement_3.s369. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, Muramoto Y, Tamura D, Sakai-Tagawa Y, Noda T, Sakabe S, Imai M, Hatta Y, Watanabe S, Li C, Yamada S, Fujii K, Murakami S, Imai H, Kakugawa S, Ito M, Takano R, Iwatsuki-Horimoto K, Shimojima M, Horimoto T, Goto H, Takahashi K, Makino A, Ishigaki H, Nakayama M, Okamatsu M, Warshauer D, Shult PA, Saito R, Suzuki H, Furuta Y, Yamashita M, Mitamura K, Nakano K, Nakamura M, Brockman-Schneider R, Mitamura H, Yamazaki M, Sugaya N, Suresh M, Ozawa M, Neumann G, Gern J, Kida H, Ogasawara K, Kawaoka Y. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460(7258):1021–5. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Manetz S, Leininger J, Luke C, Subbarao K, Murphy B, Kemble G, Coelingh KL. Toxicological evaluation of live attenuated, cold-adapted H5N1 vaccines in ferrets. Vaccine. 2007;25(52):8664–72. doi: 10.1016/j.vaccine.2007.10.032. [DOI] [PubMed] [Google Scholar]
- Joseph T, McAuliffe J, Lu B, Vogel L, Swayne D, Jin H, Kemble G, Subbarao K. A live attenuated cold-adapted influenza A H7N3 virus vaccine provides protection against homologous and heterologous H7 viruses in mice and ferrets. Virology. 2008;378(1):123–32. doi: 10.1016/j.virol.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash JC, Qi L, Dugan VG, Jagger BW, Hrabal RJ, Memoli MJ, Morens DM, Taubenberger JK. Prior infection with classical swine H1N1 influenza viruses is associated with protective immunity to the 2009 pandemic H1N1 virus. Influenza Other Respi Viruses. 2010;4(3):121–7. doi: 10.1111/j.1750-2659.2010.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendal AP, Goldfield M, Noble GR, Dowdle WR. Identification and preliminary antigenic analysis of swine influenza-like viruses isolated during an influenza outbreak at Fort Dix, New Jersey. J Infect Dis. 1977;136(Suppl):S381–5. doi: 10.1093/infdis/136.supplement_3.s381. [DOI] [PubMed] [Google Scholar]
- Maines TR, Jayaraman A, Belser JA, Wadford DA, Pappas C, Zeng H, Gustin KM, Pearce MB, Viswanathan K, Shriver ZH, Raman R, Cox NJ, Sasisekharan R, Katz JM, Tumpey TM. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science. 2009;325(5939):484–7. doi: 10.1126/science.1177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicassamy B, Medina RA, Hai R, Tsibane T, Stertz S, Nistal-Villan E, Palese P, Basler CF, Garcia-Sastre A. Protection of mice against lethal challenge with 2009 H1N1 influenza A virus by 1918-like and classical swine H1N1 based vaccines. PLoS Pathog. 2010;6(1):e1000745. doi: 10.1371/journal.ppat.1000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullers JA, Van De Velde LA, Allison KJ, Branum KC, Webby RJ, Flynn PM. Recipients of vaccine against the 1976 “swine flu” have enhanced neutralization responses to the 2009 novel H1N1 influenza virus. Clin Infect Dis. 2010;50(11):1487–92. doi: 10.1086/652441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memoli MJ, Tumpey TM, Jagger BW, Dugan VG, Sheng ZM, Qi L, Kash JC, Taubenberger JK. An early 'classical' swine H1N1 influenza virus shows similar pathogenicity to the 1918 pandemic virus in ferrets and mice. Virology. 2009 doi: 10.1016/j.virol.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, de Wit E, van den Brand JM, Herfst S, Schrauwen EJ, Bestebroer TM, van de Vijver D, Boucher CA, Koopmans M, Rimmelzwaan GF, Kuiken T, Osterhaus AD, Fouchier RA. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science. 2009;325(5939):481–3. doi: 10.1126/science.1177127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis. 2007;44(8):1084–8. doi: 10.1086/512813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RJ, Noble GR, Easterday BC, Kendal AP, Shasby DM, Nelson DB, Hattwick MA, Dowdle WR. Swine-like influenza virus infection in a Wisconsin farm family. J Infect Dis. 1977;136(Suppl):S390–6. doi: 10.1093/infdis/136.supplement_3.s390. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]
- Reuman PD, Keely S, Schiff GM. Assessment of signs of influenza illness in the ferret model. J Virol Methods. 1989;24(1-2):27–34. doi: 10.1016/0166-0934(89)90004-9. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Lee BE, Patel J, Bastien N, Grimsrud K, Seal RF, King R, Marshall F, Li Y. Swine influenza (H3N2) infection in a child and possible community transmission, Canada. Emerg Infect Dis. 2007;13(12):1865–70. doi: 10.3201/eid1312.070615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota PA, Rocha EP, Harmon MW, Hinshaw VS, Sheerar MG, Kawaoka Y, Cox NJ, Smith TF. Laboratory characterization of a swine influenza virus isolated from a fatal case of human influenza. J Clin Microbiol. 1989;27(6):1413–6. doi: 10.1128/jcm.27.6.1413-1416.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde V, Bridges CB, Uyeki TM, Shu B, Balish A, Xu X, Lindstrom S, Gubareva LV, Deyde V, Garten RJ, Harris M, Gerber S, Vagasky S, Smith F, Pascoe N, Martin K, Dufficy D, Ritger K, Conover C, Quinlisk P, Klimov A, Bresee JS, Finelli L. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005-2009. N Engl J Med. 2009;360(25):2616–25. doi: 10.1056/NEJMoa0903812. [DOI] [PubMed] [Google Scholar]
- Thompson RL, Sande MA, Wenzel RP, Hoke CH, Jr., Gwaltney JM., Jr. Swine-influenza infection in civilians. Report of two cases. N Engl J Med. 1976;295(13):714–5. doi: 10.1056/NEJM197609232951307. [DOI] [PubMed] [Google Scholar]
- Top FH, Jr., Russell PK. Swine influenza A at Fort Dix, New Jersey (January-February 1976). IV. Summary and speculation. J Infect Dis. 1977;136(Suppl):S376–80. doi: 10.1093/infdis/136.supplement_3.s376. [DOI] [PubMed] [Google Scholar]
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56(1):152–79. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells DL, Hopfensperger DJ, Arden NH, Harmon MW, Davis JP, Tipple MA, Schonberger LB. Swine influenza virus infections. Transmission from ill pigs to humans at a Wisconsin agricultural fair and subsequent probable person-to-person transmission. JAMA. 1991;265(4):478–81. doi: 10.1001/jama.265.4.478. [DOI] [PubMed] [Google Scholar]