Abstract
The need to increase the donor pool for lung transplantation is a major public health issue. We previously found that administration of mesenchymal stem cells “rehabilitated” marginal donor lungs rejected for transplantation using ex vivo lung perfusion. However, the use of stem cells has some inherent limitation such as the potential for tumor formation. In the current study, we hypothesized that microvesicles, small anuclear membrane fragments constitutively released from mesenchymal stem cells, may be a good alternative to using stem cells. Using our well established ex vivo lung perfusion model, microvesicles derived from human mesenchymal stem cells increased alveolar fluid clearance (i.e. ability to absorb pulmonary edema fluid) in a dose-dependent manner, decreased lung weight gain following perfusion and ventilation, and improved airway and hemodynamic parameters compared to perfusion alone. Microvesicles derived from normal human lung fibroblasts as a control had no effect. Co-administration of microvesicles with anti-CD44 antibody attenuated these effects, suggesting a key role of the CD44 receptor in the internalization of the microvesicles into the injured host cell and its effect. In summary, microvesicles derived from human mesenchymal stem cells were as effective as the parent mesenchymal stem cells in rehabilitating marginal donor human lungs.
Introduction
Lung transplantation remains the standard of care for an increasing number of patients with irreversible lung diseases. Since 1990, the number of lung transplantations in the world has multiplied by four, reaching 3700 cases in 2011, with a median age of recipients increasing from 45 to 55 years (1). Unfortunately, in the United States, the number of new patients on the waiting list for lung transplantation has grown from 1500 in 2006 to 2200 in 2012, with a mortality rate of 300 patients on the waiting list per year over the same period (2). Nevertheless, due to improvements in medical and surgical care, recipient survival rate at 5 years increased from 45% to 55% in the past 20 years (1).
Recently, a new technique of ex vivo lung perfusion (EVLP) has been developed to extend the donor pool size (3,4). EVLP allows “rehabilitation” of marginal donor lungs initially rejected for transplantation by allowing a short duration of perfusion and oxygenation with ventilation prior to transplantation, which in preliminary studies has reduced the incidence of primary graft dysfunction (3,4). In addition, EVLP has become an ideal method to test the effects of pharmacologic and/or gene- or cell-based therapy prior to surgery to improve the success of lung transplantation (5,6). Using a preclinical model of EVLP, we reported that intravenous administration of human bone marrow-derived mesenchymal stem (stromal) cells (MSC) restored alveolar fluid clearance (AFC) in lungs rejected for transplantation (7); in patients with acute respiratory distress syndrome, impaired AFC rate is associated with higher mortality (8). Based on our previous studies in a human EVLP model of acute lung injury induced by Escherichia coli bacteria or endotoxin (5,9), we postulated that the primary mechanisms underlying the therapeutic effect of MSC were through the secretion of soluble factors with reparative properties. However, the use of MSC or cell-based therapy in clinical practice has some potential limitations such as the risk of tumor formation, immunogenicity, and need of a bone marrow transplant facility to store and process the stem cells (10,11).
Recently, MSC have been found to release microvesicles (MV) that were as biologically active as the cells themselves. MVs are anuclear plasma membrane bound circular fragments, 50–200 nm in size, constitutively released from multiple cell types from the endosomal compartment as exosomes or shed from the plasma membrane (12). Microvesicles derived from human mesenchymal stem cells (MSC MV) express very low levels of MHC I or II antigens allowing them to become “immunoprivileged” and carry mRNA, miRNA and proteins for soluble factors with reparative properties. Bruno et al found that MSC MV accelerated the morphologic and functional recovery of glycerol-induced acute kidney injury in mice by inducing proliferation of renal tubular cells (13). MSC MV homed and incorporated into the injured tubular cells in part via the surface receptor CD44 (extracellular matrix receptor type III for hyaluronic acid), allowing the transfer of MSC MV mRNA. We also found that human MSC MV reduced pulmonary edema and lung protein permeability in an E. coli endotoxin-induced acute lung injury in mice in part through the expression of keratinocyte growth factor (KGF) mRNA in the injured alveolus (14). In the current study, we hypothesize that MSC MV would be effective in restoring AFC in human lungs rejected for transplantation using EVLP.
Materials and Methods
Selection criteria for human lungs
We used human lungs rejected for transplantation by the Northern California Transplant Donor Network and approved for research. Lungs were resected en bloc without preservative flush but heparinized, gently inflated, and stored on ice at 4°C. Lungs were used if they met these criteria: (1) a cold ischemia time <48 h, (2) no obvious parenchyma lesions, and (3) AFC >0% but <10%/h.
Ex vivo perfused human lung and measurement of alveolar fluid clearance
The pulmonary artery and bronchus was cannulated from either the right or left lung and placed in a bioreactor (Harvard Apparatus, Holliston, MA) as previously described (7). The rate of perfusion, with a solution containing DMEM without Phenol Red + 5% bovine serum albumin and kept at 37°C, was slowly increased to 250 mL/min over 30 min, allowing progressive rewarming of the lung. When the perfusate temperature reached 30°C, ventilation with a large animal respirator (Harvard Apparatus) was started (VT = 300 mL, respiratory rate = 10/min, FiO2 = 21%, and positive end-expiratory pressure = 5 cmH2O, Figure 1A). Perfusion flow, pulmonary arterial pressure (PAP), pulmonary vascular resistance (PVR), tidal volume (VT), tracheal pressure, and compliance were recorded in real time using Pulmodyn Data Acquisition software (Harvard Apparatus).
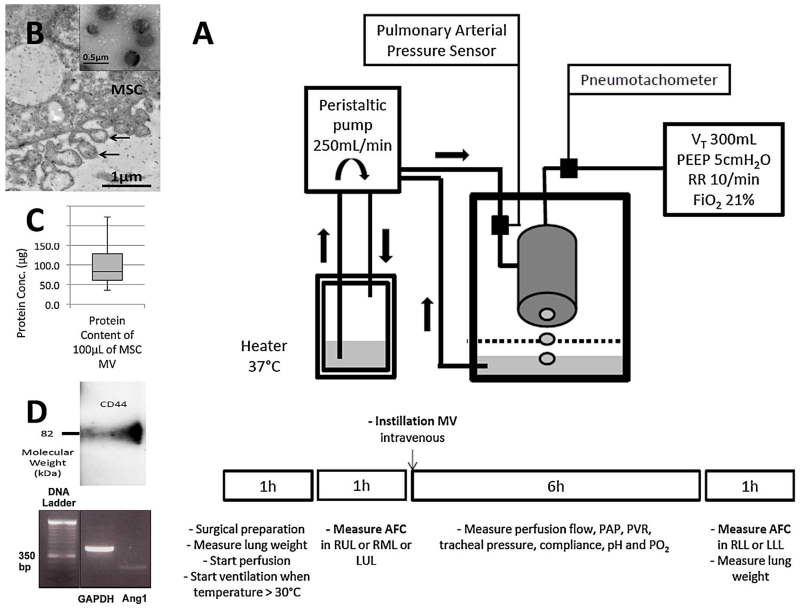
Figure 1. Characterization of mesenchymal stem cell microvesicles and the ex vivo lung perfusion model.
(A) Schematic view of the ex vivo human lung preparation as well as lung perfusion and ventilation protocol used for the experiments. (B) Electron microscopy image of human mesenchymal stem cells (MSC) constitutively releasing microvesicles (MV) after 48 h of serum starvation. Black arrows designate MV, which appear as spheroids budding off the plasma membrane. Insert shows a homogenous collection of MSC MV, which is 50 to 200 nm in size. (C) Protein concentration of 100 μL of MSC MV. Data represent the median of 24 samples of MSC MV. (D) Western Blot showing the expression of the membrane receptor CD44 by MSC MV. CD44 plays an important role in MSC MV internalization into the host cell. PCR showing the expression of Ang1 mRNA by RT-PCR.
After steady state was reached, a small catheter (Intramedic, BD Bioscience, Franklin Lakes, NJ) was introduced into the bronchus and advanced into the lung lobe of interest until gentle resistance was encountered. A volume of 125 mL of warmed normal saline solution containing 5% albumin was instilled, and the bronchoalveolar lavage (BAL) fluid was sampled after 5 and 35 min. The AFC was measured by the change in protein concentration of the BAL fluid over 30 min using the following equation: AFC (%/h) = 2(1-Ci/Cf) × 100 (Ci and Cf = protein concentration 5 and 35 min after BAL instillation respectively). If 0 <AFC <10%/h, either 100 μL of MSC MV, 200 μL of MSC MV, 200 μL of MSC MV + anti-CD44 antibody (Ab), 200 μL of MSC MV + polyclonal goat IgG, or 200 μL of microvesicles derived from normal human lung fibroblasts (NHLF MV, Lonza, Basel, Switzerland) was administered into the perfusate. The control group received perfusion only. At 6 h (T6), a second BAL was performed into the lower lobe to measure AFC (Figure 1A).
Measurement of blood gas and metabolites in the perfusate
Perfusate pH, PO2, and PCO2 were measured every hour from T0 to T6 using a blood gas machine (OptiMedical, Roswell, GA). Lactate, nitric oxide (NO), and Syndecan-1 levels were measured in the perfusate at T0 and T6 using colorimetric kits (Fisher Scientific, Hampton, NH, and Abcam, Cambridge, UK).
Measurement of angiopoietin-1 and tumor necrosis factor-α in the BAL fluid
Angiopoietin-1 (Ang1) and TNFα concentrations were measured in the BAL fluid at T0 and T6 using ELISA kits (R&D Systems, Minneapolis, MN).
Isolation of microvesicles derived from human mesenchymal stem cells or normal human lung fibroblasts
Human bone marrow derived MSC were obtained from a NIH repository at Texas A&M Institute for Regenerative Medicine; these cells fulfilled the criteria of MSC as defined by the International Society of Cellular Therapy (15).
MSC and NHLF MV were obtained from the supernatant of human MSC and NHLF respectively using ultracentrifugation (16). MSC or NHLF were first serum starved for 48 h in conditioned medium composed of α-MEM without nucleosides and 0.5% bovine serum albumin (MP Biomedicals, Santa Ana, CA). The conditioned medium was collected and centrifuged at 300 g for 20 min to remove cellular debris and then at 100 000 g for 1 h at 4°C twice (Beckman Coulter [Brea, CA] Optima L-100XP) to sediment the MV. MSC or NHLF MV were resuspended in phosphate buffered saline according to the final cell count (10 μL per 1 × 106 cells) and stored at −80°C until further use.
Characterization of microvesicles derived from human mesenchymal stem cells
MV released by monolayers of MSC were photographed with a JEOL 1200 EX transmission electron microscope operating at 80 kV as previously described (9). The protein content of 100 μL of MSC MV was measured by BCA protein assay. Western Blot analysis was performed to confirm the presence of CD44 on the MSC MV (13) using the primary anti-CD44 Ab (1 μg/mL anti-CD44, Abcam). Total RNA was isolated from MSC MV using RNeasy Mini Kit (QIAGEN Sciences, Venlo, the Netherlands), and PCR primer for human Ang1 was purchased from SABiosciences.
Uptake of fluorescent labeled MSC MV
Primary cultures of human alveolar type II cells were isolated from human lungs as previously described (17). The cells were plated on collagen I coated 24-well plates in DMEM high glucose 50%/F-12 50% mix medium containing 10% FBS and antibiotics at a concentration of 1 × 106 cells/well. Following 72 h from isolation, type 2 cells were cultured without FBS for 24 h and then exposed to 50 ng/mL of cytomix, a mixture of IL-1β, TNF-α, and IFN-γ (R&D Systems) often used as a surrogate for inflammatory injury.
For the uptake experiments, human type 2 cells were exposed with prestained MSC MV (cellmask-fluorescent labeled MV: 30 μL/well) the same day as cell injury. To investigate the role of CD44 in MV uptake, MSC MV were pre-incubated with anti-CD44 blocking Ab (BD Biosciences) or with negative control IgG (R&D Systems). After 24 h, cells were washed twice with PBS, cytospined on glass slides, fixed 10 min with 4% paraformaldehyde and mounted with fluorescent medium (Vectaschield fluorescent mounting medium, VWR, Visalia, CA). The cells were examined by fluorescence microscopy (Leica DM 1000 microscope). Fluorescence intensities from images of 20 randomly selected microscopic fields of cells from each condition were analyzed by densitometry (ImageJ software, NIH Image, Bethesda, MD).
CD44 neutralizing antibody and polyclonal goat IgG control
In CD44 neutralizing Ab experiments, 1 μg/mL of CD44 neutralizing Ab (BD Biosciences) was mixed with 200 μL of MSC MV and incubated at 4°C for 30 min prior to administration. For control, 1 μg/mL of polyclonal goat IgG (R&D Systems) was used.
Statistical analyses
Results are expressed as mean ± SD if normally distributed and as median [Q1;Q3] if not. Comparisons between several groups were made using the analysis of variance (ANOVA). Comparisons between two groups were made using either ANOVA or the unpaired Student t-test. Comparisons with a sample over time were made by repeated measures of ANOVA using the Bonferroni correction. We used the software GraphPad Prism.
Results
Baseline data for donor lungs
The demographic, clinical data, and ischemia time for 30 donor lungs are listed in Table 1. There was no significant differences between groups for PaO2/FiO2 ratio, compliance, chest radiograph infiltrates, or lung injury score prior to organ harvest (18).
Table 1.
Donor clinical data and ischemia time
| Perfusion only |
MSC MV 100 μL |
MSC MV 200 μL |
MSC MV 200 μL + anti-CD44 Ab |
MSC MV 200 μL + IgG |
NHLF MV 200 μL |
|
|---|---|---|---|---|---|---|
| Variable | (n = 6) | (n = 6) | (n = 6) | (n = 4) | (n = 4) | (n = 4) |
| Mean age (year) | 57 ± 8 | 49 ± 11 | 43 ± 16 | 56 ± 9 | 57 ± 9 | 46 ± 18 |
| Male (%) | 33 | 67 | 67 | 75 | 50 | 25 |
| Chest radiograph infiltrates | 2 ± 0 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 2 |
| Donor PaO2/FiO2 ratio (mmHg) | 259 ± 107 | 275 ± 155 | 294 ± 137 | 309 ± 102 | 270 ± 142 | 414 ± 73 |
| PEEP (cmH2O) | 8 ± 3 | 6 ± 2 | 8 ± 4 | 8 ± 2 | 7 ± 2 | 6 ± 2 |
| Donor lung compliance (ml/cmH2O) | 35.6 ± 4.9 | 40.5 ± 10.1 | 34.0 ± 11.2 | 36.8 ± 7.6 | 41.6 ± 13.3 | 35.5 ± 14.8 |
| Lung injury score | 1.75 ± 0.40 | 1.71 ± 0.90 | 1.75 ± 0.97 | 1.69 ± 0.52 | 1.63 ± 0.48 | 1.44 ± 0.31 |
| Ischemic time (hours:minutes) | 21:26 ± 10:29 | 20:35 ± 16:40 | 28:01 ± 11:03 | 33:26 ± 4:16 | 27:25 ± 16:35 | 34:55 ± 8:36 |
Ab, antibody; MSC MV, microvesicles derived from mesenchymal stem cells; NHLF MV, microvesicles derived from normal human lung fibroblasts; PEEP, positive end-expiratory airway pressure.
Data are expressed as mean ± SD.
Lung Injury Score based on maximum of 4 points for the PaO2/FiO2 ratio, the level of positive end-expiratory airway pressure, the quasi-static respiratory compliance and the extent of chest radiograph infiltrates.
Characterization of MSC microvesicles
Using electron microscopy, we found that MV was released constitutively from MSC after 48 h of serum starvation. MSC MV appeared as homogeneous spheroids between 50 and 200 nm in size (Figure 1B). The median protein concentration of 100 μL of MSC MV was 82.8 [61.0;128.4] μg; the dose used was equivalent to the dose used in other studies using MSC MV as a therapeutic (13,16) (Figure 1C). We confirmed that MSC MV expressed the membrane receptor, CD44, by Western Blot analyses and the mRNA for Ang1 by RT-PCR (Figure 1D).
MSC MV improve AFC and decrease lung weight gain following perfusion
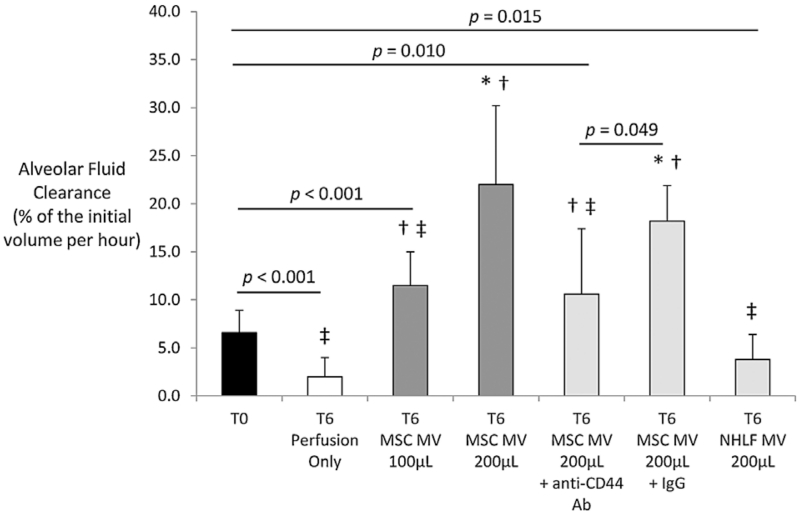
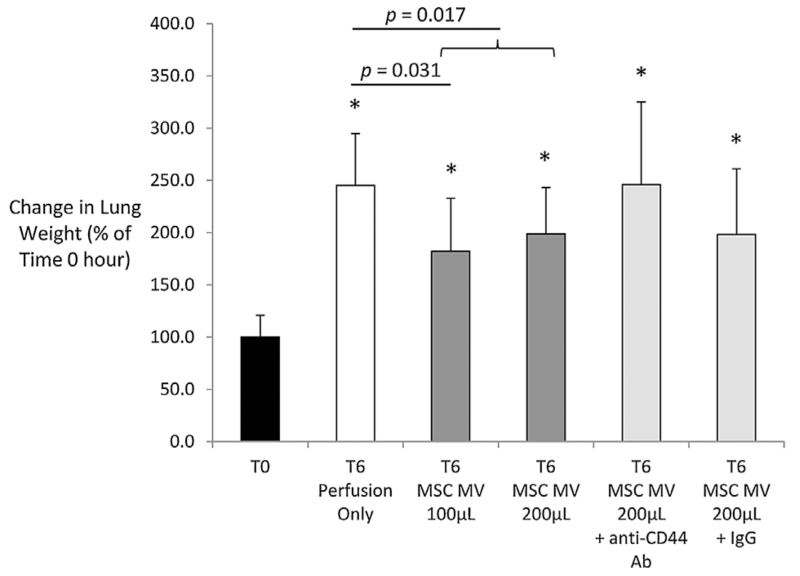
With lung perfusion and ventilation, AFC significantly decreased in the Control group from time T0 to T6. However, administration of MSC MV 100 μL or 200 μL significantly increased AFC rate in a dose-dependent manner compared to T0 (Figure 2). Lung weight increased during EVLP in all groups. However, the lung weight gain was significantly lower in the Treatment groups (MSC MV 100 μL ± 200 μL) compared to the Control at T6 (Figure 3). Administration of NHLF MV had no therapeutic effects. Perfusion alone increased Syndecan-1 levels, a marker of endothelial injury, from 38 ± 37 ng/mL (mean ± SD) at time 0 h to 139 ± 67 ng/mL at 6 h. Although not statistically significant, administration of either 100 or 200 μL MSC MV reduced Syndecan-1 levels numerically by approximately 25% at 6 h.
Figure 2. Effect of microvesicles derived from human mesenchymal stem cells on alveolar fluid clearance in donor human lungs rejected for transplantation.
Administration of MSC MV significantly improved AFC in a dose-dependent manner compared to the Control (Perfusion only) at T6. Administration of NHLF MV as a cellular control had no effect on AFC compared to the Control at T6. Inhibition of CD44 with a neutralizing Ab significantly reduced the therapeutic effect of MSC MV 200 μL, whereas administration of IgG control with MSC MV 200 μL had no significant effect. Data are presented as mean ± SD, N = 4-6 per group. *p significant by ANOVA vs. T0, †p significant by ANOVA versus T6 Perfusion Only, ‡p significant by ANOVA versus T6 MSC MV 200 μL. Abbreviations: Ab, antibody; AFC, alveolar fluid clearance; MSC, mesenchymal stem cells; MV, microvesicles; NHLF, normal human lung fibroblast.
Figure 3. Effect of microvesicles derived from human mesenchymal stem cells on lung weight gain.
Perfusion with ventilation significantly increased the total lung weight by 150%. Administration of MSC MV (100 μL or 100 μL + 200 μL groups) significantly reduced lung weight by 20–25% as compared to the Control (Perfusion only) at T6. Data was normalized to the initial weight of the lung to account for any differences in baseline lung injury or weight. Data (normalized to T0) are presented as mean ± SD, N = 4–6 per group. *p significant by ANOVA versus T0.
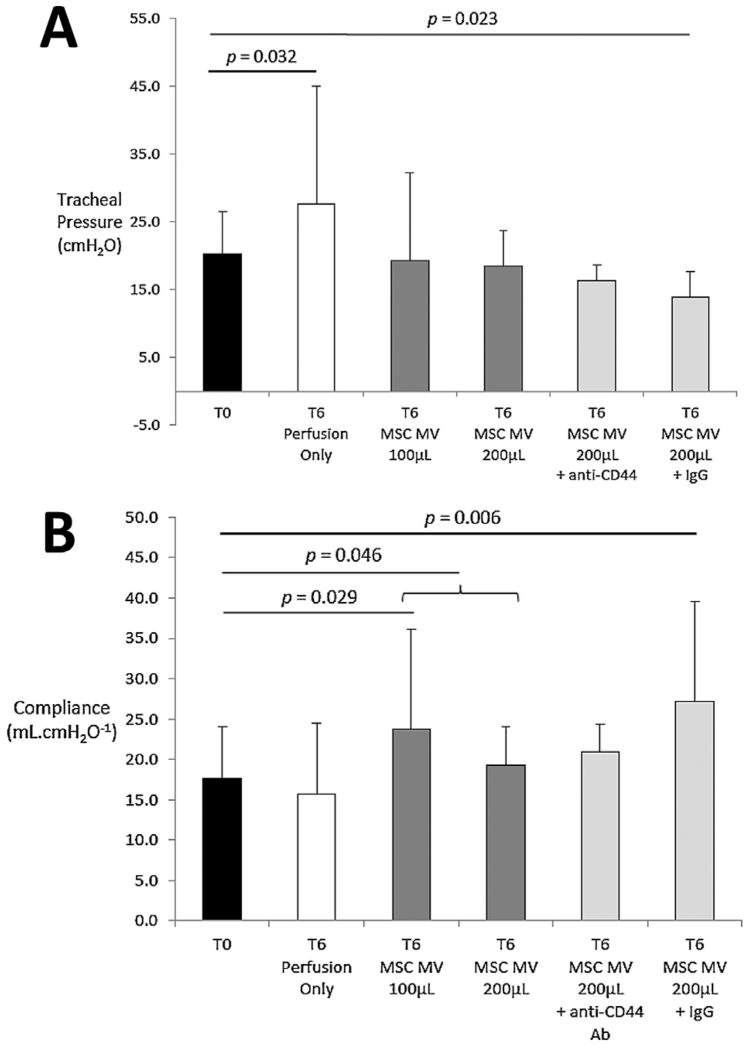
MSC MV improve airway and hemodynamic parameters
Over 6 h, the tracheal pressure in the Control group significantly increased by 37%, which was associated with a nonsignificant decrease in lung compliance at T6. However, treatment with MSC MV restored the tracheal pressure to baseline, significantly increasing the lung compliance at T6 as compared to T0 (p = 0.029 by t-test for MSC MV 100 μL and p = 0.046 by t-test for MSC MV 100 μL and 200 μL combined as a group at T6 vs. T0) (Figure 4). There was no significant effect on pulmonary artery pressure or pulmonary artery resistance with perfusion over 6 h. However, treatment with MSC MV reduced both hemodynamic parameters in a dose-dependent manner. Treatment with 200 μL MSC MV significantly reduced both PAP and PVR by 50% at T6 compared to T0 (Tables 2 and 3). Perfusate NO levels were unchanged at T6 compared to T0 in the Control group (118 ± 7% at T6 as % of Control at T0), whereas it significantly increased in both treatment groups (137 ± 13% for MSC MV 100 μL and 148 ± 9% for MSC MV 200 μL, p < 0.001 by ANOVA).
Figure 4.
Effect of microvesicles derived from human mesenchymal stem cells on lung (A) tracheal pressure and (B) compliance. Perfusion with ventilation significantly increased tracheal pressure at T6. Administration of either doses of MSC MV reduced the tracheal pressure to baseline, increasing the compliance of the lung compared to T0. Data are presented as mean ± SD, N = 4–6 per group. Abbreviations: Ab, antibody; MSC, mesenchymal stem cells; MV, microvesicles.
Table 2.
Time dependent values of mean pulmonary arterial pressure
| Perfusion only |
MSC MV μL |
MSC MV 200 μL |
MSC MV 200 μL+anti-CD44 Ab |
MSC MV 200 μL+IgG |
|
|---|---|---|---|---|---|
| Time (hours) | (n = 6) | (n = 6) | (n = 6) | (n = 4) | (n = 4) |
| 0 | 11.0 ± 6.3 | ||||
| 1 | 12.2 [4.6;20.9] | 9.0 ± 5.1 | 6.4 ± 2.3* | 9.6 ± 4.4 | 5.6 ± 2.0 |
| 2 | 5.4 [4.0;22.5] | 7.9 ± 5.9 | 5.6 ± 2.2* | 9.1 ± 6.4 | 5.0 ± 1.8* |
| 3 | 8.4 [2.8;26.6] | 11.2 ± 7.7 | 5.2 ± 2.2* | 9.4 ± 8.0 | 4.7 ± 1.4* |
| 4 | 7.2 [3.4;23.8] | 8.5 ± 7.0 | 5.3 ± 2.7* | 9.7 ± 8.6 | 4.7 ± 1.3* |
| 5 | 8.2 [2.2;26.7] | 9.3 ± 6.9 | 5.4 ± 3.0* | 10.0 ± 9.0 | 4.8 ± 1.2* |
| 6 | 7.0 [2.5;25.2] | 9.3 ± 5.1 | 5.6 ± 3.0* | 10.7 ± 8.8 | 5.0 ± 1.3* |
Ab, antibody; MSC MV, microvesicles derived from mesenchymal stem cells.
Data are expressed as mean ± SD when normally distributed and as median [Q1;Q3] if not (in mmHg).
Pulmonary arterial pressure at T0 corresponds to the mean value of each group pooled together.
p significant by Student t-test vs T0.
Table 3.
Time dependent values of mean pulmonary vascular resistance
| Perfusion only |
MSC MV 100 μL |
MSC MV 200 μL |
MSC MV 200 μL + anti-CD44 Ab |
MSC MV 200 μL + IgG |
|
|---|---|---|---|---|---|
| Time (hours) | (n = 6) | (n = 6) | (n = 6) | (n = 4) | (n = 4) |
| 0 | 44.7 ± 26.3 | ||||
| 1 | 48.9 [17.9;83.4] | 39.8 ± 28.7 | 25.5 ± 9.1* | 38.3 ± 17.6 | 22.5 ± 8.2 |
| 2 | 24.1 [16.0;89.2] | 35.9 ± 32.1 | 22.2 ± 8.7* | 36.2 ± 25.7 | 20.1 ± 7.2* |
| 3 | 33.3 [11.0;105.5] | 49.2 ± 36.8 | 20.8 ± 8.9* | 37.7 ± 32.0 | 18.9 ± 5.7* |
| 4 | 28.5 [13.8;94.4] | 38.8 ± 37.5 | 21.0 ± 11.0* | 38.7 ± 34.4 | 18.7 ± 5.3* |
| 5 | 32.3 [8.9;105.9] | 41.9 ± 37.1 | 21.8 ± 12.1* | 40.1 ± 36.1 | 19.2 ± 5.0* |
| 6 | 27.7 [10.0;100.2] | 40.4 ± 25.2 | 22.3 ± 12.1* | 42.9 ± 35.5 | 19.8 ± 5.0* |
Ab, antibody; MSC MV, microvesicles derived from mesenchymal stem cells.
Data are expressed as mean ± SD when normally distributed and as median [Q1;Q3] if not (in mmHg.L−1.min−1). Pulmonary resistances at T0 correspond to the mean value of each group pooled together.
p significant by Student t-test vs T0.
MSC MV effects on PO2, PCO2, pH, and lactate in perfusate
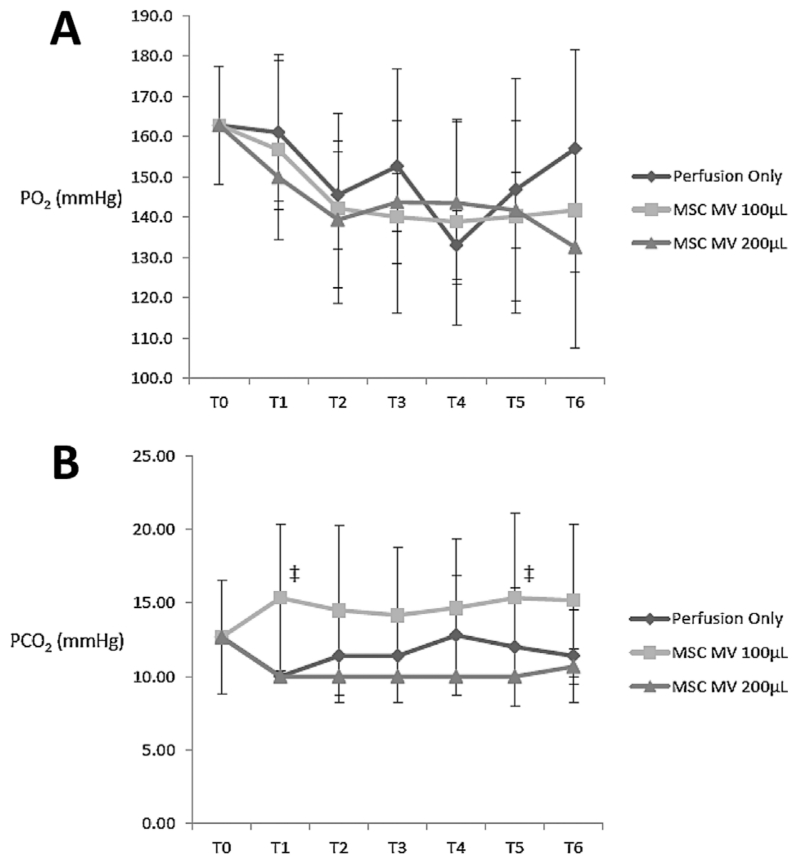
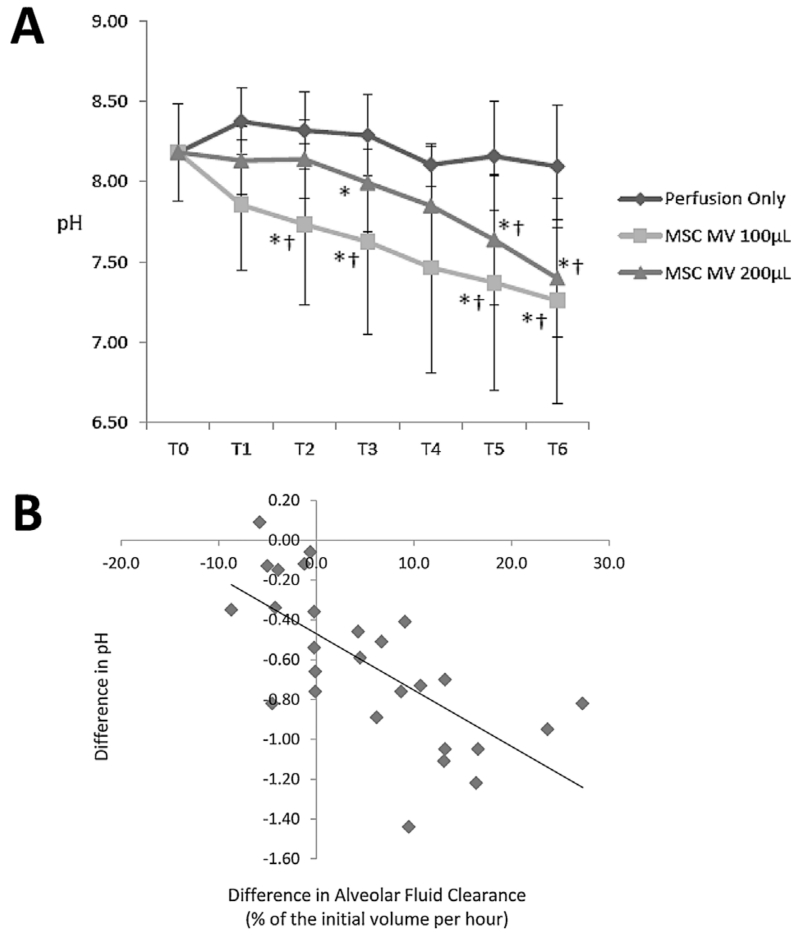
Perfusate PO2 levels decreased during EVLP with ventilation in all groups from T0 to T6 (Figure 5A). There were no significant differences between Control and Treatment groups for PO2 or PCO2 levels at T6 (Figure 5B). Perfusate pH decreased between T0 and T6 for both Treatment groups, while it remained unchanged in the Control group. At T6, pH levels were significantly lower for both Treatment groups compared to Control (p = 0.004 by ANOVA for MSC MV 100 μL and p = 0.010 by ANOVA for MSC MV 200 μL) (Figure 6A). The increase in AFC was correlated with the decrease in perfusate pH between T0 and T6 (Spearman’s rank correlation coefficient, rs = −0.7657) (Figure 6B). Perfusate lactate levels increased at T6 compared to T0 in the Control group (149 ± 36% at T6 as % of Control at T0, p = 0.010 by ANOVA), whereas it was significantly lower in both Treatment groups at T6 compared to Control group at T6 (107 ± 25% for MSC MV 100 μL, p = 0.010 by t-test, and 80 ± 24% for MSC MV 200 μL, p = 0.004 by ANOVA).
Figure 5. Effect of microvesicles derived from human mesenchymal stem cells on PO2 and PCO2 in the perfusate during ex vivo lung perfusion.
(A) In all lung groups, the PaO2 decreased with lung perfusion and ventilation. There was no significance difference in PaO2 levels at T6. (B) There was no significant difference in PaCO2 between the Treatment Groups and the Control. However, at several time points (T1-T5), the PaCO2 was higher in the MSC MV 100 μL vs. the 200 μL group. Data are presented as mean ± SD, N = 4-6 per group. ‡ p significant by ANOVA vs. T6 MV-MSC 200 μL.
Figure 6. Effect of microvesicles derived from human mesenchymal stem cells on perfusate pH during ex vivo lung perfusion.
(A) Compared to T0 and the Control (Perfusion only) at T6, administration of either dose of MSC MV reduced the perfusate pH to more physiological levels. (B) In addition, there was a strong correlation with the change in pH with the AFC, perhaps suggesting that there were increased metabolic activity in the lungs that received MSC MV. Spearman’s rank correlation coefficient, rs = −0.7657. Data are presented as mean ± SD, N = 4–6 per group. *p significant by ANOVA versus T0, †p significant by ANOVA versus Perfusion Group at the corresponding time point. Abbreviations: MSC, mesenchymal stem cells; MV, microvesicles; NHLF, normal human lung fibroblast.
MSC MV uptake is CD44-dependent
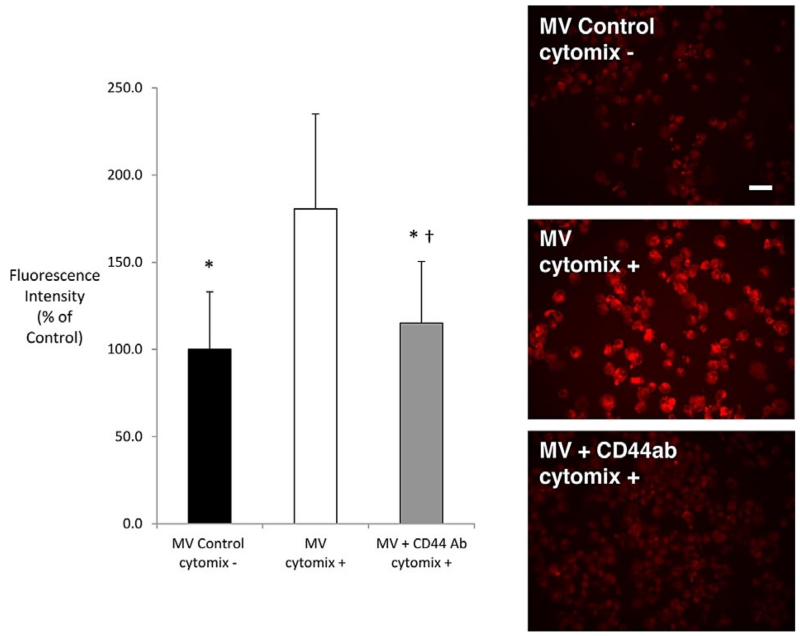
The uptake of fluorescent-labeled MSC MV was increased by 80% in human alveolar epithelial type 2 cells with injury (cytomix). Exposure to the anti-CD44 neutralizing Ab suppressed this inflammatory uptake of MSC MV (Figure 7).
Figure 7. Role of CD44 in MSC MV uptake into primary cultures of human alveolar epithelial type 2 cells.
In human alveolar epithelial type 2 cells, MSC MV uptake was dependent on CD44, the cell surface receptor for hyaluronic acid, following injury. Stimulation by an inflammatory injury (cytomix) increased the uptake of fluorescent-labeled MSC MV into alveolar epithelial type 2 cells. Fluorescence intensity was expressed as mean (% of control) ± SD for each condition, N = 179–262 cells for all groups. *p < 10 −4 versus MV/cytomix, ANOVA + †p = 0.001 versus Control Group by (Bonferroni). Photomicrographs display the pattern of fluorescence levels observed in each experimental condition. Scale bar is 20 μm.
MSC MV improve AFC in part by a CD44-dependent mechanism
The increase in AFC in EVLP using MSC MV was attenuated by administration of an anti-CD44 neutralizing Ab (p < 0.001 by ANOVA MSC MV 200 μL vs. MSC MV 200 μL + anti-CD44 Ab) but not by a control IgG (Figure 2). Co-administration of the anti-CD44 Ab with MSC MV 200 μL also eliminated any benefit with change in lung weight (Figure 3), compliance (Figure 4), or with pulmonary artery pressure and pulmonary artery resistance (Tables 2 and 3).
MSC MV effect on Ang1 and TNFα levels in the alveolus
TNFα levels in the BAL fluid significantly increased with perfusion and ventilation. However, there was no significant effect of MSC MV administration on inflammation at T6 (778 [253;3493] pg/mL for Control vs. 951 [533;4453] pg/mL for MSC MV 100 μL at T6 for TNFα level, p = 0.368 by t-test), suggesting that immunomodulation was not critical for the restoration of AFC. Although not statistically significant, administration of MSC MV increased Ang1 levels in the BAL fluid at 6 h compared to the Control (486 [232;1707] pg/mL for Control vs. 707 [297;769] pg/mL for MSC MV 100 μL at T6 for Ang1 level, p = 0.184 by t-test).
Discussion
The main findings of these studies can be summarized as follows: (1) Intravenous administration of MSC MV improved AFC rate in a dose dependent manner (Figure 2) and reduced lung weight gain (Figure 3) after 6 h of EVLP compared to perfusion alone. Administration of NHLF MV had no therapeutic effects. (2) MSC MV prevented the elevation of tracheal pressure, increased lung compliance (Figure 4), and decreased pulmonary artery pressure and artery resistance (Tables 2 and 3) following perfusion. These effects were associated with increased perfusate NO levels. (3) MSC MV decreased perfusate pH (Figure 6A) and reduced the elevation of lactate levels following perfusion, showing a correlation between MSC MV administration and improved lung metabolism. (4) CD44 neutralizing antibody eliminated the benefits of MSC MV on AFC (Figure 2), lung weight (Figure 3), compliance (Figure 4), and pulmonary artery pressure and resistance (Tables 2 and 3), demonstrating the critical role of CD44 in the therapeutic effects of MSC MV (Figure 7). (5) And, although not statistically significant, MSC MV administration was associated with a numerical reduction of Syndecan-1 levels, a marker of endothelial glycocalyx breakdown, in the perfusate and a numerical elevation of Ang1 levels in the injured alveolus, suggesting a partial restoration of lung endothelium.
This is the first study demonstrating the therapeutic effects of MSC MV in human lungs rejected for transplantation. We previously reported that human MSC given during EVLP increased AFC at 4 h after intravenous administration compared to a control group receiving perfusion only (7). In the current study, we demonstrated that MSC MV administration were as effective as MSC in restoring AFC. This is an essential point, since impaired AFC contributes to primary graft dysfunction (19,20), a major cause of morbidity and mortality after lung transplantation in the form of noncardiogenic pulmonary edema (21,22). Preserved lung barrier properties with intact AFC have been associated with better clinical outcomes in patients with posttransplant reperfusion edema or acute respiratory distress syndrome (8,19).
Although, the mechanisms underlying the effects of MSC MV on AFC in marginal donor lungs needs to be studied further, we have previously demonstrated that (1) MSC restored AFC rate in marginal donor lungs through KGF secretion following EVLP (7); (2) MSC restored protein permeability across injured human alveolar epithelial type II cells through the prevention of actin stress fiber formation by Ang1 secretion (17); and (3) MSC MV restored lung fluid balance following endotoxin induced acute lung injury in mice in part through the transfer of KGF mRNA to the injured alveolar epithelium with subsequent expression of the growth factor (14). KGF is an epithelial specific growth factor known to up-regulate alveolar fluid transport, and Ang1 is an anti-permeability factor which has been shown to prevent lung protein permeability in part through the restoration of endothelial glycocalyx (23,24). In addition, multiple preclinical studies have demonstrated the beneficial effect of MSC MV on tissue metabolism. In a model of myocardial infarction, MSC MV increased ATP levels and decreased oxidative stress, enhancing myocardial viability after ischemia/reperfusion injury (25). Several authors demonstrated the transfer of metabolic enzymes such as pyruvate kinase and glyceraldehyde 3-phosphate dehydrogenase from MSC MV to the injured myocardial tissue (25–27). Recently, Islam et al also demonstrated that MSC protect against acute lung injury by restoring alveolar bioenergetics in part through mitochondrial transfer through connexin43 channels (28). ATP restoration may be an additional mechanism for the restoration of AFC induced by MSC MV following ischemia/reperfusion injury in human lungs.
Administration of MSC MV decreased PAP and PVR following perfusion (Tables 2 and 3), which was associated with increased perfusate NO levels. Gao et al recently showed enhanced endothelial NO synthase (eNOS) activity in pulmonary microvascular endothelial cells following coculture with adipose tissue-derived stem cells (29). In a model of acute lung injury, the authors also demonstrated that intravenous instillation of adipose tissue-derived stem cells increased eNOS protein expression, which reduced the severity of acute lung injury (29). Interestingly, MSC also produced NO (30), which may be critical for their immunomodulatory properties (31). Thus, increased NO concentrations in the perfusate following MSC MV instillation may therefore be responsible for endothelial vasodilatation, decreasing PAP and PVR.
Perfusate pH decreased in the Treatment groups over 6 h of EVLP compared to the Control group (Figure 6A). However, perfusate lactate levels decreased in the Treatment groups compared to the Control group. In addition, there was a strong correlation between the decrease in pH and the increase in alveolar fluid clearance (Figure 6B). Although seemingly contradictory, we believe that MSC–MV instillation may account for these findings through several mechanisms: (1) Through an increase in perfusate NO levels, MSC MV may vasodilate cold, ischemic distal lung tissue as apposed to perfusion alone decreasing PAP and leading to the release of acids, accounting for the low pH; and (2) simultaneously, MSC MV may restore the energetics of the alveolar epithelium (25,26) whether through restoration of metabolism or prevention of apoptosis, leading to improved AFC and decreased lactate levels.
Zhu et al demonstrated that the adhesion molecule CD44, expressed on the MSC plasma membrane, was essential for the migration of MSC into the extracellular matrix (32). Bruno et al showed the presence on MSC MV of several adhesion molecules such as CD44, CD29, α4-, and α5-integrins (13). Anti-CD44 and anti-CD29 blocking antibodies inhibited MSC MV uptake in tubular epithelial cells. More importantly, the authors showed that the biological effects of MSC MV were dependent on CD44, which was responsible for the transfer of the content of the MSC MV (mRNA, miRNA, and protein) following internalization (13). In the current study, we found that CD44 expression on MSC MV was also critical for the biological effects of MSC MV. Inhibition of CD44 by anti-CD44 blocking antibody reduced the therapeutic effect of MV on AFC, lung weight, compliance, and pulmonary artery pressure and vascular resistance (Figure 7).
There are some limitations to the current study. (1) The primary endpoint was the AFC rate at 6 h, not improvement in oxygenation; instillation of large volumes of normal saline to determine AFC will lead to inaccurate blood gas measurements (i.e. PaO2) (33). (2) Although CD44 is critical for the therapeutic effects, the actual mechanisms leading to improved AFC remains to be determined further. MSC MV did not decrease alveolar inflammation as assessed by BAL TNFα levels, but there was a nonsignificant increase in Ang1 levels associated with a decrease in syndecan-1 levels in the perfusate. Shedding of the syndecan-1, a component of the endothelial glycocalyx, is associated with endothelial injury and increased protein permeability (34,35). We speculate that one of the mechanisms is through the transfer of mRNA from the MV to the injured alveolar epithelium such as possibly Ang1 with subsequent expression of the protein as we have previously shown with KGF secretion (14).
In conclusion, MSC MV administration enhanced the rate of alveolar fluid clearance in marginal donor lungs in a dose-dependent manner. This effect was associated with a decrease in lung weight gain, an increased in lung compliance, and a decrease in PAP and PVR, which was associated with increased perfusate NO levels. Lactate levels were also decreased with MSC MV treatment, suggesting an improvement in aerobic metabolism. CD44 expression on MSC MV was critical for MV uptake and the therapeutic effects. Similar to the parent stem cell, MSC MV administration may become a method of improving marginal donor lungs during EVLP for transplantation taking advantage of the beneficial attributes of mesenchymal stem cells without the inherent limitations of cell-based therapy.
Acknowledgments
This research was supported by the NHLBI Grants HL-51856 & HL-113022 (USA) and Hamilton Endowment Funds (UCSF Department of Anesthesiology, San Francisco, CA, USA).
Abbreviations
- AFC
alveolar fluid clearance
- Ang1
angiopoietin-1
- CD44
extracellular matrix receptor type III for hyaluronic acid
- EVLP
ex vivo lung perfusion
- IFNγ
interferon gamma
- IL-1β
interleukin 1 beta
- KGF
keratinocyte growth factor
- MSC
mesenchymal stem cells
- MV
microvesicles
- NHLF
normal human lung fibroblasts
- NO
nitric oxide
- TNFα
tumor necrosis factor alpha
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.ISHLT International Society of Heart and Lung Transplantation 2014. Available from: http://www.ishlt.org/
- 2.SRTR The U. S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients 2014. Available from: http://www.srtr.org/
- 3.Cypel M, Keshavjee S. The clinical potential of ex vivo lung perfusion. Expert Rev Respir Med. 2012;6:27–35. doi: 10.1586/ers.11.93. [DOI] [PubMed] [Google Scholar]
- 4.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364:1431–1440. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- 5.Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013;187:751–760. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gennai S, Souilamas R, Maignan M, et al. Effects of cyclosporine a in ex vivo reperfused pig lungs. Microcirculation. 2014;21:84–92. doi: 10.1111/micc.12082. [DOI] [PubMed] [Google Scholar]
- 7.McAuley DF, Curley GF, Hamid UI, et al. Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Physiol Lung Cell Mol Physiol. 2014;306:L809–L815. doi: 10.1152/ajplung.00358.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 9.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prockop DJ. Defining the probability that a cell therapy will produce a malignancy. Mol Ther. 2010;18:1249–1250. doi: 10.1038/mt.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gyorgy B, Szabo TG, Pasztoi M, et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu YG, Feng XM, Abbott J, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 16.Bruno S, Grange C, Collino F, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PloS ONE. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010;285:26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 19.Ware LB, Golden JA, Finkbeiner WE, Matthay MA. Alveolar epithelial fluid transport capacity in reperfusion lung injury after lung transplantation. Am J Rspir Crit Care Med. 1999;159:980–988. doi: 10.1164/ajrccm.159.3.9802105. [DOI] [PubMed] [Google Scholar]
- 20.Sugita M, Ferraro P, Dagenais A, et al. Alveolar liquid clearance and sodium channel expression are decreased in transplanted canine lungs. Am J Respir Crit Care Med. 2003;167:1440–1450. doi: 10.1164/rccm.200204-312OC. [DOI] [PubMed] [Google Scholar]
- 21.Christie JD, Bavaria JE, Palevsky HI, et al. Primary graft failure following lung transplantation. Chest. 1998;114:51–60. doi: 10.1378/chest.114.1.51. [DOI] [PubMed] [Google Scholar]
- 22.Christie JD, Sager JS, Kimmel SE, et al. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005;127:161–165. doi: 10.1378/chest.127.1.161. [DOI] [PubMed] [Google Scholar]
- 23.Thurston G, Rudge JS, Ioffe E, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 24.Salmon AH, Neal CR, Sage LM, Glass CA, Harper SJ, Bates DO. Angiopoietin-1 alters microvascular permeability coefficients in vivo via modification of endothelial glycocalyx. Cardiovasc Res. 2009;83:24–33. doi: 10.1093/cvr/cvp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Lai RC, Yeo RW, Tan KH, Lim SK. Mesenchymal stem cell exosome ameliorates reperfusion injury through proteomic complementation. Regen Med. 2013;8:197–209. doi: 10.2217/rme.13.4. [DOI] [PubMed] [Google Scholar]
- 27.Kim HS, Choi DY, Yun SJ, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11:839–849. doi: 10.1021/pr200682z. [DOI] [PubMed] [Google Scholar]
- 28.Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao P, Yang X, Mungur L, Kampo S, Wen Q. Adipose tissue-derived stem cells attenuate acute lung injury through eNOS and eNOS-derived NO. Int J Mol Med. 2013;31:1313–1318. doi: 10.3892/ijmm.2013.1328. [DOI] [PubMed] [Google Scholar]
- 30.Salvolini E, Lucarini G, Zizzi A, Orciani M, Di Benedetto G, Di Primio R. Human skin-derived mesenchymal stem cells as a source of VEGF and nitric oxide. Arch Dermatol Res. 2010;302:367–374. doi: 10.1007/s00403-009-1018-7. [DOI] [PubMed] [Google Scholar]
- 31.Oh I, Ozaki K, Sato K, et al. Interferon-gamma and NF-kappaB mediate nitric oxide production by mesenchymal stromal cells. Biochem Biophys Res Commun. 2007;355:956–962. doi: 10.1016/j.bbrc.2007.02.054. [DOI] [PubMed] [Google Scholar]
- 32.Zhu H, Mitsuhashi N, Klein A, et al. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 33.Thabut G, Mal H, Cerrina J, et al. Graft ischemic time and outcome of lung transplantation: A multicenter analysis. Am J Respir Crit Care Med. 2005;171:786–791. doi: 10.1164/rccm.200409-1248OC. [DOI] [PubMed] [Google Scholar]
- 34.Rehm M, Bruegger D, Christ F, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt EP, Yang Y, Janssen WJ, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]