Abstract
Objective
To examine the distribution of quantitative autistic traits (QATs) in an independent neurofibromatosis type I (NF1) sample, the relationships between QAT, sex, and attention deficit hyperactivity disorder (ADHD) symptomatology, and to explore evidence for QAT mutational specificity within families.
Study design
Age-appropriate versions of the Social Responsiveness Scale, second edition and the Conners Adult ADHD Rating Scales were completed for 103 patients with NF1 from the Washington University Neurofibromatosis Center.
Results
Patients with NF1 exhibited a pathologically shifted unimodal distribution for QAT. Forty-four percent of the subjects exhibited a QAT burden at or above 1 SD from the population mean; 13% scored at or above the extreme first percentile of the general population distribution. Elevations in ADHD symptomatology exhibited a distinct bimodal distribution; however, mean ADHD index scores were equivalent in patients who had been diagnosed in the community with ADHD compared with those who had not. We observed striking within-family associations for QAT, reflected by an Social Responsiveness Scale, second edition intraclass correlation of 0.77 in pairings of first degree relatives with NF1.
Conclusions
Impairments in reciprocal social behavior and attention affect a large proportion of patients with NF1 throughout life and are often clinically unrecognized. Further exploration of genotype-phenotype correlation is strongly warranted for the purpose of gaining insights into mechanisms by which specific mutational variations in the NF1 gene may influence autistic trait severity.
Neurofibromatosis type 1 (NF1), an autosomal dominant disorder with a prevalence of approximately 1 in 2500 people,1 is characterized by cognitive impairments—primarily learning disability and attention deficit hyperactivity disorder (ADHD) symptomatology—in a large proportion of affected patients.2 Recently, several studies of quantitative autistic trait (QAT) burden in individuals with NF1 have arrived at remarkably consistent prevalence estimates (40%-60%) of the broader autism phenotype in NF1.3-5 The high prevalence of autistic symptomatology observed in this monogenic condition may have important implications for understanding the developmental ontogeny of all autistic syndromes.
To date, it is entirely unknown whether the occurrence or severity of QAT relates to the specific nature of the NF1 gene mutation found in an individual or family. Fifty percent of cases of NF1 are familial in origin. NF1 arises from a germline mutation in the NF1 tumor suppressor gene (chromosome 17q11.2) in both sporadic and inherited cases.3,6 In essence, each individual or family with NF1 has a unique germline NF1 gene mutation. To date, published reports of molecular genetic testing have identified more than 1400 pathogenic mutations of various types dispersed throughout the gene.7 This degree of genetic diversity parallels its extraordinary variability in phenotypic expression and severity. Other than case reports,8,9 there are no known associations between specific NF1 genotypes and autistic syndromes, and there are no published studies in which both genotype and autistic impairment in NF1 have been characterized simultaneously. Demonstration of within-family association, if more pronounced than can be attributed to genetic background,10,11 would imply mutational specificity in the occurrence of autism spectrum disorder (ASD) symptoms. In this study, we capitalized upon the availability of assessments in pairings of first-degree relatives to explore this question.
This study contributes a large sample to a growing body of scientific investigation on autistic trait burden in NF1 summarized in Table I,3,5,6,12-14 which has capitalized on the availability of recently validated quantitative methods for rapid measurement of autistic symptomatology. Moreover, there are 3 distinct contributions of this data set: (1) it implements and analyzes QAT measurements in first-degree relatives concordantly affected by NF1; (2) it analyzes novel Diagnostic and Statistical Manual, fifth edition (DSM-5) scale scores for impairment in social communication, recently derived empirically and validated by confirmatory factor analysis15; and (3) it examines the relationship between QAT and severity measurements of ADHD in NF1 across the entire age range from childhood to adulthood.
Table I.
Recent studies of QAT burden in NF1, implementing the SRS
| Study | Sample size | Age range (y) | SRS-2 mean T-score (SD) | Proportion of subjects with SRS-2 ≥60T |
|---|---|---|---|---|
| Adviento et al (2014)12 | 78 | 4-45 | 57.0 (16.0) | 41% |
| Garg et al (2013)3,6 | 109 | 4-16 | n/a | 56% |
| Walsh et al (2013)5 | 52 | 4-18 | 57.9 (14.2) | 40% |
| Van Eeghen et al (2013)14 | 50 | 4-63 | 60.0 | 40% |
| Plasschaert et al (2014)13 | 82 | 5-17 | 69.9 (19.3) | 63% |
n/a, not applicable.
Methods
The Washington University Neurofibromatosis Center actively follows 320 individuals from 253 families. Following review and approval of a separate protocol by the Washington University Human Research Protection Office, the families were contacted to request participation in the current behavioral phenotyping study. One hundred fifty-one individuals (47.2%) agreed to participate and were sent a study packet, which included age- and rater-appropriate versions of the Social Responsiveness Scale, second edition (SRS-2), the Conners, third edition (Conners-3) for participants under the age of 18 years, and the Conners Adult ADHD Rating Scales (CAARS) for participants over the age of 18 years.16,17 For children with NF1, the ratings were completed by parents. For adults, the ratings were completed by self-report if possible; when not possible, a parent, spouse, or a close friend completed the ratings. One hundred three individuals (32.2%) completed the SRS-2; among them, 95 completed either Conners-3 or CAARS. An additional 3 subjects completed an ADHD rating but did not complete the SRS-2. Of those who did not return assessments, 5 actively declined to participate; the others were unreachable because of a combination of change-of-address, phone disconnection, or nonresponse to letters and phone messages. The final sample for whom informative data sets were available was 103; participants ranged in age from 3-77 years, with a mean age of 23.0 years and a SD of 17.5.
SRS-2
The SRS-2 is 65-item standardized measure of ASD symptoms that uses a 4-point Likert-type scale (from 0 = never true to 3 = almost always true) to derive quantitative ratings of severity of the specific traits and symptoms that characterize ASD. This study utilized a standard version for the scale typically completed by a parent or caregiver, and either of the 2 adult versions (self-report or other adult informant); typically the other adult-informant version was completed by a spouse, close relative, or friend. Norms for each version of the instrument are published in the SRS-2 manual,18 in which the psychometric properties of the SRS-2 have been previously described, summarizing data from numerous published reports. We note that in the range from school age through adulthood, there are essentially no age effects on SRS-2 scores in the general population. The instrument exhibits high internal consistency, reliability, and heritability,19 and it has been validated against a widely implemented developmental history interview, the Autism Diagnostic Interview-Revised, with strong associations (correlation on the order of 0.55) for SRS-2 scores and Autism Diagnostic Interview-Revised algorithm scores for Diagnostic and Statistical Manual, fourth edition criteria.18,20 Established thresholds reliably distinguish children with ASD from both nonaffected children and those with other child psychiatric conditions.18,21,22 Use of the SRS-2 in both general population and affected samples has demonstrated that SRS-2 scores are continuously distributed and are not related to IQ.19,23 Recently, Frazier et al conducted a confirmatory factor analysis of the SRS-2 involving over 9000 subjects representing the age range from school age through adulthood, which supported the ability of the instrument to quantify reliably the partially independent symptom domains operationalized in DSM-5: impairment in social communication and repetitive behavior/restricted interests.15 In this report, we present findings using these scale scores in addition to treatment scale scores and total SRS-2 scores as described in previous SRS-2 reports in NF1. Clinically significant ASD symptoms have been associated with T-scores ≥60; T-scores ≥75 are associated with an ASD diagnosis.18
ADHD Ratings
The Conners-3 parent report form is a 110-item standardized questionnaire measure of ADHD symptomatology that implements a 4-point Likert-like scale (from 0 = not true at all to 3 = very much true) for each item. The version of the Conners-3 generates an ADHD index score for which scores ≥65 are associated with clinically significant ADHD symptoms.16 The CAARS-Self Report Screening Version form is a 30-item standard measure of ADHD symptoms in adults that generates a comparable ADHD index for adults.17,24
Clinical Information
For the majority of patients, the clinical record provided information on the presence or absence of the following clinical features: learning disability, prior community diagnosis of ADHD, optic glioma, other brain tumor, Lisch nodules, peripheral neurofibromas, scoliosis, and epilepsy.
Results
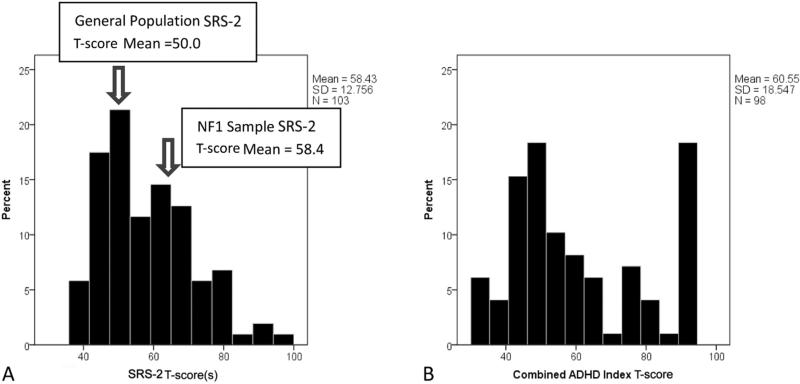
The data for total SRS-2 scores for the full sample of patients with NF1 exhibited a continuous unimodal distribution, pathologically shifted by 0.6 SD in adults and 1.0 SD in children in comparison with general population norms (Figure 1, A).
Figure 1.
Standardized quantitative trait distribution (T-scores) for SRS-2 scores, A, and ADHD symptoms, B, in the NF1 sample.
DSM-5 subscale scores for social communication/interaction and repetitive behavior/restricted interests were similarly continuously distributed. SRS-2 total scores, DSM-5 subscale scores, and all treatment scale scores on the SRS-2 were elevated in individuals with NF1 relative to the general population. The contrasts are summarized in Table II (available at www.jpeds.com) for subsets of SRS-2 data for which published standardization data exist.18 Furthermore, 43.7% of subjects with NF1 were rated with SRS-2 total scores ≥60T (ie, 1 SD above the general population mean, or above the 16th percentile for the general population distribution), and 12.7% had scores ≥75T (ie, 2.5 SDs from mean, or first percentile for the general population distribution). In marked contrast to the continuous QAT distribution, scores for ADHD symptom burden were distinctly bimodal in this sample (Figure 1, B).
Table II.
SRS-2 subscale scores in subsets of subjects with NF1; comparison to norms
| Standardization sample |
NF1 sample (N = 47) |
|||
|---|---|---|---|---|
| Adult form SRS-2 | Mean | SD | Mean | SD |
| Social Awareness | 6.19 | 3.53 | 7.21 | 2.91 |
| Social Cognition | 7.33 | 5.73 | 11.00 | 6.93 |
| Social Communication | 13.02 | 10.19 | 17.83 | 10.72 |
| Social Motivation | 7.66 | 5.97 | 11.09 | 6.75 |
| DSM-5 Restricted Interests and Repetitive Behavior | 6.08 | 5.98 | 9.15 | 6.65 |
| DSM-5 Social Communication and Interaction | 34.2 | 23.2 | 47.13 | 24.99 |
| SRS-2 total score | 40.4 | 28.4 | 56.28 | 30.58 |
| Parent-report SRS-2 on boys | Standardization sample |
NF1 sample (N = 25) |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Social Awareness | 5.69 | 3.18 | 9.12 | 5.45 |
| Social Cognition | 6.15 | 5.38 | 11.40 | 7.62 |
| Social Communication | 10.79 | 9.11 | 20.88 | 13.14 |
| Social Motivation | 5.95 | 4.87 | 10.24 | 6.58 |
| DSM-5 restricted interests and Repetitive Behavior | 5.02 | 5.67 | 11.68 | 8.99 |
| DSM-5 Social Communication and Interaction | 28.6 | 20.2 | 51.64 | 31.17 |
| SRS-2 total score | 33.6 | 25.2 | 63.32 | 39.40 |
| Parent report SRS-2 on girls | Standardization sample |
NF1 sample (N = 31) |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Social Awareness | 5.10 | 3.21 | 8.58 | 3.58 |
| Social Cognition | 5.39 | 5.13 | 10.52 | 6.40 |
| Social Communication | 8.98 | 8.71 | 18.16 | 9.95 |
| Social Motivation | 5.39 | 4.57 | 7.90 | 5.09 |
| DSM-5 Restricted Interests and Repetitive Behavior | 4.13 | 4.94 | 10.68 | 7.04 |
| DSM-5 Social Communication and Interaction | 24.9 | 19.4 | 45.16 | 22.69 |
| SRS-2 total score | 29.0 | 23.7 | 55.84 | 27.79 |
We observed a trend for sex differences in SRS-2 total, treatment scale, and DSM-5 scale scores, all in the expected direction (male greater than female), but none reached statistical significance (total score, t = 0.973, degrees of freedom [df] = 101, P < .333). SRS-2 total scores for male and female subjects with NF1 were continuously distributed, which represents a departure from the bimodal distribution observed for females in familial ASD samples.25,26
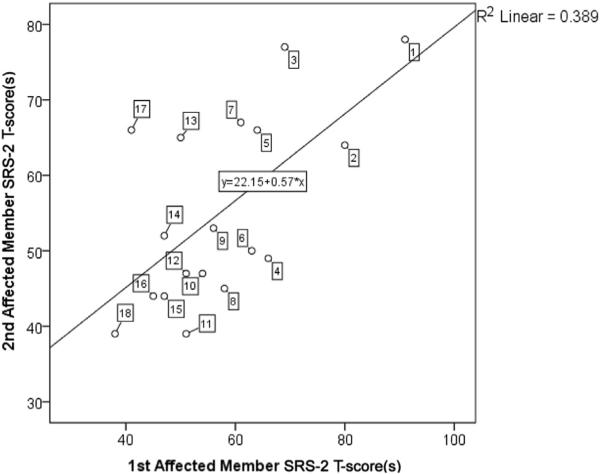
An SRS-2 intraclass correlation of 0.77 was observed for 18 available pairings of first-degree relatives with NF1, substantially higher than correlations on the order of 0.30 typically observed for first degree relatives in ASD clinical family samples11,27 and in the general population.19 Table III provides a listing of the respective ages and relationships between family members in this subanalysis, and a scatter plot of the family data is provided in Figure 2, with individual points labeled and keyed to Table III.
Table III.
Family relationships for pairings (n = 18) of first degree relatives affected by NF1
| Family number | Relationship between first and second affected members | SRS-2 informants | First affected member's sex | Second affected member's sex | First affected member's age (y) | Second affected member's age (y) |
|---|---|---|---|---|---|---|
| 1. | Full sibling | Mother | Male | Female | 13 | 10 |
| 2. | Child/parent | Mother/spouse | Female | Male | 11 | 49 |
| 3. | Full sibling | Mother | Female | Female | 15 | 12 |
| 4. | Full sibling | Self | Female | Male | 36 | 41 |
| 5. | Child/parent | Mother/self | Female | Female | 5 | 35 |
| 6. | Full sibling | Mother | Male | Female | 11 | 8 |
| 7. | Full sibling | Mother | Female | Female | 12 | 8 |
| 8. | Child/parent | Mother/self | Male | Female | 19 | 49 |
| 9. | Child/parent | Father/self | Female | Male | 9 | 40 |
| 10. | Child/parent | Mother/self | Male | Female | 10 | 33 |
| 11. | Child/parent | Mother/self | Female | Female | 6 | 28 |
| 12. | Child/parent | Mother/spouse | Female | Female | 16 | 51 |
| 13. | Child/parent | Parent/self | Male | Male | 5 | 31 |
| 14. | Child/parent | Mother/self | Male | Female | 8 | 25 |
| 15. | Child/parent | Father/spouse | Female | Male | 23 | 58 |
| 16. | Full sibling | Mother | Female | Male | 11 | 3 |
| 17. | Child/parent | Father/spouse | Male | Male | 10 | 57 |
| 18. | Twin sibling | Self | Female | Female | 19 | 19 |
Figure 2.
Scatter plot of SRS-2 total T-scores of pairings of first degree relatives affected by NF1, listed in Table III.
The correlation between continuously distributed SRS-2 scores and bimodally distributed ADHD index scores was moderate, as previously reported in population-based samples in which both variables are typically continuously distributed and did not differ significantly between children and adults with NF1. ADHD index scores exhibited an intraclass correlation of 0.46 for pairings of first-degree relatives with NF1. When comparing subjects above vs below an ADHD index T-score of 65, SRS-2 mean t-scores differed markedly, both among children (t = 4.54; df = 46; P < .0001) and among adults (t = 4.05; df = 45; P < .0001). Of note, the ADHD scores, though substantially elevated among individuals with NF1 did not differ significantly between those with vs without community diagnoses of ADHD. Out of 10 subjects who scored at 99% probability on the Conners 3 ADHD index, 6 reported no prior ADHD diagnosis.
Comparisons of individuals with and without specific medical features of NF1 (restricting analysis to those with frequencies that allowed valid comparisons) are provided in Table IV (available at www.jpeds.com). These included optic pathway glioma, other brain tumor, presence/absence of Lisch nodules, peripheral neurofibroma, scoliosis, “learning disorder,” and epilepsy. Although statistical power was limited to examine some of the features, none exhibited a statistically significant association with QAT.
Table IV.
Comparison of SRS-2 scores between individuals with vs without specific medical features of NF1
| Condition status |
No |
Yes |
t test |
|||||
|---|---|---|---|---|---|---|---|---|
| T-scores | n | Mean (SD) | n | Mean (SD) | t | df | P | |
| Learning disability | SRS-2 total | 43 | 58.5 (14.1) | 14 | 64.5 (13.9) | –1.379 | 55 | .173 |
| DSM-5 SCI | 58.5 (13.7) | 64.7 (12.7) | –1.504 | 55 | .138 | |||
| DSM-5 RRB | 60.4 (15.7) | 66.9 (11.4) | –1.669 | 30 | .105 | |||
| Prior community diagnosis of ADHD | SRS-2 total | 45 | 58.5 (13.9) | 18 | 61.8 (13.7) | –0.852 | 61 | .398 |
| DSM-5 SCI | 57.9 (13.8) | 63.6 (11.4) | –1.553 | 61 | .126 | |||
| DSM-5 RRB | 59.3 (15.0) | 64.6 (14.0) | –1.278 | 61 | .206 | |||
| Optic pathway glioma | SRS-2 total | 83 | 57.9 (12.6) | 11 | 59.9 (15.2) | –0.483 | 92 | .631 |
| DSM-5 SCI | 58.0 (12.3) | 59.1 (14.3) | –0.265 | 92 | .792 | |||
| DSM-5 RRB | 58.6 (12.8) | 62.4 (18.7) | –0.646 | 11 | .531 | |||
| Other brain tumor | SRS-2 total | 88 | 58.0 (12.9) | 11 | 60.7 (11.6) | –0.495 | 92 | .622 |
| DSM-5 SCI | 58.1 (12.6) | 59.0 (11.2) | –0.171 | 92 | .864 | |||
| DSM-5 RRB | 58.5 (13.4) | 66.7 (16.0) | –1.427 | 92 | .157 | |||
| Lisch nodules | SRS-2 total | 9 | 59.3 (20.6) | 54 | 58.2 (12.1) | 0.162 | 8 | .875 |
| DSM-5 SCI | 59.0 (19.7) | 58.8 (11.8) | 0.025 | 8 | .981 | |||
| DSM-5 RRB | 60.0 (21.1) | 58.5 (12.3) | 0.197 | 8 | .848 | |||
| Peripheral neurofibromas | SRS-2 total | 33 | 55.1 (10.7) | 61 | 59.8 (13.6) | –1.701 | 92 | .092 |
| DSM-5 SCI | 55.9 (10.0) | 59.3 (13.6) | –1.265 | 92 | .209 | |||
| DSM-5 RRB | 57.6 (12.8) | 59.8 (14.1) | –0.758 | 92 | .450 | |||
| Scoliosis | SRS-2 total | 84 | 58.6 (13.0) | 10 | 54.4 (11.2) | 0.978 | 92 | .331 |
| DSM-5 SCI | 58.6 (12.6) | 54.3 (11.6) | 1.030 | 92 | .306 | |||
| DSM-5 RRB | 59.6 (13.8) | 54.6 (10.9) | 1.098 | 92 | .275 | |||
| Epilepsy | SRS-2 total | 90 | 57.6 (12.4) | 4 | 70.0 (17.6) | –1.916 | 92 | .058 |
| DSM-5 SCI | 57.6 (12.1) | 69.5 (17.1) | –1.881 | 92 | .063 | |||
| DSM-5 RRB | 58.6 (13.2) | 69.3 (19.5) | –1.546 | 92 | .126 | |||
SCI, social communication and interaction; RRB, restricted interests/repetitive behaviors.
Discussion
We confirm prior reports of substantial autistic trait burden in individuals with NF1: 44% with QAT burden at or above 1 SD from the population mean, and 13% at or above the extreme first percentile of the general population distribution. These data also provide several fundamental new insights into the association between NF1, ADHD, and autistic symptomatology. First, although the distribution of total autistic trait scores appears fully continuous, there is marked bimodality in the distribution of ADHD impairments. These observations suggest that a subset of individuals with NF1 are particularly affected by ADHD, whereas the entire NF1 population exhibits a pathologic shift in QAT burden. Second, the association in the degree of autistic impairment endorsed in pairings of first degree relatives is striking (intraclass correlation = 0.77), especially when considering the diversity in age of subjects in any given pair (Table III) and substantially higher than what has previously been observed for QAT on the basis of family genetic background. This finding raises the intriguing possibility of mutational specificity for autistic symptomatology in the vast diversity of one-of-a-kind mutations that comprise the population burden of NF1. Such mutational specificity is also suggested by the bimodal distribution of ADHD index scores, but trait correlations for concordantly affected first degree relatives are not as strong as those observed for QAT in this sample. Third, as observed by Walsh et al,5 the association between QAT burden and ADHD index scores is substantial and equivalent for children and adults with NF1 who manifest ADHD. Fourth, in marked contrast to what has been observed for other inherited autistic syndromes, QAT scores in individuals with NF1 exhibit minimal sex differences (these did not reach statistical significance), and the total score distributions for both sexes were unimodal. This contrasts with familial autistic syndromes in which trait distributions for females in ASD-affected families are distinctly bimodal and suggestive of a general “female protective effect” for most familial ASDs28,29 that may not be operative in the setting of NF1.
In addition, these data have important clinical and scientific implications, both for patients with NF1 and broadly for all individuals affected by autistic syndromes. First and foremost, these results imply substantial under-recognition of both ADHD and ASD symptoms in patients with NF1, and support the implementation of systematic screening for clinical level symptomatology of ASD and ADHD among children affected with NF1. Early surveillance and intervention for these behavioral syndromes are likely to improve the adaptation and outcome of affected individuals. Developmental therapies, positive behavior support planning at home and at school, specific provisions in educational curricula, psychoeducation, parent training for families, and psychopharmacologic intervention when appropriate (for inattention, hyperactivity, aggression, or irritability) have all proved beneficial for individuals affected by these behavioral syndromes, across an increasingly recognized diversity of causal mechanisms that result in ASD. The presence of these behavioral comorbidities represents an opportunity for more effective clinical intervention for at-risk individuals with NF1.
Autism has historically been infrequently entertained in NF1; however, these and other data from several recent reports indicate that clinical level autistic symptomatology occurs in upwards of 40% of all patients with NF1. We also observed a substantial burden of ADHD symptomatology in children with NF1 never diagnosed with ADHD, comparable to patients with NF1 who had received community diagnoses. This ADHD trait burden appears to persist well into adulthood, invoking careful clinical consideration of the potential benefit of treatment on adaptive functioning for adults with NF1, as has been reported for adults with ADHD.30 Future prevalence studies of ADHD and learning disability in NF1 that compare rates of community diagnosis with prevalence derived from systematic screening and diagnostic confirmation will provide clearer estimation of the magnitude of the under-recognition problem. Although our study featured quantitative trait data from standardized measurement of ADHD, association with medical record diagnosis is complicated by the fact that ADHD and learning disability may be conceptualized differently and/or ascertained more or less completely by different clinicians, depending upon clinical practice and how systematic the methods were for documenting these conditions.
These data highlight unique characteristics of autistic symptomatology within the NF1 population: relative absence of sex differences, continuous distributions of total autistic trait scores for both males and females, and the strong possibility of germline NF1 gene mutational specificity. In this study, the degree of association for QAT between first-degree relatives affected by NF1 far exceeded what has been observed in the general population and in clinical ASD samples.12 Still, data from unaffected family members of patients with NF1, which were not available in the current data set, will be of value in future studies in order to specify more precisely the incremental increase over familial/polygenic background that is contributed by an NF1 mutation shared between 2 family members. The inferred mutational specificity and the repeated observations of autistic trait burden in NF1 make it a particularly important monogenic disease model for autism, similar to fragile X syndrome, Rett syndrome, and tuberous sclerosis. The identification and detailed characterization of subsets of genotypes associated with ASD, how they differ from and/or, exhibit similarity to the other monogenic syndromes that give rise to autism, and how the underlying mechanisms of impairment in NF1 might overwhelm the usual phenomenon of sex differentiation referred to as the “female protective effect” in autism29 could lend major insights into understanding molecular mechanisms underlying all autistic syndromes. Elucidation of those mechanisms could lead to new opportunities for novel, higher-impact interventions for this devastating group of disorders.28
The limitations of this study include constraints on the size of the accumulated sample, the use of single-informant behavioral questionnaire data, and the lack of availability of data on unaffected family members. This is, however, one of the larger collections of patients with NF1 in whom QAT data have been collected to date, and limitations in sample size are mitigated by the consistency of findings across several comparably sized studies (Table I). The instrument used in this research, the SRS-2, has been used in most previous studies of QAT in NF1, has been extensively validated in relation to research diagnosis of ASD, and its prior validation included examination for the possibility of rater bias effects that would inflate estimations of associations between family members whose SRS-2 reports were completed by the same individual (eg, spouse and child): fortunately, prior analyses of genetically informative family data involving thousands of subjects have indicated that rater bias is essentially absent in the ratings.19,31 Regarding self-report, there is evidence that some adults with NF1 demonstrate reduced self-awareness with regard to deficits in social skills. For example, Pride et al found that observer reports completed by friends and family members of adults with NF1 indicated lower rates of prosocial behavior than self-reports, whereas control subjects showed the opposite trend.32 This possible confound in the adult self-report data would operate to minimize the ascertainment of QAT elevations, so the aggregations observed among self-reporting adults (which were indeed slightly less pronounced than those observed for children by parent-report) would represent conservative estimates, which were still very significant (effect size 0.6). Ideally, clinical level aggregations of autistic traits as measured by the SRS-2 in this study would be confirmed by structured diagnostic interviews and observation for case confirmation. Although it is a relative limitation of the study that these were not implemented, we note that previous research in ASD18,20,22 and on autistic symptomatology in NF16 has established the strong association between clinical level symptom elevation as measured by the SRS-2 and diagnostic confirmation, and this study builds upon that background. Similarly, we did not implement specific measures of cognitive function/impairment in this study; however, there was no association between SRS-2 scores and presence/absence of learning disability, and previous large studies have demonstrated the independence of SRS-2 ratings from indices of general cognition within the typical range of variation of IQ in the population.20,23
In summary, we conclude that correlated impairments in reciprocal social behavior and attention affect a large proportion of patients with NF1 throughout life and may often go clinically unrecognized. Systematic ascertainment and intervention for these behavioral morbidities are likely to improve adaptation and outcome in individuals affected with NF1. Further exploration of a potential genotype-phenotype correlation is strongly warranted for the purpose of gaining insights into mechanisms by which specific mutational variations in the NF1 gene may influence, and possibly predict, autistic trait severity.
Acknowledgments
Research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number P30HD062171 to the Intellectual and Developmental Disabilities Research Center at Washington University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.C. receives royalties from Western Psychological Services for commercial sales and distribution of the Social Responsive ness Scale-2.
Glossary
- ADHD
Attention deficit hyperactivity disorder
- ASD
Autism spectrum disorder
- CAARS
Conners Adult ADHD Rating Scales
- Conners-3
Conners, third edition
- df
Degrees of freedom
- DSM-5
Diagnostic and Statistical Manual, fifth edition
- NF1
Neurofibromatosis type 1
- QAT
Quantitative autistic trait
- SRS-2
Social Responsiveness Scale, second edition
Footnotes
The other authors declare no conflicts of interest.
References
- 1.Tonsgard JH. Clinical manifestations and management of neurofibromatosis type 1. Semin Pediatr Neurol. 2006;13:2–7. doi: 10.1016/j.spen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Hyman SL, Shores EA, North KN. Learning disabilities in children with neurofibromatosis type 1: subtypes, cognitive profile, and attention-deficit-hyperactivity disorder. Dev Med Child Neurol. 2006;48:973–7. doi: 10.1017/S0012162206002131. [DOI] [PubMed] [Google Scholar]
- 3.Garg S, Lehtonen A, Huson SM, Emsley R, Trump D, Evans DG, et al. Autism and other psychiatric comorbidity in neurofibromatosis type 1: evidence from a population-based study. Dev Med Child Neurol. 2013;55:139–45. doi: 10.1111/dmcn.12043. [DOI] [PubMed] [Google Scholar]
- 4.Lehtonen A, Howie E, Trump D, Huson SM. Behaviour in children with neurofibromatosis type 1: cognition, executive function, attention, emotion, and social competence. Dev Med Child Neurol. 2013;55:111–25. doi: 10.1111/j.1469-8749.2012.04399.x. [DOI] [PubMed] [Google Scholar]
- 5.Walsh KS, Velez JI, Kardel PG, Imas DM, Muenke M, Packer RJ, et al. Symptomatology of autism spectrum disorder in a population with neurofibromatosis type 1. Dev Med Child Neurol. 2013;55:131–8. doi: 10.1111/dmcn.12038. [DOI] [PubMed] [Google Scholar]
- 6.Garg S, Green J, Leadbitter K, Emsley R, Lehtonen A, Evans DG, et al. Neurofibromatosis type 1 and autism spectrum disorder. Pediatrics. 2013;132:e1642–8. doi: 10.1542/peds.2013-1868. [DOI] [PubMed] [Google Scholar]
- 7.Uusitalo E, Hammais A, Palonen E, Brandt A, Makela VV, Kallionpaa R, et al. Neurofibromatosis type 1 gene mutation analysis using sequence capture and high-throughput sequencing. Acta Derm Venereol. 2014;94:663–6. doi: 10.2340/00015555-1843. [DOI] [PubMed] [Google Scholar]
- 8.Marui T, Hashimoto O, Nanba E, Kato C, Tochigi M, Umekage T, et al. Association between the neurofibromatosis-1 (NF1) locus and autism in the Japanese population. Am J Med Genet B Neuropsychiatr Genet. 2004;131B:43–7. doi: 10.1002/ajmg.b.20119. [DOI] [PubMed] [Google Scholar]
- 9.Mbarek O, Marouillat S, Martineau J, Barthelemy C, Muh JP, Andres C. Association study of the NF1 gene and autistic disorder. Am J Med Genet. 1999;88:729–32. [PubMed] [Google Scholar]
- 10.Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 2005;57:655–60. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Lyall K, Constantino JN, Weisskopf MG, Roberts AL, Ascherio A, Santangelo SL. Parental social responsiveness and risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2014;71:936–42. doi: 10.1001/jamapsychiatry.2014.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adviento B, Corbin IL, Widjaja F, Desachy G, Enrique N, Rosser T, et al. Autism traits in the RASopathies. J Med Genet. 2014;51:10–20. doi: 10.1136/jmedgenet-2013-101951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plasschaert E, Descheemaeker MJ, Van Eylen L, Noens I, Steyaert J, Legius E. Prevalence of autism spectrum disorder symptoms in children with neurofibromatosis type 1. Am J Med Genet B Neuropsychiatr Genet. 2015;168B:72–80. doi: 10.1002/ajmg.b.32280. [DOI] [PubMed] [Google Scholar]
- 14.van Eeghen AM, Pulsifer MB, Merker VL, Neumeyer AM, van Eeghen EE, Thibert RL, et al. Understanding relationships between autism, intelligence, and epilepsy: a cross-disorder approach. Dev Med Child Neurol. 2013;55:146–53. doi: 10.1111/dmcn.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frazier TW, Ratliff KR, Gruber C, Zhang Y, Law PA, Constantino JN. Confirmatory factor analytic structure and measurement invariance of quantitative autistic traits measured by the Social Responsiveness Scale-2. Autism. 2014;18:31–44. doi: 10.1177/1362361313500382. [DOI] [PubMed] [Google Scholar]
- 16.Conners CK. Conners. 3rd edition Multi-Health Systems; Toronto, Ontario, Canada: 2008. [Google Scholar]
- 17.Conners CK, Erhardt D, Sparrow E. CAARS Adult ADHD Rating Scales. Multi-Health Systems; Toronto, Ontario, Canada: 1999. [Google Scholar]
- 18.Constantino JN, Gruber CP. Social Responsiveness Scale. Second Edition Western Psychological Services; Los Angeles, CA: 2012. [Google Scholar]
- 19.Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60:524–30. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 20.Constantino JN, Lavesser PD, Zhang Y, Abbacchi AM, Gray T, Todd RD. Rapid quantitative assessment of autistic social impairment by classroom teachers. J Am Acad Child Adolesc Psychiatry. 2007;46:1668–76. doi: 10.1097/chi.0b013e318157cb23. [DOI] [PubMed] [Google Scholar]
- 21.Constantino JN, Przybeck T, Friesen D, Todd RD. Reciprocal social behavior in children with and without pervasive developmental disorders. J Dev Behav Pediatr. 2000;21:2–11. doi: 10.1097/00004703-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Frazier TW, Youngstrom EA, Embacher R, Hardan AY, Constantino JN, Law P, et al. Demographic and clinical correlates of autism symptom domains and autism spectrum diagnosis. Autism. 2013;18:571–82. doi: 10.1177/1362361313481506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hus V, Bishop S, Gotham K, Huerta M, Lord C. Factors influencing scores on the social responsiveness scale. J Child Psychol Psychiatry. 2013;54:216–24. doi: 10.1111/j.1469-7610.2012.02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hines JL, King TS, Curry WJ. The Adult ADHD Self-report Scale for Screening for Adult Attention Deficit-Hyperactivity Disorder (ADHD). J Am Board Fam Med. 2012;25:847–53. doi: 10.3122/jabfm.2012.06.120065. [DOI] [PubMed] [Google Scholar]
- 25.Lowe JK, Werling DM, Constantino JN, Cantor RM, Geschwind DH. Social responsiveness, an autism endophenotype: genomewide significant linkage to two regions on chromosome 8. Am J Psychiatry. 2015;172:266–75. doi: 10.1176/appi.ajp.2014.14050576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virkud YV, Todd RD, Abbacchi AM, Zhang Y, Constantino JN. Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:328–34. doi: 10.1002/ajmg.b.30810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Constantino JN, Lajonchere C, Lutz M, Gray T, Abbacchi A, McKenna K, et al. Autistic social impairment in the siblings of children with pervasive developmental disorders. Am J Psychiatry. 2006;163:294–6. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- 28.Constantino JN, Charman T. Gender bias, female resilience, and the sex ratio in autism. J Am Acad Child Adolesc Psychiatry. 2012;51:756–8. doi: 10.1016/j.jaac.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Gockley J, Willsey AJ, Dong S, Dougherty JD, Constantino JN, Sanders SJ. The female protective effect in autism spectrum disorder is not mediated by a single genetic locus. Mol Autism. 2015;13:6–25. doi: 10.1186/s13229-015-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lichtenstein P, Halldner L, Zetterqvist J, Sjolander A, Serlachius E, Fazel S, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2012;367:2006–14. doi: 10.1056/NEJMoa1203241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Constantino JN. The quantitative nature of autistic social impairment. Pediatr Res. 2011;69:55R–62R. doi: 10.1203/PDR.0b013e318212ec6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pride NA, Crawford H, Payne JM, North KN. Social functioning in adults with neurofibromatosis type 1. Res Dev Disabil. 2013;34:3393–9. doi: 10.1016/j.ridd.2013.07.011. [DOI] [PubMed] [Google Scholar]