Abstract
Objectives
The objective of this study was to describe the cortical evoked response to silent gaps in a group of young adults with normal hearing using stimulus conditions identical to those used in psychophysical studies of gap detection. Specifically, we sought to examine the P1-N1-P2 auditory evoked response to the onsets of stimuli (markers) defining a silent gap for within-channel (spectrally identical markers) and across-channel (spectrally different markers) conditions using four perceptually-equated gap durations. It was hypothesized that (1) P1, N1, and P2 would be present and consistent for 1st marker (before the gap) onsets; (2) for within-channel markers, P1, N1, and P2 would be present for 2nd marker (after the gap) onsets only when the gap was of a duration equal to or larger than the behaviorally measured gap detection threshold; and (3) for the across-channel conditions, P1, N1, and P2 would be present for 2nd marker onsets regardless of gap duration. This is expected due to the additional cue of frequency change following the gap.
Design
Twelve young adults (mean age 26 years) with normal hearing participated. Within-channel and across-channel gap detection thresholds were determined using an adaptive psychophysical procedure. Next, cortical auditory evoked potentials (P1-N1-P2) were recorded with a 32-channel Neuro-scan™ electroencephalogram system using within-channel and across-channel markers identical to those used for the psychophysical task and four perceptually weighted gap durations: (1) individual listener's gap detection threshold; (2) above gap detection threshold; (3) below gap detection threshold; and (4) a 1-ms gap identical to the gap in the standard interval of the psychophysical task. P1-N1-P2 peak latencies and amplitudes were analyzed using repeated-measures analyses of variance. A temporal-spatial principal component analysis was also conducted.
Results
The latency of P2 and the amplitude of P1, N1, and P2 were significantly affected by the acoustic characteristics of the 2nd marker as well as the duration of the gap. Larger amplitudes and shorter latencies were generally found for the conditions in which the acoustic cues were most salient (e.g., across-channel markers, 1st markers, large gap durations). Interestingly, the temporal-spatial principal component analysis revealed activity elicited by gap durations equal to gap detection threshold in the latency regions of 167 and 183 ms for temporal-parietal and right-frontal spatial locations.
Conclusions
The cortical response to a silent gap is unique to specific marker characteristics and gap durations among young adults with normal hearing. Specifically, when the onset of the 2nd marker is perceptually salient, the amplitude of the P1-N1-P2 response is relatively larger and the P2 latency is relatively shorter than for nonsalient 2nd marker onsets, providing noninvasive, nonbehavioral indicators of the neural coding of this important temporal cue in the thalamic-cortical region of the central auditory system. Gap duration appears to be most clearly indicated by P1 and T-complex amplitude.
Temporal resolution may be defined as the ability to follow rapid changes in intensity and frequency over time, a skill that is thought to be important for understanding speech in noise (Dubno, Horwitz, & Ahlstrom, 2003; Gordon-Salant & Fitzgibbons, 1993, 1999; Peters, Moore, & Baer, 1998; Stuart & Phillips, 1996). Temporal resolution has been studied extensively using psychophysical tasks of silent gap detection in which at least one standard stimulus, comprised of one continuous or two contiguous sounds (markers), and a target stimulus, comprised of two markers separated by a silent gap, are presented. The shortest gap that a listener can detect (relative to the standard) is called a gap detection threshold (GDT). Psychophysical GDTs are influenced by a number of stimulus factors, including marker bandwidth (Eddins, Hall, & Grose, 1992; Snell, Ison, & Frisina, 1994), marker duration (He, Horwitz, Dubno, & Mills, 1999), monotic, diotic, or dichotic presentation modes (Gordon-Salant & Fitzgibbons, 1999; He, et al., 1999; Lister & Roberts, 2005), and the spectral similarity of the markers before and after the gap (Lister, Besing & Koehnke, 2002; Oxenham, 2000).
Behavioral studies have shown that, when the stimuli that mark the silent gap are noise bands of similar frequency, known as within-channel gap detection, the task is relatively easy and GDTs are small (Lister & Roberts, 2005; Lister, et al., 2002). When the noise bands that mark the gap are of different frequencies, known as across-channel gap detection, the task is more difficult and GDTs are larger (Lister & Roberts, 2005; Lister, et al., 2002). Because the phonemes that precede and follow temporal cues in speech are never identical, across-channel gap detection is thought to be more representative of the temporal cues important for speech perception than within-channel gap detection (Formby, Gerber, Sherlock, & Magder, 1998; Phillips, Taylor, Hall, Carr, & Mossop, 1997); therefore, this condition has received great interest in recent years.
It has been suggested that detection of gaps between markers that are close (or overlap) in frequency is relatively easy because it requires monitoring activity in a single neural channel. For gaps between markers that differ in frequency, the listener must make a decision regarding the relative timing between the offset of neural activity in one channel and the onset in an entirely different channel (Formby, et al., 1998; Grose, Hall, Buss, & Hatch, 2001).
Results from animal studies suggest that an important cue to gap detection is the onset of the 2nd (after the gap) marker (Abeles & Goldstein, 1972; Barsz, Ison, Snell, & Walton, 2002; Ison, Castro, Allen, Virag, & Walton, 2002). In the within-channel case, this onset would stimulate a previously active neural channel, whereas in the across-channel case, this onset would stimulate a previously inactive neural channel. Therefore, across-channel conditions should result in a more robust neural response than within-channel conditions. It is clear, however, from behavioral data that this robust response does not result in smaller GDTs. On the contrary, it seems to interfere with gap detection.
Although behavioral methods are popular for the measurement of temporal resolution, they are influenced by many factors (e.g., memory, cognition, motivation, task, response criteria), making it difficult to draw conclusions about the underlying physiological deficit(s). This point is important because there is tremendous variability among individuals in terms of behavioral performance, suggesting that the underlying physiological deficit might vary across individual listeners (e.g., Lister & Roberts, 2005). Therefore, a temporal resolution measure that does not rely on behavioral responses is needed and may be quite useful.
Cortical auditory evoked potentials (CAEPs), such as the P1-N1-P2 response, can be used to measure temporal resolution, in the absence of a behavioral response. The P1-N1-P2 response, also called the acoustic change complex, is a physiological response that signals the neural detection of acoustic change at the level of the auditory cortex and corresponds well with perceptual thresholds for the same acoustic change (Kaukoranta, Hari, & Lounasmaa, 1987; Martin & Boothroyd, 1999; Ostroff, Martin, & Boothroyd, 1998). Therefore, the P1-N1-P2 is particularly well suited for studying a number of acoustic cues important for the perception of speech, including silent gaps. These peaks occur approximately 50 ms (P1), 100 ms (N1), and 200 ms (P2) after stimulus onset and are thought to represent synchronous neural firing in the thalamic-cortical segment of the central auditory system in response to the onset of acoustic change (for review see Key, Dove, & Maguire, 2005; also Naatanen & Picton, 1987; Wolpaw & Penry, 1975; Woods, 1995).
Specifically, the P1 is thought to be generated in the superior temporal gyrus, and is associated with auditory inhibition and sensory gating (Huotilainen, et al., 1998; Thoma, et al., 2003; Waldo, Gerhardt, Baker, Drebing, Adler, & Freedman, 1992). The N1 component is thought to reflect stimulus characteristics such as intensity and timing (Naatanen & Picton, 1987), and may be generated by activity in the superior temporal plane as well as other sources in the temporal and frontal lobes (Knight, Scabini, Woods, & Clayworth, 1988; Papanicolaou, Bau-mann, Rogers, Saydjari, Amparo, & Eisenberg, 1990; Scherg, Vajsar, & Picton, 1989). The P2 component also appears to be affected by stimulus characteristics such as frequency and intensity (Hegerl & Juckel, 1993; Hillyard & Picton, 1987; Novak, Ritter, & Vaughan, 1992), and has sources in the primary and secondary auditory cortices that may or may not be distinct from those of the N1 (Knight, et al., 1988; Zouridakis, Simos, & Papanicolaou, 1998).
The P1-N1-P2 response has been reliably evoked and recorded in individuals listening to speech (Friesen & Tremblay, 2006; Tremblay, Friesen, Martin, & Wright, 2003) and nonspeech stimuli (Segalowitz & Barnes, 1993; Walhovd & Fjell, 2002), and has been used to study the neural detection of acoustic change in a number of clinical populations, including older adults (Tremblay, Billings, & Rohila, 2004), children (Ponton, Eggermont, Kholsa, Kwong, & Don, 2002), individuals with hearing loss (Korczak, Kurtzberg, & Stapells, 2005), individuals with auditory neuropathy (Michalewski, Starr, Nguyen, Kong, & Zeng, 2005), and individuals with cochlear implants (Friesen & Tremblay, 2006). Most importantly, the P1-N1-P2 response corresponds well to the behavioral detection of frequency, intensity, and temporal changes in a stimulus (for review see Hyde, 1997), making it a particularly useful tool for the assessment of perception among patients who are unable to perform behavioral tasks.
CAEPs have been used to assess temporal resolution in three studies (Heinrich, Alain, & Schneider, 2004; Michalewski, et al., 2005; Pratt, Bleich, & Mittelman, 2005). Heinrich et al. (2004) found that N1 and P2 responses were similar for near-threshold gap durations for both within- and across-channel conditions using very brief pure-tone markers. Michalewski et al. (2005) found that N1 and P2 responses to gaps in broadband noise (i.e., within-channel) were unaffected by attention and corresponded well with gap detection thresholds measured behaviorally for adults with normal hearing and adults with auditory neuropathy. Pratt et al. (2005) described the N1 response to the onset and offset of gaps in broadband noise (i.e., within-channel). They found that, for relatively long gap durations, the N1 response to gap onset (marker offset) was double-peaked and distinct from the N1 response that occurred to gap offset (marker onset). As mentioned above, the onset of the 2nd marker (gap offset) is thought to be the most important cue for silent gap detection and was, therefore, the focus of the present study.
Despite the sensitivity of the P1-N1-P2 response, there have been few investigations of the individual P1-N1-P2 responses to the onsets of 1st and 2nd markers and using stimuli typical to psychophysical gap detection tasks (e.g., relatively long markers and brief gaps, across-channel conditions, standard and target stimuli). This direction of research is important because of the potential contribution to the psychoacoustic literature. For example, when long markers are used to elicit the P1-N1-P2, the physiologic response to each marker onset should be visible. With this information, one could determine the relative saliency of each onset and perhaps, in turn, identify clinical populations with impaired temporal resolution. Additionally, the inclusion of across-channel conditions as well as the standard (small or nonexistent gap) and target (gap) intervals typical of psychophysical GDT tasks could provide information regarding the neural representation of each element involved in the behavioral detection of a silent gap.
Therefore, the purpose of this study was to examine the P1-N1-P2 response to the onsets of 1st (before the gap) and 2nd (after the gap) markers for within-channel and across-channel conditions using a variety of gap durations in a group of young adults with normal hearing. We hypothesized the following: (1) For all conditions, P1, N1, and P2 would be present and consistent for 1st marker onsets, providing a baseline response for each participant; (2) For the within-channel conditions, P1, N1, and P2 would be present for 2nd marker onsets only when the gap is behaviorally perceptible; and (3) For the across-channel conditions, P1, N1, and P2 would be present for 2nd marker onsets regardless of gap duration due to the additional cue of frequency change following the gap; however, we hoped to see some difference in the response across gap duration.
Materials and Methods
Participants
Twelve young adults with normal hearing (pure tone air conduction thresholds ≤20 dB HL from 250 to 8000 Hz) in both ears participated in the study. All participants were native English speakers and had no history of middle ear infections, oto-toxic medication use, excessive noise exposure, or neurological disorders. Descriptive data for all participants are shown in Table 1.
TABLE 1.
Descriptive data for all participants*
| Detection threshold (dB SPL) |
Gap detection threshold (ms) |
|||||
|---|---|---|---|---|---|---|
| Participant | Age | Gender | 1000 Hz NBN | 2000 Hz NBN | Within-channel | Across-channel |
| P01 | 33 | Female | 5 | 1 | 8 | 22 |
| P02 | 25 | Male | 8 | 13 | 12 | 26 |
| P03 | 22 | Female | –13 | –4 | 7 | 9 |
| P04 | 24 | Female | 17 | 6 | 8 | 50 |
| P05 | 40 | Female | 4 | 6 | 7 | 32 |
| P06 | 24 | Female | 1 | 7 | 15 | 30 |
| P07 | 25 | Female | –5 | 0 | 10 | 22 |
| P08 | 21 | Male | –4 | 3 | 10 | 42 |
| P09 | 24 | Female | –4 | –3 | 12 | 25 |
| P10 | 23 | Male | 2 | –2 | 12 | 22 |
| P11 | 25 | Female | –5 | 8 | 11 | 59 |
| P12 | 24 | Female | 3 | –4 | 6 | 11 |
| Mean (SE) | 25.8 (1.5) | 0.8 (2.2) | 2.6 (1.6) | 9.8 (0.8) | 29.2 (4.3) | |
Standard errors are shown in parentheses.
NBN, narrow band noise.
Stimuli
One-quarter-octave narrowband noise (NBN) markers, geometrically centered on 2000 or 1000 Hz, were generated using Tucker Davis Technologies (TDT) System 3 hardware (20 kHz sampling rate) and locally produced software. The NBN markers were shaped with a cos2 window to create a 1-ms rise-fall time around the gap (offset of 1st marker, onset of 2nd marker) and a more gradual 10-ms rise-fall time on the onset of the 1st marker and the offset of the 2nd marker. These stimuli and rise-fall times are similar to those used in behavioral gap detection tasks (e.g., Lister & Roberts, 2005; Phillips, et al., 1997). Markers were presented at a level of 70 dB SPL via Sennheiser HD 265 linear circumaural headphones (behavioral tasks) or via Etymotic ER-2 linear insert earphones (CAEP task). Sound pressure levels were calibrated by playing out individual 400-ms noise bursts at each center frequency and recording the level using a Bruel and Kjaer Type I sound level meter (model 2235, linear setting, fast mode) with a ½ inch condenser microphone (model 4134). The sound level meter was connected to a Bruel and Kjaer artificial ear (model 4153) with a flat plate adapter for the circumaural earphones used for the behavioral tasks. The sound level meter was connected to a Bruel and Kjaer 2cc coupler (model DB 0138) to calibrate the insert earphones used for the CAEP task.
Psychophysical Gap Detection
GDTs were obtained monotically from the right ear using stimuli and methods identical to those used previously in our laboratory (e.g., Lister & Roberts, 2005; Lister, et al., 2002) and similar to those used by others (e.g., Phillips, et al., 1997). There were two stages in obtaining behavioral GDTs. In the first stage, behavioral detection thresholds were obtained for both NBN markers using a standard psychophysical paradigm. This was done to ensure audibility of the stimuli in subsequent experiments as Fitzgibbons and Gordon-Salant (1987) have suggested that sensation levels of 25 to 30 dB are necessary for optimum gap detection. Detection thresholds for all listeners are presented in Table 1.
In the second stage, GDTs were measured for two “channel” conditions: (1) within-channel in which the 1st and 2nd markers were centered on 2000 Hz, and (2) across-channel in which the 1st marker was centered on 2000 Hz and the 2nd marker was centered on 1000 Hz. The order of conditions was counterbalanced. The paired NBN markers were passed through attenuators (TDT PA4) to set the overall level and a low-pass filter with an 8-kHz cutoff (TDT FT6). The signals were presented to the right ear via Sennheiser HD 265 linear headphones at 70 dB SPL.
GDTs were obtained by using a two-interval, two-alternative forced choice (2I/2AFC) paradigm using a two-down, one-up rule targeting 70.7% (Levitt, 1971) correct detection. In the standard (incorrect answer) interval, the markers were separated by a 1-ms gap to insure that similar gating transients were present in both intervals and preclude the use of short-term spectral cues for interval selection (Phillips, et al., 1997). In the target interval (correct answer), the markers were separated by a gap that was decreased by a factor of 1.2 (after Lister, et al., 2002; Phillips, et al., 1997) following two correct answers and increased by a factor of 1.2 following one incorrect answer as dictated by the two-down, one-up rule mentioned above. Presentation order of the standard and target intervals was randomized across trials. Marker duration was varied randomly within a range of 250 to 350 ms to prevent use of marker duration cues to select the target interval (Lister & Tarver, 2004).
The listener's task was to indicate which one of two stimulus intervals contained the marker pair separated by a silent gap. The adaptive procedure continued until 10 reversals occurred; GDTs were calculated as the average gap size of the final eight reversals minus the standard 1-ms gap. The interval between marker pairs within a trial was fixed at 500 ms, and an intertrial interval of 500 ms occurred following the listener's response. Three runs of each channel condition were completed. Average GDTs for each listener and channel condition are shown in Table 2.
TABLE 2.
Mean P1, N1, and P2 peak amplitude and latency for each marker, channel condition, and gap duration (N = 12)*
| Amplitude |
Latency |
|||||||
|---|---|---|---|---|---|---|---|---|
| Marker | Channel condition | Gap duration | P1 | N1 | P2 | P1 | N1 | P2 |
| 1st | Within-channel | Standard | 0.7 (0.2) | –0.8 (0.5) | 1.4 (0.2) | 47.8 (3.2) | 101.8 (3.2) | 165.3 (6.8) |
| Subthreshold | 0.6 (0.1) | –0.8 (0.3) | 1.3 (0.2) | 51.0 (3.5) | 104.9 (3.9) | 167.9 (6.4) | ||
| GDT | 0.7 (0.1) | –1.1 (0.3) | 1.2 (0.2) | 52.0 (4.1) | 100.0 (3.5) | 171.5 (6.6) | ||
| Suprathreshold | 0.8 (0.2) | –0.8 (0.2) | 1.1 (0.2) | 59.6 (3.8) | 105.2 (4.3) | 176.8 (6.7) | ||
| Across-channel | Standard | 0.4 (0.1) | –0.8 (0.2) | 1.3 (0.2) | 54.8 (4.4) | 100.2 (5.1) | 170.7 (5.7) | |
| Subthreshold | 0.8 (0.2) | –1.1 (0.3) | 1.0 (0.2) | 56.9 (3.5) | 105.6 (3.8) | 179.5 (7.2) | ||
| GDT | 0.9 (0.2) | –1.1 (0.3) | 1.2 (0.3) | 55.9 (5.3) | 109.5 (4.6) | 185.7 (6.4) | ||
| Suprathreshold | 0.8 (0.2) | –0.9 (0.3) | 1.0 (0.2) | 51.5 (3.7) | 107.0 (3.9) | 176.2 (5.5) | ||
| 2nd | Within-channel | Standard | 0.0 (0.1) | –0.4 (0.2) | 0.6 (0.1) | 58.6 (5.9) | 103.2 (4.7) | 179.2 (7.4) |
| Subthreshold | 0.0 (0.2) | –0.7 (0.2) | 0.6 (0.1) | 47.7 (5.4) | 101.1 (5.3) | 191.4 (5.8) | ||
| GDT | 0.4 (0.1) | –0.9 (0.2) | 0.8 (0.2) | 55.7 (5.4) | 107.9 (4.3) | 186.8 (6.6) | ||
| Suprathreshold | 0.3 (0.1) | –1.0 (0.1) | 0.7 (0.1) | 51.0 (5.2) | 99.9 (3.9) | 175.3 (7.6) | ||
| Across-channel | Standard | 0.3 (0.2) | –1.6 (0.3) | 1.0 (0.1) | 51.1 (5.7) | 103.2 (3.4) | 173.3 (5.8) | |
| Subthreshold | 0.4 (0.3) | –1.1 (0.2) | 1.2 (0.2) | 58.6 (4.4) | 105.3 (3.3) | 171.0 (4.9) | ||
| GDT | 1.1 (0.2) | –1.4 (0.3) | 0.8 (0.4) | 55.1 (4.6) | 104.4 (3.1) | 164.2 (5.1) | ||
| Suprathreshold | 0.7 (0.1) | –1.3 (0.3) | 1.0 (0.2) | 52.3 (5.1) | 110.1 (3.6) | 178.8 (5.7) | ||
Standard errors are shown in parentheses.
P1-N1-P2 Peak Measurements
CAEPs were measured for eight conditions using stimuli identical to those used for the behavioral task with one exception: for AEP measurements, marker duration was fixed at 300 ms and did not vary randomly between 250 and 350 ms, as for the behavioral task. Fixed stimulus duration is typical for AEP tasks as a large number of identical stimuli must be presented and the subsequent responses averaged per condition and participant. Stimuli were presented monotically via Etymotic ER-2 linear insert earphones at a level of 70 dB SPL to the right ear. The listener was seated comfortably in a sound-attenuated booth and watched a closed caption video. The video monitor was placed outside the booth and was viewed through the booth window. The listener was asked to ignore the sounds and focus on the video.
As for the behavioral task, two channel conditions were used: (1) within-channel and (2) across-channel. In addition, four conditions of gap duration were used: (1) gap = individual listener's behavioral GDT; (2) gap = 2.4 X GDT (considered suprathreshold); (3) gap = GDT/2.4 (considered subthreshold); and (4) gap = 1 ms (identical to the standard interval in the psychophysical task). These four conditions were termed GDT gap, suprathreshold gap, subthreshold gap, and standard gap, respectively. The multiplier 2.4 was selected because it was twice as large as the step size used in the behavioral task (1.2) and, therefore, produced gap durations that were well above and below the 70.7% GDT on the psychometric function. Four hundred tokens were presented (prior to artifact rejection) for each of the eight conditions. The interstimulus interval was 900 ms. Data were collected for within-channel in a single 40-minute session. Listeners returned on a separate day for across-channel. Gap duration order was randomized within each channel condition.
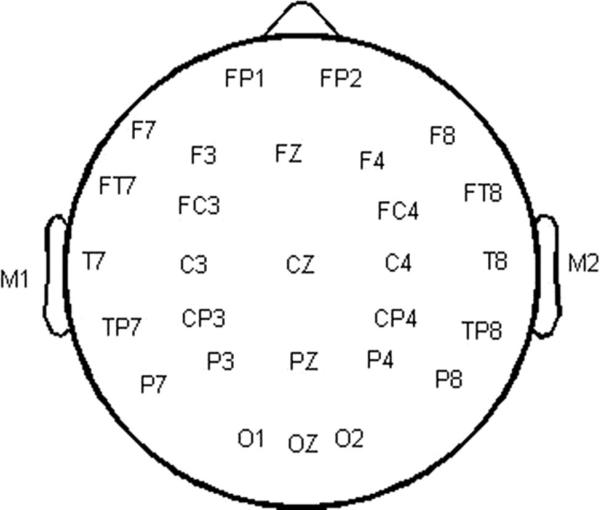
Continuous electroencephalogram (EEG) activity was recorded from 32 sintered Ag/AgCl electrodes (see Fig. 1). Triggers were time-locked to the onset of the 2nd marker. A nose electrode served as reference and a high forehead electrode served as ground. Four electrodes, one on the outer canthus of each eye, and one on both the supraorbital and infraorbital ridges of the left eye were used to monitor eye blink activity. A PC-based Neuroscan™ system (SCAN version 4.3.1) with SynAmps2 (amplifiers) was used to record EEG activity from all electrodes at a sampling rate of 1000 Hz. Evoked responses were band-pass filtered online from 0.1 to 100 Hz (12 dB/octave roll-off). Offline analysis of the continuous EEG began with manual artifact rejection. EEG epochs of 600 ms (−100 to 500 ms) were obtained, baseline corrected (−100 to 0 ms), and averaged separately for each marker, stimulus condition, and listener. Epochs containing artifacts greater than ±100 μV were rejected from averaging. Averaged waveforms were digitally band-pass filtered between 1 and 30 Hz with a 48 dB/octave roll-off.
Fig. 1.
Illustration of the recording montage for the 32 sintered Ag/AgCl electrodes. Electrodes not shown: nose electrode (reference), high forehead electrode (ground), four electrodes placed around the eyes to monitor eye blink activity.
Visual inspection of the entire scalp topography was used to ensure that the response fit the expected pattern (i.e., larger amplitudes frontally/centrally reversing in polarity over mastoids) (Vaughan & Ritter, 1970). As expected, P1, N1, and P2 were most easily identified at Fz. Therefore, the waveforms measured from this electrode were used in determining the peak latencies and amplitudes of these responses. Specifically, P1 was defined as the largest positivity occurring between 25 and 75 ms after marker onset. N1 was defined as the largest negativity occurring between 90 and 130 ms. P2 was defined as the largest positivity occurring between 150 and 210 ms. Peak amplitude and latency measurements of P1, N1, and P2 were measured at Fz, separately for each participant for each marker in each condition. Next, in order to test whether these responses were modulated by the experimental conditions, the peak amplitude and latency measurements for each response (P1, N1, and P2, respectively) were submitted to a repeated-measures ANOVA, with channel condition (within-channel, across-channel), marker (1st, 2nd), and gap duration (standard, subthreshold, GDT, suprathreshold) as within-subjects factors.
Temporal-Spatial Principal Component Analysis
Peak amplitude and latency measurements at a single electrode, as described above, focus on highly specific portions of the signal, allowing for some degree of standardization in measurement and, hence, comparison across studies. However, the time course and scalp topography of evoked potentials are much more complex than those observed at specific time points at a single location on the scalp. This fact is recognized in the design of modern systems that make it possible to record evoked potentials with high spatial and temporal resolution. As the number of recording electrodes and sampling rates have increased, alternative methods have been sought for describing and statistically analyzing the voltage activity captured in high-resolution AEP data sets. One approach is principal component analysis (PCA), which can be used to identify patterns of variance-covariance in the voltage activity recorded across several consecutive time points (in the temporal domain), or at topographically coherent clusters of electrodes on the scalp (in the spatial domain) (see Dien & Frishkoff, 2005 for further details on this approach). To make maximal use of our data set, and to extend our understanding of the complex brain dynamics involved in auditory temporal resolution, we submitted the averaged responses to the 2nd marker to a covariance-based, two-step, temporal-spatial PCA (see Hestvik, Maxfield, Schwartz, & Shafer, 2007, for a similar application of this approach).
For step 1 of this analysis, the averaged ERP data were combined into a single data matrix comprised of 501 columns (one column for each of the sampling points in the window extending from 0 to 500 ms after 2nd marker onset) and 2880 rows (the averaged ERP voltages for 12 participants, at each of 30 electrodes, in each of the eight conditions). This matrix was used as input to a temporal PCA. The aim of this initial, temporal PCA was to identify distinct windows of time in the ERP averages (hereafter, temporal factors) during which similar voltage variance was registered. In step 2, a spatial PCA was performed on the factor scores of the meaningful temporal factors, that is, the scores for each temporal factor (representing the voltage variance within a specific time window) were reconfigured into a matrix with 30 columns (one column per electrode) and 96 rows (scores for the temporal factor, for each of the 12 participants, in each of the 8 different conditions). This matrix was then submitted to a spatial PCA, in order to identify topo-graphically coherent regions of voltage activity (hereafter, spatial factors) within the time window represented by each temporal factor.
The following specific procedures were used to conduct both the initial, temporal PCA, and each of the subsequent, spatial PCAs. First, in order to determine how many dominant-variance components were extracted by each PCA, we used Rule N (Preisendorfer & Mobley, 1988). Rule N estimates how many components extracted from a real data set account for more variance than corresponding components extracted from a data set of normally distributed, randomly sampled noise having the same dimensions as the real data set. For each PCA, the number of components indicated by this rule was retained and rotated to simple structure using Promax (Hendrickson & White, 1964) with Kaiser normalization and k = 2 (Richman, 1986; Tataryn, Wood, & Gorsuch, 1999). All PC analyses and Promax rotations were completed using the Matlab-based PCA Toolbox (Dien, 2005).
To test for experimental effects, the factor scores associated with specific pairs of temporal and spatial factors were submitted to a repeated-measures ANOVA, with channel (within-channel, across-channel) and gap duration (standard, subthreshold, GDT, suprathreshold) as within-subjects factors. When the sphericity assumption was violated, the degrees of freedom were corrected (Greenhouse & Geisser, 1959). This correction is reflected in the reported p values. For all statistically significant main effects of gap duration, and for two-way interactions, post hoc comparisons of the scores for the different factor levels were made using Tukey Honest Significant Difference (HSD) tests.
Results
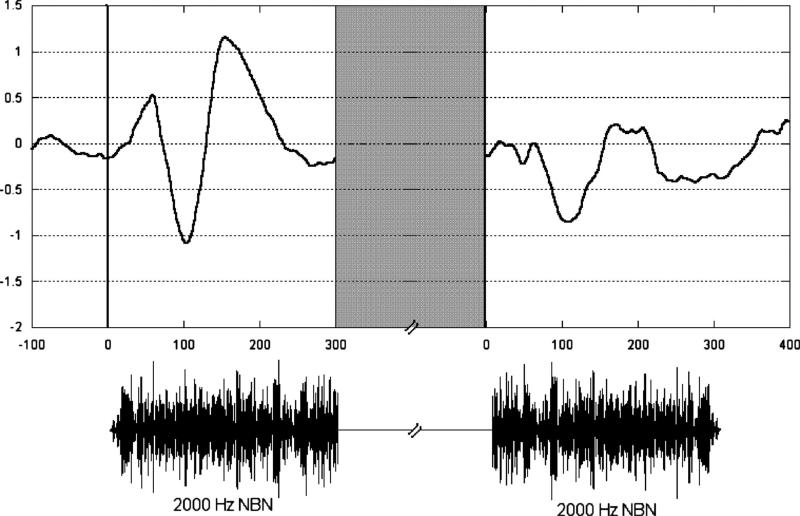
Representative individual responses for each marker are shown in Figure 2 with the time wave-forms of the markers. As shown in the figure, there were two time windows of interest, one following the onset of the 1st marker and one following the onset of the 2nd marker. Peak latencies are described relative to the onset of each marker.
Fig. 2.
Representative auditory evoked responses (AEPs) following each marker onset recorded from electrode Fz for a single participant. The stimulus condition was within-channel suprathreshold gap. Marker time waveforms are shown below AEPs. Gray area represents duration of gap, which varied across listeners and conditions.
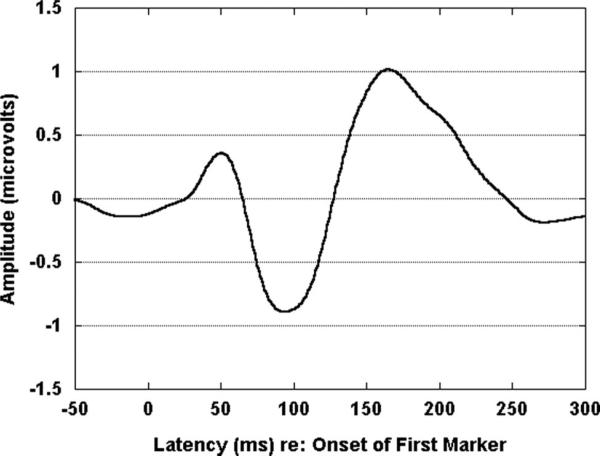
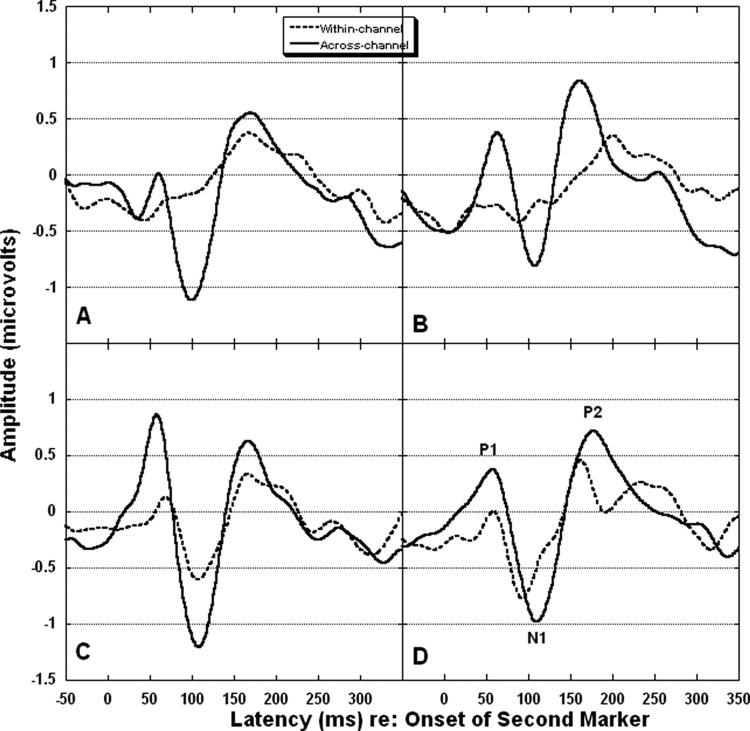
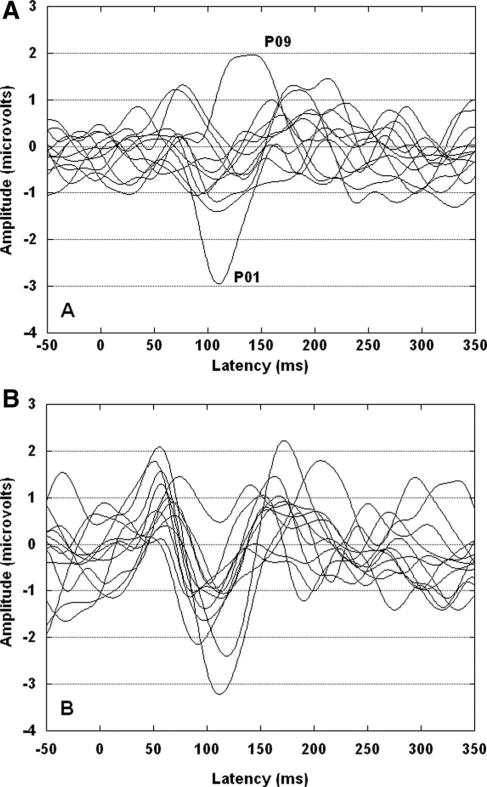
The average response for the 1st marker is shown in Figure 3. P1, N1, and P2 were easily identified and present across conditions for this marker. In the four panels of Figure 4, the average responses to the 2nd marker are shown for each channel condition and gap duration. Table 2 shows mean amplitudes and latencies for each peak for each marker, channel condition, and gap duration. As shown in Figure 4, P1, N1, and P2 are apparent for all across-channel conditions. For within-channel, however, the waveform morphology is relatively poor for the standard, subthreshold, and GDT gap conditions. In Figure 5 are shown the 12 individual responses for the within-channel (panel A) and across-channel (panel B) GDT gap conditions. For the within-channel GDT gap conditions (panel A), two individual waves stand out as less representative of the group mean response than the others. These two waves are identified by the associated participant numbers (P01 and P09). Both were female participants with unremarkable noise band detection thresholds and gap detection thresholds (see Table 1). Both had participated previously in behavioral gap detection experiments. This variability may be reflective of the following: (1) individual variation in activity across electrode locations (i.e., Fig. 5 shows activity at Fz only); (2) individual variation in performance on the behavioral task that generated the GDT used to set the gap durations (e.g., although controlled to a great extent by the adaptive psychophysical procedure, P01 may have responded conservatively on the behavioral task and her resulting GDT was slightly above true gap threshold).
Fig. 3.
Group mean response to 1st marker recorded from electrode Fz (N = 12).
Fig. 4.
Group mean responses to the 2nd marker recorded from electrode Fz (N = 12). Dotted lines represent within-channel. Solid lines represent across-channel. Panel A shows standard gap. Panel B shows subthreshold gap. Panel C shows GDT gap. Panel D shows suprathreshold gap. Peaks are labeled in panel D.
Fig. 5.
Individual waveforms for the 12 participants for two conditions recorded from Fz. Panel A represents the within-channel GDT gap condition and panel B represents the across-channel GDT gap condition. In panel A, two waves are identified with participant numbers and discussed in the text.
For each peak, amplitudes and latencies were analyzed in separate analyses of variance (ANOVAs) with channel condition, marker (1st, 2nd), and gap duration as within-subjects factors. For all peaks, latencies were measured relative to marker onset. The assumption of sphericity was not violated for any of the analyses.
P1-N1-P2 Latency
Latency did not differ across channel condition, marker, or gap duration for P1 or N1 (p > 0.05). For P2 latency, the interaction between channel condition and marker [F(1,11) = 17.56, p = 0.002] as well as the three-way interaction between channel condition, marker, and gap duration [F(3,33) = 3.41, p = 0.030] were significant. Tukey HSD post hoc analyses indicated that, for within-channel, P2 latency was significantly longer for the 2nd marker than for the 1st marker (p = 0.010). For 2nd markers only, P2 latency was significantly longer for within-channel than for across-channel (p = 0.020). None of the other comparisons of interest was significant (p > 0.05).
P1-N1-P2 Amplitude
For P1, amplitude was significantly larger for across-channel than for within-channel [F(1,11) = 8.10, p = 0.016]. Amplitude was also significantly larger for the 1st marker than for the 2nd marker [F(1,11) = 7.98, p = 0.017]. The effect of gap duration was also significant [F(3,33) = 7.86, p < 0.001]. A Tukey HSD post hoc analysis indicated that P1 amplitude was significantly smaller for standard gap than for suprathreshold gap (p = 0.015) and GDT (p = 0.001). P1 amplitude was also significantly smaller for subthreshold gap than for GDT (p = 0.013). The interaction between channel condition and marker did not quite reach statistical significance [F(1,11) = 4.33, p = 0.061]. A Tukey post hoc analysis indicated that the effect of channel condition was significant for the 2nd marker (p = 0.020) but not for the 1st marker (p = 0.921) and the effect of marker was significant for within-channel (p = 0.010) but not for across-channel (p = 0.736).
For N1, amplitude was significantly larger for across-channel than for within-channel [F(1,11) = 7.63, p = 0.019]. Also, the interaction between channel condition and marker [F(1,11) = 6.77, p = 0.025] was significant. A Tukey HSD post hoc analysis indicated that, for the 2nd marker, amplitudes were significantly larger for across-channel than for within-channel (p = 0.005). This difference between channel conditions was not significant for the 1st marker (p = 0.889).
For P2, amplitudes were significantly larger for the 1st marker than for the 2nd marker [F(1,11) = 5.04, p = 0.046]. The interaction between channel condition and marker did not reach statistical significance [F(1,11) = 3.32, p = 0.096]. A Tukey HSD post hoc analysis indicated that the marker effect was significant for within-channel (p = 0.028) but not for across-channel (p = 0.865). None of the other effects was statistically significant (p > 0.05).
Temporal-Spatial Principal Component Analysis
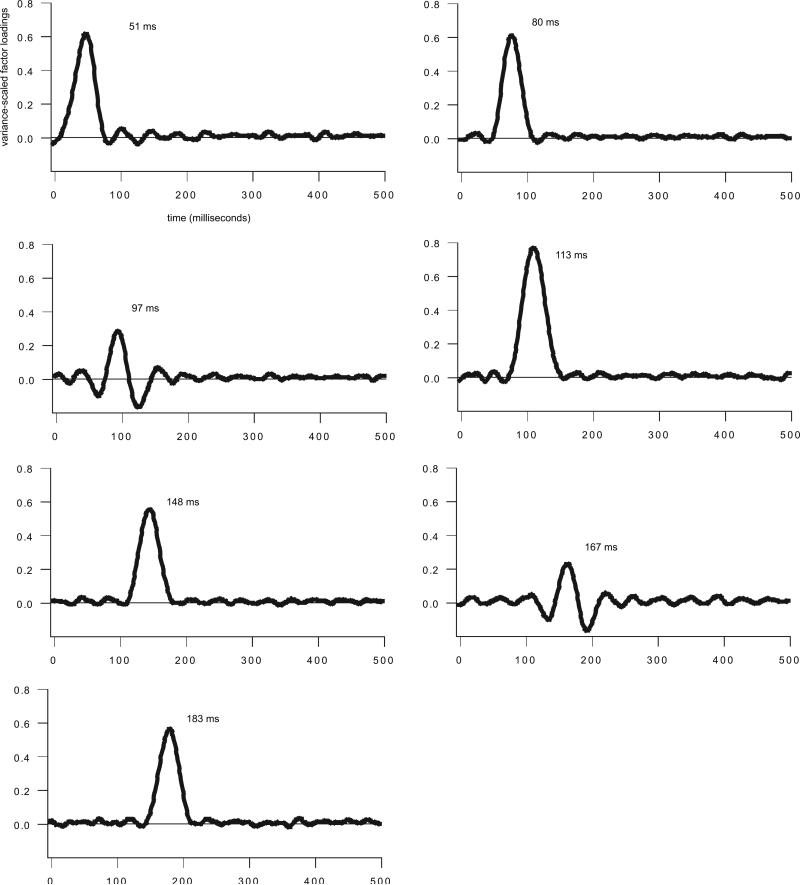
A temporal-spatial PCA was conducted using the averaged CAEP responses to the 2nd marker. For the initial, temporal PCA, a total of 22 factors were retained and Promax-rotated. The 22 temporal factors accounted for 84.74% of the variance in the data set. As we were interested in the latency region of the P1-N1-P2 response, 7 of the 22 temporal factors were retained for further analysis because their peak latencies occurred between 25 and 210 ms after 2nd marker onset (region of interest for single electrode analysis described above), and they were associated with experimental effects. These seven temporal factors occurred at latencies between 51 and 183 ms (see Fig. 6 for the time course and peak latency of each meaningful temporal factor). A subsequent spatial PCA, performed on the factor scores of each temporal factor, identified topographically coherent regions of voltage activity (spatial factors) within the time window represented by each temporal factor. Only those temporal-spatial factor combinations associated with statistically significant experimental effects are reported (see Fig. 7 for temporal-spatial factor combinations associated with experimental effects). Each temporal factor is labeled by its latency (e.g., T51 occurred 51 ms following 2nd marker onset).
Fig. 6.
Temporal factor loadings showing the time course and peak latency for each of the seven meaningful temporal factors. The largest consecutive loadings indicate the time interval of CAEP activation for each factor.
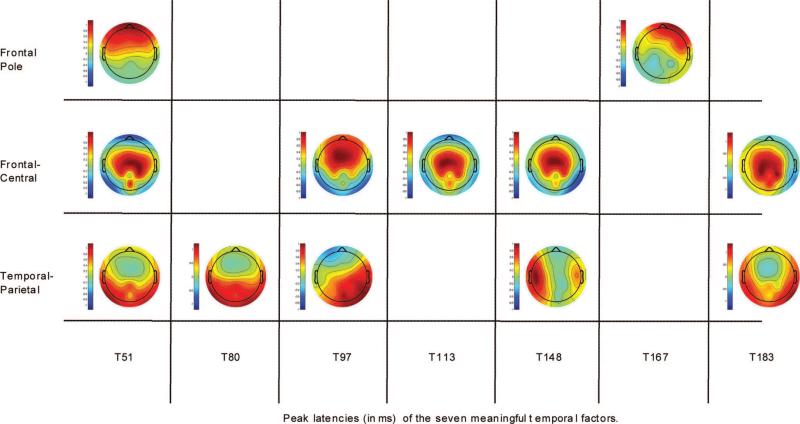
Fig. 7.
Spatial factor loadings showing the scalp topography of spatial factors, at each temporal factor, associated with statistically significant experimental effects. The maps are organized loosely by region. Spatial factor loadings range in amplitude from +1 (extreme red) to −1 (extreme blue).
T51
The first temporal factor had a peak latency of 51 ms (see Fig. 6). For T51, three spatial factors were retained and Promax-rotated, partitioning the voltage variance within this window into three scalp regions. All three spatial factors were associated with statistically significant effects. One spatial factor was localized at frontal pole electrodes (see Fig. 7, T51, frontal pole). The factor scores representing the voltage variance within the 51-ms time window, at this frontal pole region, yielded a main effect of channel [F(1,11) = 10.75, p = 0.007]. Across-channel scores were more positive in amplitude than within-channel scores. Given its time course and scalp topography, this effect is consistent with a P1 (also called P50), sensory gating-type response (Key et al., 2005).
The second spatial factor at this latency had a bilateral temporal-parietal scalp topography (see Fig. 7, T51, temporal-parietal). The factor scores representing the voltage variance within the 51-ms time window, at this bilateral temporal-parietal region, yielded a main effect of gap duration [F(3,33) = 5.48, p = 0.003]. A Tukey HSD post hoc analysis showed a statistically significant difference between suprathreshold gap and each of the other three gap durations (p < 0.05). The suprathreshold scores were more negative in amplitude than the scores for the other three gap durations. Thus, it appears that suprathreshold gap was initially coded at the same latency as was across-channel, but by a different CAEP. Given the time course and scalp topography of this potential, it may represent an early component of the T-complex, a series of peaks in the latency region of 70 to 160 ms that are recorded at temporal electrodes (e.g. Tonnquist-Uhlen, Ponton, Eggermont, Kwong, & Don, 2003; Wolpaw & Penry, 1975).
The third spatial factor at this latency had a bilateral central scalp topography (see Fig. 7, T51, frontal-central). The factor scores representing the voltage variance within the 51-ms time window, at this bilateral central region, yielded a main effect of channel [F(1,11) = 9.32, p = 0.011]. Across-channel scores were more positive in amplitude than within-channel scores. This effect inverted in polarity at frontal pole sites (see inversion of loading for the two spatial locations), indicating that it was different from P1 activation. Thus, it appears that across-channel was associated with two distinct potentials during the 51-ms window.
T80
The second temporal factor had a peak latency of 80 ms (see Fig. 6). For this time window, a total of two spatial factors were retained and Promax-rotated, partitioning the T80 voltage variance into two scalp regions. Just one spatial factor, with a bilateral temporal-parietal scalp topography (see Fig. 7, T80, temporal-parietal), was associated with statistically significant effects. The factor scores summarizing the voltage variance within the 80-ms time window, at this bilateral temporal-parietal region, yielded both a main effect of channel [F(1,11) = 13.50, p = 0.003] and a main effect of gap duration [F(3,33) = 5.02, p = 0.005]. For the channel effect, across-channel scores were more negative in amplitude than within-channel scores. For the gap effect, a Tukey HSD post hoc analysis showed that the suprathreshold scores were statistically different from the scores for the other three gap durations (p < = 0.05). Here again, as for the T51 gap effect, the suprathreshold scores were more negative in amplitude than the scores for the other three gap durations.
Both the negative-going channel effect and the negative-going gap effect reported here are consistent with early N1 activation. Early N1 activation (~75 ms), when seen at temporal electrodes, is associated with processing of the physical characteristics of stimuli (Naatanen & Picton, 1987) and may also be considered the Na component of the T-complex (e.g., Tonnquist-Uhlen, et al., 2003; Wolpaw & Penry, 1975). Therefore, it appears that both across-channel (spectral) change between markers and a long duration (suprathreshold) gap between markers, are physical cues that elicit an early negativity in the temporal scalp region.
T97
The next temporal factor had a peak latency of 97 ms (see Fig. 6). For this temporal factor, a total of three spatial factors were retained and Promax-rotated, partitioning the T97 voltage variance into three scalp regions. Two of those spatial factors were associated with statistically significant effects. One had a mid-frontal scalp distribution (see Fig. 7, T97, frontal-central). The factor scores summarizing the voltage variance within the 97-ms time window, at this mid-frontal region, yielded an interaction of channel and gap duration [F(3,33) = 5.40, p = 0.003]. A Tukey HSD post hoc analysis revealed no differences in the amplitude of the scores between gap durations within each channel condition (p > 0.05). However, a statistically significant difference was detected for within-channel versus across-channel at standard gap (p < 0.05). The scores for across-channel, standard gap were more negative in amplitude than the scores for within-channel, standard gap. This effect is consistent with another N1-type potential. N1 activation, when seen at frontal-central electrodes at ~100 ms after stimulus onset, is also associated with processing of the physical characteristics of stimuli (Naatanen & Picton, 1987).
The second spatial factor at this latency had a right temporal-parietal scalp topography (see Fig. 7, T97, temporal-parietal). The factor scores summarizing the voltage variance within the 97-ms window, at this right temporal-parietal region, yielded a main effect of channel [F(1,11) = 10.35, p = 0.008]. Across-channel scores were more negative in amplitude than within-channel scores. Thus, it appears that, while across-channel, standard gap was associated with a large frontal-central N1 activation during the 97-ms window, a right temporal-parietal CAEP was elicited by across-channel during this window for all gap durations. The right temporal-parietal potential does not appear to be related to the T-complex as the Ta component of that complex that occurs at ~100 ms is of positive, not negative, orientation.
T113
The next temporal factor had a peak latency of 113 ms (see Fig. 6). For T113, a total of three spatial factors were retained and Promax-rotated, partitioning the voltage variance for this time window into three scalp regions. Just one spatial factor, with a frontal-central topography (see Fig. 7, T113, frontal-central), was associated with a statistically significant effect. The factor scores summarizing the voltage variance within the 113-ms window, at this frontal-central region, yielded a main effect of channel [F(1,11) = 28.76, p < 0.001]. Here, again, across-channel scores were more negative in amplitude than within-channel scores. This effect, too, is consistent with N1 activation which, as noted above, has been associated with processing of the physical characteristics of stimuli when seen at frontal-central electrodes. What seems to be the case is that frontal-central N1 activation was associated with across-channel for all gap durations during the 113-ms window, whereas it was also associated with across-channel, standard gap at a slightly earlier (97 ms) latency, reported above.
T148
The next temporal factor had a peak latency of 148 ms (see Fig. 6). For this temporal factor, four spatial factors were retained and Promax-rotated, partitioning the T148 voltage variance into four scalp regions. Two of those four spatial factors were associated with statistically significant experimental effects. The first had a frontal-central scalp topography (see Fig. 7, T148, frontal-central), similar to the frontal-central N1-type activations reported above. The scores representing the voltage variance within the 148-ms window, at this frontal-central region, yielded an interaction of channel and gap duration [F(3,33) = 5.19, p = 0.004]. A Tukey HSD post hoc analysis revealed no differences in the amplitude of the scores between gap durations within each channel condition (p > 0.05). However, statistically significant differences were seen for the within-channel versus across-channel for sub-threshold and GDT gap. For these two gap durations, across-channel scores were more positive in amplitude than within-channel scores. These effects are consistent with P2 activation. P2, like N1, is sensitive to physical properties of stimuli, such as loudness and pitch (Key, et al., 2005). In the present experiment, then, it appears that P2 activation was enhanced when a spectral change occurred between stimuli, but only when those stimuli were separated by certain gap durations.
The second spatial factor at this latency had a distinct, left temporal scalp topography (see Fig. 7, T148, temporal-parietal). The factor scores summarizing the voltage variance within the 148-ms window, at this left temporal region, also yielded an interaction of channel and gap duration [F(3,33) = 3.71, p = 0.020]. Here, again, a Tukey HSD post hoc analysis revealed no differences between the scores of the different gap duration levels within each channel condition (p > 0.05). However, a statistically significant difference was detected for within-channel versus across-channel at subthreshold gap (p < 0.05). Across-channel, sub-threshold scores were more negative in amplitude than within-channel, subthreshold scores. This effect is consistent with the Tb component of the T-complex, a negativity that occurs between 140 and 160 ms, and is seen only for across-channel markers following a subthreshold gap.
T167
The next temporal factor had a peak latency of 167 ms (see Fig. 6). For this time window, four spatial factors were retained and Promax-rotated, partitioning the T167 voltage variance into four different scalp regions. Just one spatial factor, with a right anterior scalp topography (see Fig. 7, T167, frontal pole), was associated with a statistically significant effect. The factor scores summarizing the voltage variance within the 167-ms window, at this right anterior region, yielded a main effect of gap duration [F(3,33) = 2.86, p = 0.050]. A Tukey HSD post hoc analysis revealed a significant difference between the standard scores versus GDT scores (p < 0.05). Positive-going scores were seen for GDT but not for the other three gap durations. Therefore, it appears that this temporal-spatial factor combination is a novel AEP correlate of GDT.
T183
The final temporal factor had a peak latency of 183 ms (see Fig. 6). For this temporal factor, three spatial factors were retained and Promax-rotated, partitioning the T183 voltage variance into three scalp regions. Two of those spatial factors were associated with statistically significant effects. The first had a bilateral, temporal-parietal scalp topography (see Fig. 7, T183, temporal-parietal). The factor scores summarizing the voltage variance within the 183-ms time window, at this bilateral temporal-parietal region, yielded a main effect of gap duration [F(3,33) = 4.70, p = 0.007]. A Tukey HSD post hoc analysis revealed a statistically significant difference between the GDT and suprathreshold scores (p < 0.05). GDT scores were more negative in amplitude than suprathreshold scores. Although this component does not fall within the latency region traditionally considered for the T-complex (i.e., 70–160 ms), it may represent a late component of this complex. The significance of this effect, if any, is as yet unclear.
The second spatial factor at this latency had a bilateral central scalp topography (see Fig. 7, T183, frontal-central). The factor scores summarizing the voltage variance within the 183-ms window, at this bilateral central region, yielded a main effect of channel [F(1,11) = 7.67, p = 0.018]. Across-channel scores were more positive in amplitude than within-channel scores. This effect may reflect a later P2-type activation, serving as yet another marker of across-channel stimulation.
Discussion
This study was designed to describe the neural representation of the elements of a typical psychophysical gap detection task, including conditions of across-channel markers that have received much interest in recent years. As expected, behavioral GDTs were significantly larger for across-channel markers than for within-channel markers. GDTs were consistent with those measured previously (e.g., Lister & Roberts, 2005) for similar within-channel (~6 ms) and across-channel (~30 ms) conditions. Also as expected, P1, N1, and P2 were consistently identified for 1st markers and did not differ significantly in latency or amplitude across channel and gap conditions for that marker.
For within-channel 2nd marker onsets, we expected that P1, N1, and P2 would be present only when the gap was behaviorally perceptible. For across-channel, we expected that P1, N1, and P2 would be present for all 2nd marker onsets regardless of gap duration due to the additional cue of frequency change following the gap. Figure 4 illustrates these effects. For across-channel standard gap (panel A), the P1-N1-P2 response is apparent despite the fact that the gap duration (1 ms) is well below behavioral threshold. We believe this response reflects activity related to the change in frequency across the gap and not the 1 ms gap because P1-N1-P2 is known to occur in response to any acoustic change. For within-channel standard gap, the P1-N1-P2 response is not discernible. These two waveforms denote the cortical representation of a typical “standard” stimulus in a psychophysical gap detection task. The neural representations of this stimulus may be compared to those of the target stimulus and a decision made regarding which interval to select. For within-channel, this comparison seems simple as the 2nd marker of the standard gap stimulus does not produce a P1-N1-P2 response. For across-channel, the comparison is less obvious as both intervals produce a robust 2nd marker response.
For the 2nd marker, across-channel markers produced shorter P2 latencies and larger P1 and N1 amplitudes than within-channel. For within-channel markers, 2nd markers produced longer P2 latencies and smaller P1 and P2 amplitudes than 1st markers. In addition, P1 amplitude was larger for larger gap duration conditions (e.g., suprathreshold and GDT) than smaller gap duration conditions (e.g., standard and subthreshold).
Michalewski et al. (2005) and Pratt et al. (2005) measured AEPs using gaps in broadband noise (i.e., within-channel). They found that the N1 to marker onset was single-peaked, increased in amplitude with gap duration, and was present for gaps equal to or larger than behavioral gap detection threshold. This is consistent with the present findings. For relatively long gap durations (200–800 ms), Pratt et al. observed a “N(egation)-process” or “N1b” that occurred as part of a double-peaked N1 following marker offset, distinct from the single-peaked N1 to marker onset. It is likely, given the relatively short gap durations (1–59 ms for standard, subthreshold, and GDT gaps; 14–142 ms for suprathreshold gap) used in the present study, that the N1 responses we measured represent some combination of the offset and onset N1 responses described by Pratt et al.
Heinrich et al. (2004) used brief (20 ms) pure tones of 1000 or 2000 Hz to mark silent gaps. N1 and P2 latencies and amplitudes were similar for near-threshold gap durations for both within- and across-channel conditions. In contrast to Heinrich et al., we observed significant effects of channel condition for P2 latency, P1 amplitude, and N1 amplitude. However, these effects occurred only for the 2nd and not the 1st marker. Heinrich et al. measured peaks relative to the onset of the 1st tone burst, and markers were very brief; therefore, the response to the onset of the 2nd marker could not be observed separately from that of the 1st marker. We are not aware of any other P1-N1-P2 studies of across-channel gap detection.
The temporal-spatial PCA uncovered a number of experimental effects. First, clear channel-related effects were seen at all but one (T167) of the temporal factors examined. Across-channel markers were associated with significantly more positive-going voltage activity than within-channel for T51 (frontal and frontal-central), T148 (frontal-central), and T183 (frontal-central). Across-channel markers were associated with significantly more negative-going voltage activity than within-channel for T80 (temporal-parietal), T97 (temporal-parietal and frontal-central), T113 (frontal-central), and T148 (temporal-parietal). For channel effects, then, the temporal-spatial PCA was useful for decomposing evoked potentials typically associated with the P1-N1-P2 complex into individual components with differing scalp topographies. Although a source localization was not performed, the projection of these components to different regions of the scalp over time suggests the involvement of multiple neural generators responding to the same across-channel markers. Given the time course and scalp regions of these potentials, they likely represent components of both the traditional P1-N1-P2 complex and the T-complex (Tonnquist-Uhlen, et al., 2003; Wolpaw & Penry, 1975).
Gap duration effects were also seen for three temporal-parietal factors. At the earliest latencies (T51 and T80), suprathreshold gap appeared to elicit more negative-going activity than any other gap duration. Later, for T167 (right frontal), a significant difference was found between standard and GDT gap, with GDT gap associated with a more positive-going voltage than the other gap durations. Finally, for the latest temporal factor (T183, temporal-parietal), a significant difference was found between GDT and suprathreshold gap, with GDT gap associated with a more negative-going voltage than the other gap durations. These primarily temporal-parietal effects were missed by our initial, single-electrode analysis. Though restricted to the temporal-parietal region, the slightly varying scalp topographies of the components within this region are, again, suggestive of different neural generators sensitive to gap duration effects, likely components of the T-complex (Tonnquist-Uhlen, et al., 2003; Wolpaw & Penry, 1975).
Further still, interactions between channel condition and gap duration were found for T97 and T148. For T97 (frontal-central), a significant channel effect was found only for standard gap. For T148 (frontal-central and left temporal-parietal), channel effects were found for GDT and subthreshold gap. This pattern of voltage activity suggests that across-channel standard gap elicited a strong frontal-central response 97 ms following 2nd marker onset, whereas longer gaps (GDT and subthreshold) in the across-channel condition elicited a strong frontal-central and temporal-parietal response ~50 ms later.
In all, the PCA results provide a more detailed illustration of stimulus effects than the initial analyses of peak latency and amplitude measured from a single electrode. Although channel condition appeared to have effects across the temporal and spatial factor combinations identified by the PCA, gap duration effects were found primarily in the temporal-parietal region at 51, 80, and 183 ms, and probably reflect components of the T-complex. This expands the results of the initial, single (Fz) electrode, analysis, which revealed an overall effect of channel but only one effect of gap duration at the earliest latency region of interest, P1 (25–75 ms). Most interesting were the positive-going activity at T167 and the negative-going activity at T183 that appeared to be uniquely associated with the threshold (GDT) gap duration for both channel conditions. Also interesting but requiring further exploration were the interaction effects suggesting activity associated with specific channel condition and gap duration combinations for particular temporal and spatial factor combinations. These data are consistent with the existence of a central timing mechanism that must monitor the activity in a variety of temporal and spatial regions to detect a gap between spectrally different markers (e.g., Phillips, Hall, Harrington, & Taylor, 1998).
Clearly, further research including PCA is necessary to understand the physiological underpinnings of across-channel gap detection. A simple one to one relationship between a single-electrode auditory cortical response and behavioral perception is not apparent for across-channel markers. Overall, the pattern of results suggests that larger amplitudes and shorter latencies were generally found for the conditions in which the acoustic cues are most salient. For the stimuli used in this study, across-channel markers, 1st markers, and large gap durations represented more salient cues. In the across-channel case, the onset of the 2nd marker is of a different frequency than the 1st marker. Therefore, the population of neurons that respond to the onset of the 2nd marker may be different from the neural population that responded to the 1st marker onset. For longer gap durations, the 2nd marker onset is occurring after a relatively long silent period, providing a refractory period for neuron recovery. Different and well rested neural populations are able to respond robustly to a stimulus onset. This hypothesis is supported by the finding of significantly smaller amplitudes for the 2nd marker onset than for the 1st marker onset for within-channel. For those conditions, the additional cue of frequency change after the gap is not present and the gap durations are relatively short. Therefore, the same population of neurons that responded to the onset of the 1st marker is called upon (after a brief recovery period) to respond again to the onset of the 2nd marker. It is apparent from these data that such a response is less robust than the one generated by across-channel and large gap conditions.
It is important to describe the normal neural representation of the elements of a typical psychophysical gap detection task so that we may begin to explore the underlying physiological differences in populations known to have poor temporal resolution. Such populations include but are not limited to older adults (Lister & Tarver, 2004; Lister, et al., 2002), adults with hearing loss (Lister & Roberts, 2005; Roberts & Lister, 2004), adults and children with dyslexia (Conlon, Sanders, & Zapart, 2004; Meyler & Breznitz, 2005; Van Ingelghem, van Wieringen, Wouters, Vandenbussche, Onghena, & Ghesquière, 2001), and adults and children with auditory neuropathy (Kraus, Bradlow, Cheatham, Cunningham, King, Koch, et al., 2000; Zeng, Oba, Garde, Sininger, & Starr, 1999).
Preliminary data in our laboratory and published studies from others (e.g., Tremblay, Piskosz, & Souza, 2003; Werner, Folsom, Mancl, & Syapin, 2001) suggest that both psychophysical and cortical responses may differ significantly from normal in the aforementioned populations. Most interesting in light of the present results are the behavioral findings for older adults. This group often has poorer within- and across-channel gap detections than young adults; yet, age-group differences are much larger for across-channel than within-channel conditions (e.g., Lister & Roberts, 2005; Lister, et al., 2002). As within- and across-channel performance was not correlated in the present study (r = 0.22, p = 0.50) or others (Phillips & Smith, 2004), it is likely that different mechanisms are involved in the two tasks. The present study represents a first step in the investigation of the physiological underpinnings of across-channel gap detection.
Key to further exploration of the physiological underpinnings of behaviorally measured perceptual deficits is use of stimuli that parallel those used in the behavioral tasks as well as the measurement of physiological and behavioral responses in the same participants. In the area of temporal resolution, future studies should use relatively long stimuli to mark silent gaps and examine onset and offset responses to each marker individually. Future studies may also include comparison of peak to peak amplitudes for the 1st marker versus the 2nd marker. In this manner, an individual listener's response to the 1st marker onset may serve as a reference to which the 2nd marker response may be compared to determine relative response magnitude.
Conclusion
This study was designed to provide a description of the cortical response to silent gaps in a group of young adults with normal hearing using within-channel and across-channel stimulus conditions identical to those used in psychophysical studies of gap detection. The results suggest that the latency of P2 and the amplitude of P1, N1, and P2 are affected by acoustic characteristics of the 2nd marker as well as the duration of the gap. Specifically, larger amplitudes and shorter latencies were generally found for the conditions in which the acoustic cues are most salient (e.g., across-channel markers, 1st markers, large gap durations). Although the average waveforms measured at Fz showed clear gap duration effects for the within-channel conditions, gap duration effects were not apparent for the across-channel conditions. A subsequent PCA revealed activity elicited by threshold gap durations in the regions of 167 and 183 ms for temporal-parietal and right-frontal spatial locations. Gap duration appeared to be most clearly indicated by P1 and T-complex amplitude. These data provide noninvasive, nonbehavioral indicators of the neural coding of an important temporal cue in the thalamic-cortical region of the central auditory system.
Acknowledgments
The authors thank Lauren Stack and Victoria Gonzalez for their assistance in data collection.
This work was supported by grant R03AG024589 from the National Institute on Aging.
References
- Abeles M, Goldstein M. Responses of single units in the primary auditory cortex of the cat to tones and to tone pairs. Brain Research. 1972;42:337–352. doi: 10.1016/0006-8993(72)90535-5. [DOI] [PubMed] [Google Scholar]
- Barsz K, Ison J, Snell K, Walton J. Behavioral and neural measures of auditory temporal acuity in aging humans and mice. Neurobiology of Aging. 2002;23:565–578. doi: 10.1016/s0197-4580(02)00008-8. [DOI] [PubMed] [Google Scholar]
- Conlon E, Sanders M, Zapart S. Temporal processing in poor adult readers. Neuropsychologia. 2004;42:142–157. doi: 10.1016/j.neuropsychologia.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Dien J. PCA Toolbox [Computer software] (Version 1.93) Lawrence, KA: 2005. Available at: http://www.people.ku.edu/~jdien/dowloads.html. [Google Scholar]
- Dien J, Frishkoff GA. Principal components analysis of event-related potential datasets. In: Handy T, editor. Event-related potentials: a methods handbook. MIT Press; Cambridge: 2005. [Google Scholar]
- Dubno J, Horwitz A, Ahlstrom J. Recovery from prior stimulation: masking of speech by interrupted noise for younger and older listeners with normal hearing. The Journal of the Acoustical Society of America. 2003;113:2084–2094. doi: 10.1121/1.1555611. [DOI] [PubMed] [Google Scholar]
- Eddins D, Hall J, Grose J. The detection of temporal gaps as a function of frequency region and absolute noise bandwidth. The Journal of the Acoustical Society of America. 1992;91:1069–1077. doi: 10.1121/1.402633. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons P, Gordon-Salant S. Minimum stimulus levels for temporal gap resolution in listeners with sensorineural hearing loss. The Journal of the Acoustical Society of America. 1987;81:1542–1545. doi: 10.1121/1.394506. [DOI] [PubMed] [Google Scholar]
- Formby C, Gerber M, Sherlock L, Magder L. Evidence for an across-channel frequency, between channel process in asymptotic monaural temporal gap detection. The Journal of the Acoustical Society of America. 1998;103:3554–3560. doi: 10.1121/1.423084. [DOI] [PubMed] [Google Scholar]
- Friesen L, Tremblay K. Acoustic change complexes recorded in adult cochlear implant listeners. Ear and Hearing. 2006;27:678–685. doi: 10.1097/01.aud.0000240620.63453.c3. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons P. Temporal factors and speech recognition performance in young and elderly listeners. Journal of Speech, Language, and Hearing Research. 1993;36:1276–1285. doi: 10.1044/jshr.3606.1276. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons P. Profile of auditory temporal profiling in older listeners. Journal of Speech, Language, and Hearing Research. 1999;42:300–311. doi: 10.1044/jslhr.4202.300. [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Grose J, Hall J, Buss E, Hatch D. Gap detection for similar and dissimilar gap markers. The Journal of the Acoustical Society of America. 2001;109:1587–1595. doi: 10.1121/1.1354983. [DOI] [PubMed] [Google Scholar]
- He N, Horwitz A, Dubno J, Mills J. Psychometric functions for gap detection in noise measured from young and aged subjects. The Journal of the Acoustical Society of America. 1999;106:966–978. doi: 10.1121/1.427109. [DOI] [PubMed] [Google Scholar]
- Hegerl U, Juckel G. Intensity dependence of auditory evoked potentials as an indicator of central serontonergic neurotransmission: a new hypothesis. Biological Psychiatry. 1993;33:173–187. doi: 10.1016/0006-3223(93)90137-3. [DOI] [PubMed] [Google Scholar]
- Heinrich A, Alain C, Schneider B. Within- and between-channel gap detection in the human auditory cortex. Neuroreport. 2004;15:2051–2056. doi: 10.1097/00001756-200409150-00011. [DOI] [PubMed] [Google Scholar]
- Hendrickson AE, White PO. Promax: a quick method for rotation to oblique simple structure. The British Journal of Mathematical and Statistical Psychology. 1964;17(1):65–70. doi: 10.1111/j.2044-8317.1966.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Hestvik A, Maxfield N, Schwartz RG, Shafer V. Brain responses to filled gaps. Brain and Language. 2007;100(3):301–316. doi: 10.1016/j.bandl.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard S, Picton T. Electrophysiology of cognition. In: Mountastle VB, Plum F, Geiger SR, editors. Handbook of physiology. Section 1: The nervous system. Vol. 5. American Physiology Society; Washington, DC: 1987. pp. 519–584. [Google Scholar]
- Huotilainen M, Winkler I, Alho K, Escera C, Virtanen J, Ilmoniemi R, et al. Combined mapping of human auditory EEG and MEG responses. Electroencephalography and Clinical Neurophysiology. 1998;108:370–379. doi: 10.1016/s0168-5597(98)00017-3. [DOI] [PubMed] [Google Scholar]
- Hyde M. The N1 response and its applications. Audiology & Neuro-Otology. 1997;2:281–307. doi: 10.1159/000259253. [DOI] [PubMed] [Google Scholar]
- Ison J, Castro J, Allen P, Virag T, Walton J. The relative detectability for mice of gaps having different ramp durations at their onset and offset boundaries. The Journal of the Acoustical Society of America. 2002;112:740–747. doi: 10.1121/1.1490352. [DOI] [PubMed] [Google Scholar]
- Kaukoranta E, Hari R, Lounasmaa OV. Responses of the human auditory cortex to vowel onset after fricative consonants. Experimental Brain Research. 1987;69:19–23. doi: 10.1007/BF00247025. [DOI] [PubMed] [Google Scholar]
- Key A, Dove G, Maguire M. Linking brainwaves to the brain: an ERP primer. Developmental Neuropsychology. 2005;27:183–215. doi: 10.1207/s15326942dn2702_1. [DOI] [PubMed] [Google Scholar]
- Knight R, Scabini D, Woods D, Clayworth C. The effects of lesions of superior temporal gyrus and inferior parietal lobe on temporal and vertex components of the human AEP. Electroencephalography and Clinical Neurophysiology. 1988;70:499–508. doi: 10.1016/0013-4694(88)90148-4. [DOI] [PubMed] [Google Scholar]
- Korczak P, Kurtzberg D, Stapells D. Effects of sensorineural hearing loss and personal hearing aids on cortical event-related potentials and behavioral measures of speech–sound processing. Ear and Hearing. 2005;26:165–185. doi: 10.1097/00003446-200504000-00005. [DOI] [PubMed] [Google Scholar]
- Kraus N, Bradlow A, Cheatham M, Cunningham J, King C, Koch D, et al. Consequences of neural asynchrony: a case of auditory neuropathy. Journal of the Association for Research in Otolaryngology. 2000;1:33–45. doi: 10.1007/s101620010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H. Transformed up–down methods in psychoacoustics. The Journal of the Acoustical Society of America. 1971;49:467–477. [PubMed] [Google Scholar]
- Lister J, Roberts R. Effects of age and hearing loss on gap detection and the precedence effect: narrow-band stimuli. Journal of Speech, Language, and Hearing Research. 2005;48:482–493. doi: 10.1044/1092-4388(2005/033). [DOI] [PubMed] [Google Scholar]
- Lister J, Tarver K. Effect of age on silent gap discrimination in synthetic speech stimuli. Journal of Speech, Language, and Hearing Research. 2004;47:257–269. doi: 10.1044/1092-4388(2004/021). [DOI] [PubMed] [Google Scholar]
- Lister J, Besing J, Koehnke J. Effects of age and frequency disparity on gap duration discrimination. The Journal of the Acoustical Society of America. 2002;111:2793–2800. doi: 10.1121/1.1476685. [DOI] [PubMed] [Google Scholar]
- Martin B, Boothroyd A. Cortical, auditory, event-related potentials in response to periodic and aperiodic stimuli with the same spectral envelope. Ear and Hearing. 1999;20:33–44. doi: 10.1097/00003446-199902000-00004. [DOI] [PubMed] [Google Scholar]
- Meyler A, Breznitz Z. Visual, auditory and cross-modal processing of linguistic and nonlinguistic temporal patterns among adult dyslexic readers. Dyslexia. 2005;11:93–115. doi: 10.1002/dys.294. [DOI] [PubMed] [Google Scholar]
- Michalewski H, Starr A, Nguyen T, Kong Y, Zeng F. Auditory temporal processes in normal-hearing individuals and in patients with auditory neuropathy. Clinical Neurophysiology. 2005;116:669–680. doi: 10.1016/j.clinph.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Novak G, Ritter W, Vaughan H. Mismatch detection and the latency of temporal judgments. Psychophysiology. 1992;29:398–411. doi: 10.1111/j.1469-8986.1992.tb01713.x. [DOI] [PubMed] [Google Scholar]
- Ostroff J, Martin B, Boothroyd A. Cortical evoked responses to acoustic change within a syllable. Ear and Hearing. 1998;19:290–297. doi: 10.1097/00003446-199808000-00004. [DOI] [PubMed] [Google Scholar]
- Oxenham A. Influence of spatial and temporal coding on auditory gap detection. The Journal of the Acoustical Society of America. 2000;107:2215–2223. doi: 10.1121/1.428502. [DOI] [PubMed] [Google Scholar]
- Papanicolaou A, Baumann S, Rogers R, Saydjari C, Amparo E, Eisenberg H. Source estimation of late components of emitted tone evoked magnetic fields. In: Hoke M, Enre S, Okada Y, Romani G, editors. Biomagnetism: clinical aspects. Elsevier; Amsterdam: 1990. pp. 177–180. [Google Scholar]
- Peters R, Moore BCJ, Baer T. Speech reception thresholds in noise with and without spectral dips for hearing-impaired and normally hearing people. The Journal of the Acoustical Society of America. 1998;103:577–587. doi: 10.1121/1.421128. [DOI] [PubMed] [Google Scholar]
- Phillips D, Smith J. Correlations among within-channel and between-channel auditory gap-detection thresholds in normal listeners. Perception. 2004;33:371–378. doi: 10.1068/p5116. [DOI] [PubMed] [Google Scholar]
- Phillips D, Hall S, Harrington I, Taylor T. “Central” auditory gap detection: a spatial case. The Journal of the Acoustical Society of America. 1998;103:2064–2068. doi: 10.1121/1.421353. [DOI] [PubMed] [Google Scholar]
- Phillips D, Taylor T, Hall S, Carr M, Mossop J. Detection of silent intervals between noises activating different perceptual channels: some properties of central auditory gap detection. The Journal of the Acoustical Society of America. 1997;101:3694–3705. doi: 10.1121/1.419376. [DOI] [PubMed] [Google Scholar]
- Ponton C, Eggermont J, Khosla D, Kwong B, Don M. Maturation of human central auditory system activity: separating auditory evoked potentials by dipole source modeling. Clinical Neurophysiology. 2002;113:407–420. doi: 10.1016/s1388-2457(01)00733-7. [DOI] [PubMed] [Google Scholar]
- Pratt H, Bleich N, Mittelman N. The composite N1 component to gaps in noise. Clinical Neurophysiology. 2005;116:2648–2663. doi: 10.1016/j.clinph.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Preisendorfer RW, Mobley CD. Principal component analysis in meteorology and oceanography. Elsevier; New York: 1988. [Google Scholar]
- Richman MB. Rotation of principal components. Journal of Climatology. 1986;6:293–335. [Google Scholar]
- Roberts R, Lister J. Effects of age and hearing loss on gap detection and the precedence effect: broad-band stimuli. Journal of Speech, Language, and Hearing Research. 2004;47:965–978. doi: 10.1044/1092-4388(2004/071). [DOI] [PubMed] [Google Scholar]
- Scherg M, Vajsar J, Picton T. A source analysis of the late human auditory evoked potentials. Journal of Cognitive Neuroscience. 1989;1:336–355. doi: 10.1162/jocn.1989.1.4.336. [DOI] [PubMed] [Google Scholar]
- Segalowitz S, Barnes K. The reliability of ERP components in the auditory oddball paradigm. Psychophysiology. 1993;30:451–459. doi: 10.1111/j.1469-8986.1993.tb02068.x. [DOI] [PubMed] [Google Scholar]
- Snell K, Ison J, Frisina D. The effects of signal frequency and absolute bandwidth on gap detection in noise. The Journal of the Acoustical Society of America. 1994;96:1458–1464. doi: 10.1121/1.410288. [DOI] [PubMed] [Google Scholar]
- Stuart A, Phillips D. Word recognition in continuous and interrupted broadband noise by young normal-hearing, older normal-hearing, and presbyacusic listeners. Ear and Hearing. 1996;17:478–489. doi: 10.1097/00003446-199612000-00004. [DOI] [PubMed] [Google Scholar]
- Tataryn DJ, Wood JM, Gorsuch RL. Setting the value of K in Promax: a Monte Carlo study. Educational and Psychological Measurement. 1999;59(3):384–391. [Google Scholar]
- Thoma R, Hanlon F, Moses S, Edgar J, Huang M, Weisend M, et al. Lateralization of auditory sensory gating and neuropsychological dysfunction in schizophrenia. The American Journal of Psychiatry. 2003;160:1595–1605. doi: 10.1176/appi.ajp.160.9.1595. [DOI] [PubMed] [Google Scholar]
- Tonnquist-Uhlen I, Ponton C, Eggermont J, Kwong B, Don M. Maturation of human central auditory system activity: the T-complex. Clinical Neurophysiology. 2003;114:685–701. doi: 10.1016/s1388-2457(03)00005-1. [DOI] [PubMed] [Google Scholar]
- Tremblay K, Billings C, Rohila N. Speech evoked cortical potentials: effects of age and stimulus presentation rate. Journal of the American Academy of Audiology. 2004;15:226–237. doi: 10.3766/jaaa.15.3.5. [DOI] [PubMed] [Google Scholar]
- Tremblay K, Friesen L, Martin B, Wright R. Test–retest reliability of cortical evoked potentials using naturally produced speech sounds. Ear and Hearing. 2003;24:225–232. doi: 10.1097/01.AUD.0000069229.84883.03. [DOI] [PubMed] [Google Scholar]
- Tremblay K, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clinical Neurophysiology. 2003;114:1332–1343. doi: 10.1016/s1388-2457(03)00114-7. [DOI] [PubMed] [Google Scholar]
- Van Ingelghem M, van Wieringen A, Wouters J, Vandenbussche E, Onghena P, Ghesquière P. Psychophysical evidence for a general temporal processing deficit in children with dyslexia. Neuroreport. 2001;12:3603–3607. doi: 10.1097/00001756-200111160-00046. [DOI] [PubMed] [Google Scholar]
- Vaughan H, Ritter W. The sources of auditory evoked responses recorded from the human scalp. Electroencephalography and Clinical Neurophysiology. 1970;28:360–367. doi: 10.1016/0013-4694(70)90228-2. [DOI] [PubMed] [Google Scholar]
- Waldo M, Gerhardt G, Baker N, Drebing C, Adler L, Freedman R. Auditory sensory gating and catecholamine metabolism in schizophrenic and normal subjects. Psychiatry Research. 1992;44:21–32. doi: 10.1016/0165-1781(92)90066-c. [DOI] [PubMed] [Google Scholar]
- Walhovd K, Fjell A. One year test re-test reliability of auditory ERPs in young and old adults. International Journal of Psychophysiology. 2002;46:29–40. doi: 10.1016/s0167-8760(02)00039-9. [DOI] [PubMed] [Google Scholar]
- Werner L, Folsom R, Mancl L, Syapin C. Human auditory brainstem response to temporal gaps in noise. Journal of Speech, Language, and Hearing Research. 2001;44:737–750. doi: 10.1044/1092-4388(2001/058). [DOI] [PubMed] [Google Scholar]
- Wolpaw J, Penry J. A temporal component of the auditory evoked response. Electroencephalography and Clinical Neurophysiology. 1975;39:609–620. doi: 10.1016/0013-4694(75)90073-5. [DOI] [PubMed] [Google Scholar]
- Woods D. The component structure of the N1 wave of the human auditory evoked potential. Electroencephalography and Clinical Neurophysiology. 1995;44:102–109. [PubMed] [Google Scholar]
- Zeng F, Oba S, Garde S, Sininger Y, Starr A. Temporal and speech processing deficits in auditory neuropathy. Neuroreport. 1999;10:3429–3435. doi: 10.1097/00001756-199911080-00031. [DOI] [PubMed] [Google Scholar]
- Zouridakis G, Simos P, Papanicolaou A. Multiple bilaterally asymmetric cortical sources for the auditory N1m component. Brain Topography. 1998;10:183–189. doi: 10.1023/a:1022246825461. [DOI] [PubMed] [Google Scholar]