Abstract
It is known that Rab1 regulates the expression and function of beta-adrenoceptors (β-ARs) in many cells. However, the effect of these changes in rat pulmonary microvascular endothelial cells (RPMVECs) is not known. In the present study, we investigated the role of Rab1, a Ras-like GTPase that coordinates protein transport from the endoplasmic reticulum (ER) to the Golgi body and regulates the cell-surface targeting and function of endogenous β-ARs in RPMVECs in the presence of lipopolysaccharide (LPS).
We found that lentivirus-driven expression of wild-type Rab1 (Rab1WT) in RPMVECs strongly enhanced the amount of β-ARs on the cell surface, whereas the dominant-negative mutant Rab1N124I significantly attenuated β-ARs expression on the cell surface. In addition, LPS stimulation significantly reduced β-ARs expression on the cell surface in RPMVECs; however, this effect was reversed by over-expression of wild-type Rab1WT. Fluorescent microscopy analysis demonstrated that expression of Rab1N124I and Rab1 small interfering RNA (siRNA) significantly induced the accumulation of green fluorescent protein (GFP)-tagged β2-AR in the ER. Consistent with their effects on β-ARs export, Rab1WT and Rab1N124I differentially modified the β-AR-mediated activation of extracellular signal-regulated kinase1/2 (ERK1/2). Importantly, over-expression of Rab1WT markedly reduced LPS-induced hyper-permeability of RPMVECs by increasing the expression of β2-AR on the cell surface. These data reveal that β-ARs function in RPMVECs could be modulated by manipulating β-ARs traffic from the ER to the Golgi body. We propose the ER-to-Golgi transport as a regulatory site for control of permeability of RPMVECs.
Keywords: Rab1 GTPase, Beta-adrenergic receptors, Permeability, Lipopolysaccharide, Small Interfering RNA
1. Introduction
Beta-adrenergic receptors (β-ARs) play a critical role in the regulation of the permeability and function of vascular endothelial cells in both normal and pathological conditions (Minnear et al., 1993; McDonald, 2001). All β-ARs subtypes, namely β1-, β2-, and β3-AR, have been described as having a role in the vasculature (Guimaraes and Moura, 2001). These receptors belong to the seven transmembrane-spanning receptor superfamily and are coupled to heterotrimeric G proteins (Dixon et al., 1986; Frielle et al., 1987; Emorine et al., 1989). A number of studies have demonstrated that the increased endothelial permeability that occurs after the administration of inflammatory mediators could be mediated by activation of β2-AR (Allen and Coleman, 1995; Kwan et al., 2001; Mehta et al., 2004). However, the activation of β2-AR cannot completely reverse the endothelial hyper-permeability induced by inflammatory mediators (van Nieuw Amerongena and van Hinsbergh, 2003; Bogatcheva et al., 2009; Mehta and Malik, 2006). Few specific therapies are available to attenuate the endothelial hyper-permeability, and current therapies are often unsuccessful. Nevertheless, new insights into the mechanisms underlying the cell-surface expression of β-AR offer potential novel targets and strategies for pharmacological intervention.
The intracellular trafficking of β-ARs is a critical event in regulating its function. After it is synthesized, folded, and assembled in the endoplasmic reticulum (ER), the β-ARs is transported to the Golgi apparatus, where it is post-translationally modified (Pierce et al., 2002; Drake et al., 2006). It is then transported to the plasma membrane. Once β-ARs arrive at the plasma membrane, they may undergo internalization to the endosome upon stimulation by their ligands (Pierce et al., 2002). The internalized receptors may then be transported to the lysosome for degradation or recycled back to the plasma membrane (Reiter and Lefkowitz, 2006). Thus, the amount of β-ARs at the plasma membrane is determined by the overall balance of β-ARs export to the cell surface, internalization, recycling, and degradation. Because most studies on β-ARs trafficking have focused on the events involved in its internalization, recycling, and degradation (Pierce et al., 2002; Drake et al., 2006; Reiter and Lefkowitz, 2006), the export of β-ARs from the ER through the Golgi to the cell surface and regulation of receptor function by these processes remain poorly understood.
Intracellular protein trafficking between organelles is coordinated by Rab proteins, which are members of the Ras superfamily of monomeric GTPases (Zerial and McBride, 2001). Recently, more than 60 Rab GTPases have been identified in the human genome. Nearly all of these enzymes are involved in signaling vesicle formation, motility, docking, or fusion, events critical to endocytosis and exocytosis in endothelial cells (Zerial and McBride, 2001; Segev, 2001). Endothelial cells express Rab 1–9, 11, 13, 14, 15, 18, 22, and 30 (DE Leeuw et al., 1998; Karniguian et al., 1993; Schäfer et al., 2000; Wilson and Wilson, 1992). Rab1 is one of the most extensively studied Rab GTPases, involved in the regulation of vesicular protein transport between intracellular organelles. Rab1 is localized in the ER and Golgi, and regulates antegrade protein transport specifically from the ER to the Golgi and among the Golgi compartments (Martinez and Goud, 1998; Short et al., 2005; Duvernay et al., 2005; Wittinghofer, 2006; Saraste and Goud, 2007). It is well known that Rab1 regulates the transport of G protein–coupled receptors (GPCRs) from the ER to the cell surface in HEK293T cells and cardiocytes (Duvernay et al., 2004; Wu et al., 2003; Wu et al., 2001; Filipeanu et al., 2006). These findings suggest that an alteration in Rab1 function might play an essential role in regulation of the cell-surface expression of β-ARs and subsequently change endothelial permeability.
In the present study, we determined whether modified ER-to-Golgi transport could alter the cell-surface expression and function of endogenous β-ARs in the RPMVECs. The role of Rab1-mediated reduction in endothelial permeability through β-ARs was also investigated. We demonstrated that lentivirus-mediated gene transfer of Rab1WT and its dominant-negative mutant elicited opposite effects on the ER-to-Golgi transport and cell-surface expression of β-ARs as well as β-AR-mediated signaling. Notably, over-expression of Rab1WT significantly reduced LPS-induced hyper-permeability of RPMVECs. These data strongly indicate that β-ARs function in RPMVECs can be modulated through manipulating β-ARs traffic from the ER to the Golgi body.
2. Materials and methods
2.1 Materials
Rats were obtained from Chongqing City Laboratory Animal Center, Chongqing, China. Dulbecco's modified Eagle's medium (DMEM), trypsin and fetal bovine serum (FBS) were purchased from GIBCO (Invitrogen, Carlsbad, CA). [3H]CGP12177 (specific activity, 49Ci/mmol) were from GE Healthcare (Little Chalfont, Buckinghamshire, UK). isoproterenol (ISO), atenolol, ICI 118,551, LPS (from E. coli 0111:B4), and anti-FLAG M2 monoclonal antibody were obtained from Sigma (St. Louis, MO). Antibodies against Rab1, antibodies against phospho-ERK1/2 and antibodies against ERK1/2 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Wild type Rab1-pcDNA3, Rab1N124I (dominant negative mutant)-pcDNA3, pDsRed2-ER, an ER marker, and human β2-AR tagged with green fluorescent protein (GFP), were kind gifts of Dr. Wu (Department of Pharmacology and Experimental Therapeutics, Louisiana State University Health Sciences Center, New Orleans, Louisiana). Biotin conjugated BSA (biotin–BSA) was purchased from Pierce Biotechnology, Inc. (Rockford, IL). All other materials were described elsewhere (Wu et al., 2003; Wang et al., 2008).
2.2 Cell culture and LPS challenge
Isolation and culture of RPMVECs were performed according to a method developed by Chen, S.F. et al. and modified by Zhang, H. et al (Chen and Li, 1995; Zhang and Sun, 2005). Briefly, the fresh lungs, isolated from the sacrificed rats, were cut into small pieces and cultured with DMEM containing 20% heat-inactivated low endotoxin FBS, 90 μg/ml heparin, 4 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in a humidified atmosphere containing 95% air-5% CO2. After 60 h, the tissue was removed and thereafter, the culture medium was replaced every 3 days, and after reaching confluence, the cells were passaged by harvesting with 0.25% trypsin containing 0.02% EDTA and cultured in 25-cm2 flask in DMEM with 10% FBS. Cells were identified according to morphological and functional criteria, and used for experiments between passages 2 and 4. RPMVECs, either experiment group or control group, were grown in plates or flasks to 95% confluence and then were treated with LPS at concentration 10μg/ml for different periods of time.
2.3 Lentivirus vectors for wild type rab1 and rab1N124I and infection of RPMVECs
Rab1 and Rab1N124I lentivirus gene transfer vector encoding the FLAG epitope and green fluorescent protein (GFP) sequence were constructed by Genechem Co., Ltd, Shanghai, China, using a transient transfection procedure as described previously (Tiscornia et al., 2006; Sena-Esteves et al., 2004; Reiser, 2000). The recombinant lentivirus of Rab1 and Rab1N124I and the control lentivirus (GFP-lentivirus) were prepared and titered to 109TU/mL (transfection unit). The cells were transiently transfected with control parent lentivirus or lentivirus expressing Rab1WT or its dominant-negative mutant Rab1N124I (multiplicity of infection of 5 to 50). After infection for 4 h, the medium was changed, and the RPMVECs were further cultured in DMEM with 10% FBS. The efficiency of lentivirus infection of RPMVECs was evaluated by fluorescent microscopy.
2.4 Measurement of Cell Surface Receptors
Cell-surface expression of β-ARs in RPMVECs was measured by intact cell ligand binding as described previously (Wang et al., 2008; McLean et al., 1999; Calls et al., 2000). RPMVECs were cultured on 12-well plates at a density of 5×105 cells/well and infected for 48 h. The RPMVECs were then incubated with the ligand [3H] CGP12177 at a concentration of 20 nM for 2 h at room temperature. To measure expression of individual AR subtypes, RPMVECs were preincubated for 30 min with the AR subtype-selective antagonists: ICI 118,551 (β2-AR), atenolol (β1-AR) (10 μM). Nonspecific binding was determined in the presence of alprenolol (β-ARs) (20 μM). The cells were washed twice with ice-cold phosphate-buffered saline and digested with 500 μl of 1 M NaOH. All ligand binding assays were performed in triplicate. The radioactivity was counted by liquid scintillation spectrometry.
2.5 Immunoblotting
Western blotting was carried out as described previously (Duvernay et al., 2004). Forty μg of protein samples was separated by 12% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The signal was detected by enhanced chemiluminescence detectionsystem (ECL Plus, Amersham, England). Densitometric analysis was performed using Quantity One software (Bio-Rad). The phosporylation of ERK is normalized to their total protein expression.
2.6 Measurement of ERK1/2 Activation
Activation of ERK1/2 was measured as described previously (Wu et al., 2003; Filipeanu et al.,2004). RPMVECs were cultured on six-well plates at a density of 1 ×106 cells/well and infected for 48 h. Cells were stimulated with ISO (10μM) for 8 min with or without pretreatment with the β2-AR antagonists ICI 115,881 or β1-AR antagonists atenolol (100 nM) for 30 min. The reaction was terminated by the addition of 600μl of 1× SDS gel loading buffer. After solubilizing the cells, 40 μg of total cell lysates was separated by 12% SDS-polyacrylamide gel electrophoresis. ERK1/2 activation was determined by immunoblotting to measure their phosphorylation with phospho-specific antibodies. The membranes were stripped and reprobed with anti-ERK1/2 antibodies to determine the total amount of kinases and to confirm equal loading of proteins. The signal was detected by enhanced chemiluminescence detectionsystem (ECL Plus, Amersham, Buckinghamshire, England). Densitometric analysis was performed using Quantity One software (Bio-Rad). The phosporylation of ERK is normalized to their total protein expression.
2.7 Fluorescent Microscopy
RPMVECs were grown on coverslips in six-well plates and infected with control, Rab1 or Rab1N124I lentivirus as described above. After 10 h of infection, the medium was removed and the cells were transiently transfected using LipofectAMINE 2000 reagent (Invitrogen) as described previously (Wu et al., 2003; Filipeanu et al., 2004). One microgram of GFP-tagged AR with or without 1μg of pDsRed2-ER construct were diluted into 125 μl of serum-free Opti-MEM in a tube. In another tube, 3 μl of LipofectAMINE was diluted into 125 μl of serum-free Opti-MEM. Five minutes later both solutions were mixed and incubated for another 20 min. The transfection mixture was added to culture dishes containing 0.8 ml of fresh Dulbecco's modified Eagle's medium and 10% fetal bovine serum without antibiotics. After transfection 48 h, the cells were fixed with a mixture of 4% paraformaldehyde and 4% sucrose in phosphate-buffered saline for 15 min. The coverslips were mounted, and fluorescence was detected with a Leica DMRA2 epifluorescence microscope. Images were deconvoluted using Slide-Book software and the nearest-neighbors deconvolution algorithm (Intelligent Imaging Innovations, Denver, CO) as described previously (Wu et al., 2003).
2.8 Double-stranded Small Interfering
siRNA of human Rab1 and a control nonsilencing siRNA were purchased from QIAGEN Inc. (Valencia, CA). RPMVECs were plated on 6-well dishes at a density of 2×105 cells/well for 14 h before transfection. Control and Rab1 siRNA were delivered into RPMVECs using LipofectAMINE 2000 reagent as described previously (Wu et al., 2003). In brief, 8 μl of LipofectAMINE 2000 and 6 μl of 20 μM siRNA plus 1 μg of individual receptor plasmids were added separately to 100 μl of Opti-MEM. After incubation for 5 min, both solutions were mixed for 20 min. The transfection mixture was added to culture dishes containing 0.8 ml of fresh Dulbecco's modified Eagle's medium and 10% fetal bovine serum without antibiotics. The cells were incubated with the transfection mixture for 10 h and then medium was changed to standard culture medium. After 48 h, the cells were processed for fluorescence microscopy as described above.
2.9 Measurement of the endothelial cell monolayer permeability
Permeability assays to assess barrier function of mono-layers were performed using a modified protocol described by Chang et al (Chang et al., 2000; Murphy et al., 2001). Briefly, RPMVECs mono-layers were seeded (105 cells per insert) on 12-well cell cultured dishes (Costar, Cambridge, MA) lined with polycarbonate filters (pore size 0.4μm). The filters were treated for 20 min with 0.1% acetic acid, then for 1 h with 0.1% gelatin, and air-dried before seeding endothelial cells. The medium in the wells was changed every day. Typically, monolayer cells were monitored for 5–7 days post-seeding. The DMEM serum was removed for a period of 24 h prior to studies of monolayer permeability were done. The permeability of RPMVEC mono-layers was measured by diffusion of biotinylated bovine serum albumin (biotin-BSA). The upper chamber was filled with 0.5 mL of appropriate DMEM media. Sufficient media was added to each lower chamber to cover the membrane. When confluent, cultures were infected with control and Rab1WT lentivirus for 2 days. Select cultures were then subjected to LPS 64.6 (ng/ml) [This concentration was ½ maximal effective dosing for LPS (10μg/ml) determained in control group.] and LPS (10μg/ml), respectively for 2 h. ISO (10 μM), ISO plus ICI 118,551 (10 μM) and ISO plus atenolol (10 μM) were added to upper chamber wells simultaneously with 500 μg/mL biotin-BSA. 100 μL aliquots of lower chamber media were aspirated at 0.5 h and biotin-BSA concentrations were determined by enzyme-linked immunosorbent assay.
2.10 Statistical Analysis
Differences were evaluated using one-way analysis of variance, and p < 0.05 was considered as statistically significant. Data are expressed as means ± S.E.
3. Results
3.1 RPMVECs Characteristics
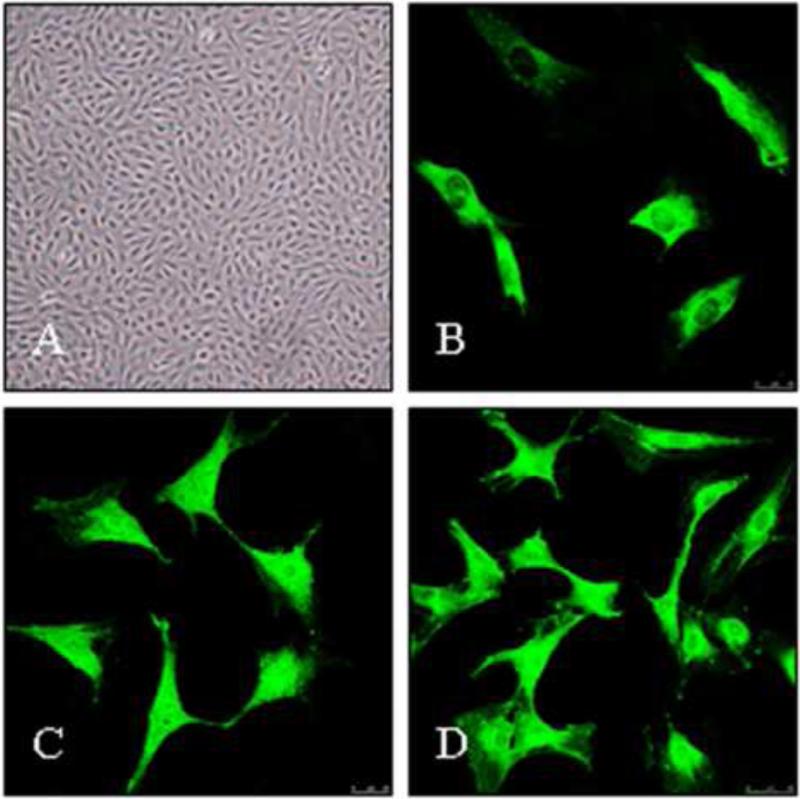
The cells grew initially as capillary-like structures and assumed the typical cobblestone morphology of endothelial cells at confluence (Fig. 1A). The cells were characterized as endothelial cells by factor VIII-related antigen and CD34 antigen expression. Using immunofluorescence, positive expression of factor VIII-related antigen and CD34 antigen in RPMVECs was demonstrated by green particles in the cytoplasm (Fig. 1B and C). The cells demonstrated the intense lectin binding criteria for microvascular endothelial cells. The cells bound FITC-labeled BSI and displayed positive staining by lectin BSI (Fig. 1D).
Fig. 1.
Rat pulmonary microvascular endothelial cells (RPMVECs) morphology. Cells were stained with anti-human factor VIII-related antigen and anti-CD34 antigen and binding of Lectin BSI was determined. (A) Normal primary RPMVEC morphology was observed under phase-contrast microscopy (magnification ×100); (B) and (C), Positive expression of factor VIII-related antigen and CD34 antigen in RPMVECs was demonstrated as green particles in the cytoplasm by immunofluorescence (magnification ×400); (D) RPMVECs bound to FITC-labeled BSI under fluorescence microscopy (magnification ×400).
3.2 Effect of LPS on the expression of Rab1 and β-ARs and the β-ARs subtypes β1-AR and β2-AR in RPMVECs
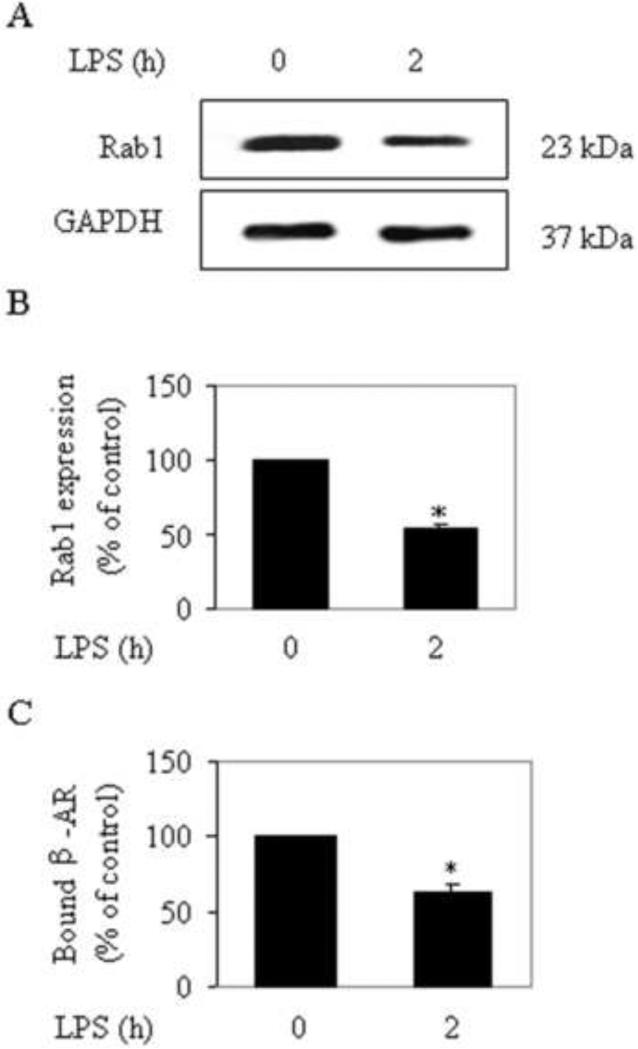
RPMVECs were cultured and subjected to LPS for 2 h. The expression of Rab1 was determined by Western blotting using the Rab1 antibodies as described in the section 2.5 (Materials and methods).The cell-surface expression of β-ARs was determined by intact cell ligand binding. The expression of Rab1 and β-ARs (especially of β2-AR) was dramatically decreased at 2 h in RPMVECs after LPS challenge (Fig. 2A and B). These results indicate that LPS induces a reduction of the expression of Rab1 and β-ARs in RPMVECs.
Fig. 2.
Effect of LPS on the expression of Rab1 and β-ARs in RPMVECs. (A) RPMVECs were subjected to LPS for 2 h, and the expression of Rab1 was determined by Western blotting using Rab1 antibodies. Representative blots show Rab1 (upper panel) and GAPDH expression (lower panel). (B) Quantitative data of Rab1 after LPS challenge. (C) RPMVECs were subjected to LPS for 2 h, and the cell-surface expression of β-ARs was determined by intact cell ligand binding as described in the Materials and methods section. The mean values of specific [3H]CGP12177 binding were 2107.00 ± 202.36, and 1324.00±130.01 cpm (n=3 each in duplicate) from the RPMVECs in control group and subjected to LPS, respectively. The data are expressed as the percentage of the mean value obtained from the control (time 0) and presented as the means ± SE of three separate experiments. *P < 0.05 versus control (time 0).
3.3 Effect of Rab1 on cell-surface expression of β-AR in RPMVECs
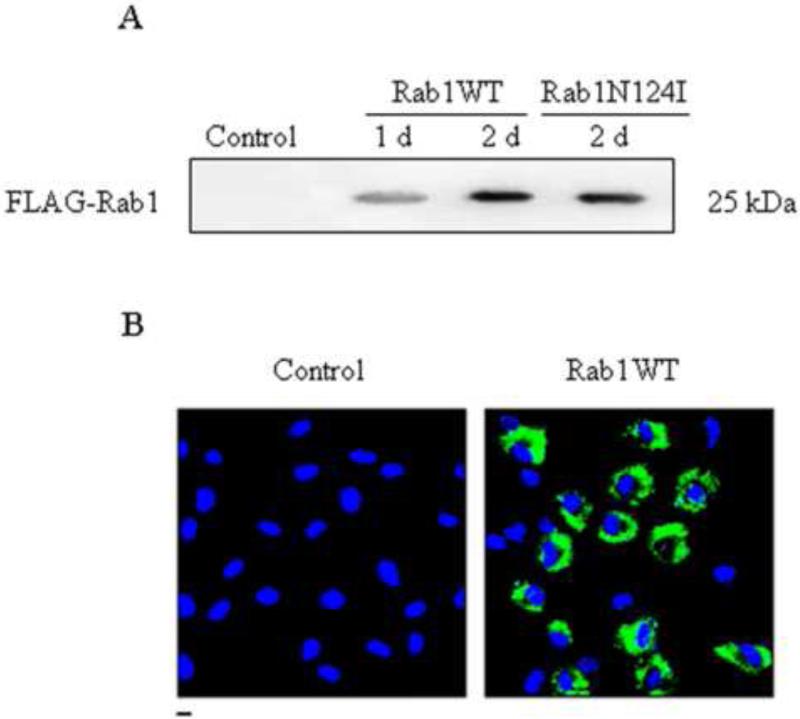
To determine whether Rab1 regulates the export of endogenous β-ARs from the ER to the cell surface, we first determined the effect of lentivirus-mediated expression of Rab1 on β-ARs expression at the cell surface. Recombinant lentiviruses encoding FLAG-tagged Rab1WT or its dominant-negative mutant Rab1N124I were generated to infect RPMVECs. Rab1 expression was determined by Western blotting using a FLAG high affinity monoclonal antibody (Fig. 3A). The control lentiviral vector expression cassette allowed for the permanent expression of GFP in transduced cells. GFP expression and the percentage of GFP expressing cells were determined by Fluorescent microscopy. Figure 3B indicates that greater than 60% of the RPMVECs were infected after 2 d transfection. These data indicate that Rab1 was successfully transferred into primary cultures of RPMVECs with lentivirus.
Fig. 3.
Lentivirus-mediated expression of Rab1 in RPMVECs. (A) Western blot analysis of expression of wild-type Rab1 (WT) and dominant-negative mutant Rab1N124I driven by lentivirus. RPMVECs were infected with empty lentivirus vector (Control) or recombinant FLAG-Rab1WT lentivirus for 1 and 2 days or with Rab1N124I for 2 days at an m.o.i. of 20. 40 μg of whole RPMVECs was separated by 12% SDS-PAGE, and FLAG-Rab1 expression was detected by immunoblotting with anti-FLAG antibody. The immunoblot is representative of results obtained in two different experiments. (B) localization of Rab1WT and estimation of infection efficiency. RPMVECs were cultured on coverslips and infected with control or GFP-Rab1WT lentivirus. Localization of GFP-Rab1WT in infected RPMVECs was revealed by fluorescence microscopy as described in the Materials and methods section. Similar results were obtained in three separate experiments.
3.4 Effect of lentivirus-mediated gene transfer of Rab1 on the cell-surface expression of β-AR using [3H]CGP12177 ligand binding in intact RPMVECs
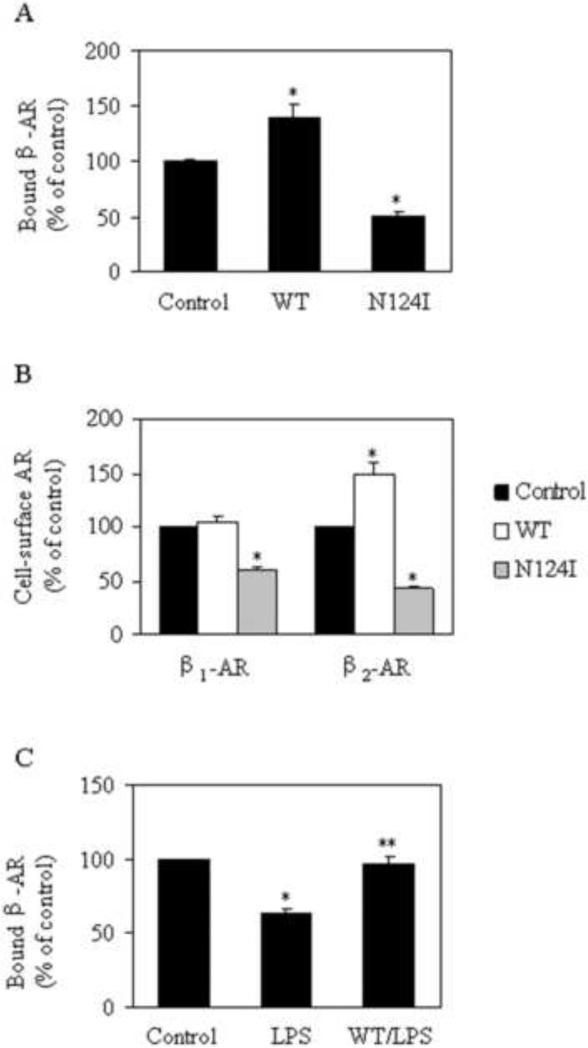
The cell-surface expression of total β-ARs and β2-AR was significantly augmented by 38.3% and 49.0% (Fig. 4A and B), respectively, in RPMVECs infected with Rab1WT lentivirus as compared with that from RPMVECs infected with control lentivirus. Cell-surface expression of β1-AR was not significantly altered by Rab1. In contrast, β2-AR expression at the cell surface was markedly attenuated by 49.2% in RPMVECs infected with the dominant-negative mutant Rab1N124I lentivirus (Fig. 4A). It is noteworthy that the cell-surface expression of β-ARs after an LPS challenge of 2 h in RPMVECs infected with Rab1WT lentivirus was significantly augmented compared with cells infected with control lentivirus (Fig. 4C). These data indicate that manipulation of Rab1 function modifies the cell-surface expression of endogenous β-ARs in RPMVECs.
Fig. 4.
Effect of Rab1 on cell-surface expression of β-ARs and the β-ARs subtypes β1-AR and β2-AR in RPMVECs. (A) and (B) Quantitation of cell-surface number of β-ARs, β1-AR and β2-AR in RPMVECs. RPMVECs were cultured and infected with control, Rab1WT, or Rab1N124I lentivirus for 2 days. The cell-surface expression of β-ARs, β1-AR and β2-AR was determined by binding to ligands [3H]CGP12177 as described in Materials and methods section. The mean values of specific [3H]CGP12177 binding of β-ARs were 2048.00±207.64, 2833.00±203.07, and 1040.00± 91.59 cpm (n=3 each in duplicate) from the cells infected with control, Rab1WT, or Rab1N124I lentivirus, respectively. The mean values of specific [3H]CGP12177 binding of β1-AR and β2-AR were 517.78±29.65, 591.22±18.29,and 364.11±20.68; 1627.90±112.27, 2262.10±169.33 and 694.33± 29.79 cpm (n=3 each in duplicate) from the cells infected with control, Rab1WT, or Rab1N124I lentivirus, respectively. (C) The modulation of the cell-surface expression of β-ARs by over-expressing Rab1WT in RPMVECs in the presence of LPS challenge. RPMVECs were cultured and infected with control and Rab1WT lentivirus for 2 days. The RPMVECs were then subjected to LPS for 2 h. The cell-surface expression of β-ARs was determined by intact cell ligand binding as described in Materials and methods. The mean values of specific [3H]CGP12177 binding of β-ARs were 2093.00±186.73, 1313.00±102.35, and 1997.60± 96.09 cpm (n=3 each in duplicate) from the cells infected with control lentivirus, the cells subjected to LPS and the cells infected with Rab1WT lentivirus and subjected to LPS. Data shown are the percentage of the mean value obtained from the RPMVECs infected with control lentivirus and are presented as the means ± S.E (n = 3 each in triplicate). *p < 0.05 versus RPMVECs infected with control lentivirus; **p < 0.05 versus RPMVECs challenged with LPS.
3.5 Effect of Rab1 on the subcellular localization of the β2-AR
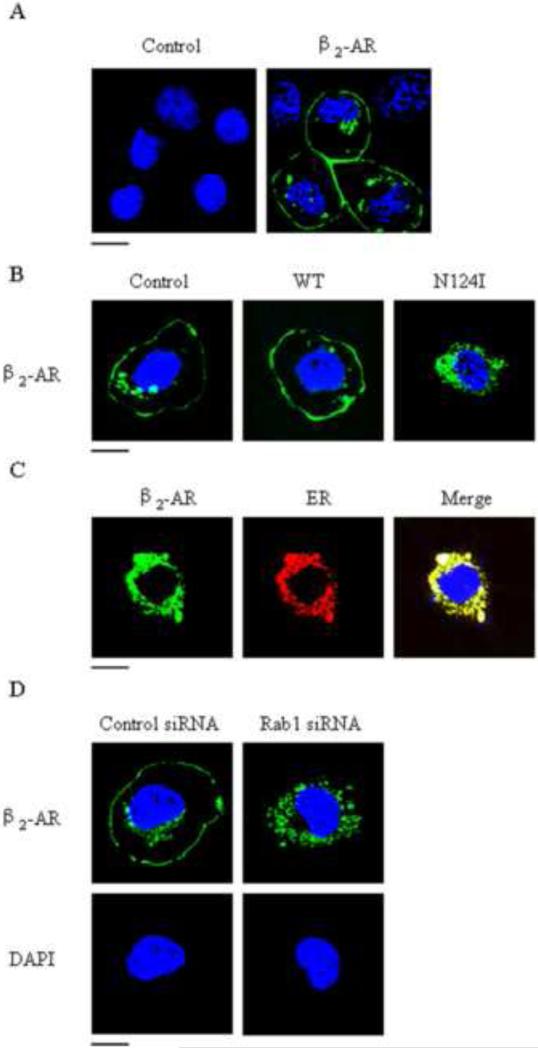
To further characterize the functional role of Rab1 in regulating β2-AR expression at the cell surface, we sought to determine the effect of transient expression of the dominant-negative mutant Rab1N124I on the subcellular distribution of β2-AR. In this respect, GFP-tagged β2-AR was transiently transfected into RPMVECs after infection with control or Rab1N124I lentivirus. Fluorescent microscopic analyses revealed that about 60% of the cells were infected (Fig. 5A). As anticipated, β2-AR-GFP was mainly localized at the cell surface in cells infected with control lentivirus. In contrast, β2-AR-GFP accumulated in the perinuclear regions and colocalized with the ER marker-pDsRed2 and could not be transported to the cell surface in cells infected with Rab1N124I (Fig. 5B and C).
Fig. 5.
Effect of Rab1 on the subcellular localization of β2-AR in RPMVECs. (A) localization of β2-AR and estimation of infection efficiency. RPMVECs were cultured on coverslips and transfected with control and GFP-tagged β2-AR using LipofectAMINE 2000. Localization of GFP-tagged β2-AR in transfected RPMVECs was revealed by fluorescence microscopy as described in Materials and methods. (B) The effect of lentivirus expression of Rab1 on the subcellular distribution of β2-AR. RPMVECs were cultured and infected as above. The subcellular distribution of the receptor was revealed by fluorescence microscopy as described in Materials and methods. (C) Colocalization of receptors with the ER marker. RPMVECs were grown on coverslips and infected with Rab1N124I lentivirus. The cells were then transfected with GFP-tagged β2-AR together with the ER marker pDsRed2-ER. (D) The effect of transient expression of Rab1 siRNA on the subcellular distribution of β2-AR. RPMVECs were transiently transfected with GFP-tagged β2-AR together with control siRNA (left) or Rab1 siRNA (right). The data are representative images of three separate experiments. Blue, DNA staining with DAPI (nuclear); green, GFP-tagged receptor; red, ER marker pDsRed2-ER; yellow, colocalization of GFP-tagged receptors with the ER marker.
We also determined the effect of siRNA-mediated depletion of Rab1 on the subcellular localization of β2-AR. Wu et al (Wu et al., 2003) previously demonstrated that transient transfection of Rab1 siRNA in HEK293T cells selectively reduced the expression of endogenous Rab1. Consistent with the effect of Rab1N124I, transfection of Rab1 siRNA induced an accumulation of β2-AR in the perinuclear regions when these cells were compared with cells transfected with control siRNA (Fig. 5D). These data strongly indicate that a normal Rab1 level is required for the transport of β2-AR and to the cell surface.
3.6 Modulation of β-AR Signaling by Rab1 in RPMVECs
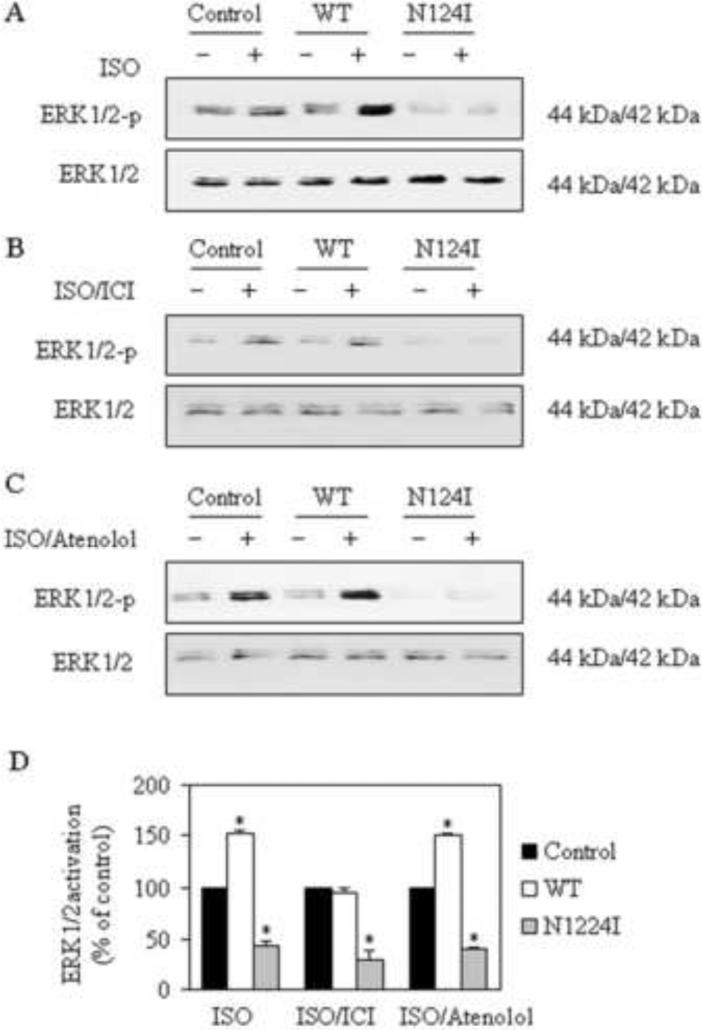
To determine whether Rab1 can regulate β-ARs signaling through modifying the ER-to-Golgi transport of these receptors, we determined the effect of Rab1 on the β-AR-mediated activation of ERK1/2. RPMVECs infected with control, Rab1, or Rab1N124I lentiviruses were stimulated with the nonselective β-ARs agonist ISO in the absence or presence of atenolol (β1-AR antagonists) or ICI 118,551 (β2-AR antagonists). Lentivirus-mediated Rab1WT expression significantly enhanced ERK1/2 activation in response to stimulation with ISO in RPMVECs compared with cells infected with control lentiviruses (Fig. 6A). In contrast, expression of dominant-negative Rab1N124I markedly reduced ISO-induced ERK1/2 activation compared with cells infected with control lentiviruses (Fig. 6A). ISO-mediated ERK1/2 activation in the absence or presence of the antagonists was similarly inhibited in cells in fected with Rab1N124I compared with cells infected with control lentiviruses. In contrast, ISO-mediated ERK1/2 activation in the absence or presence of the antagonists was selectively augmented in cells in fected with Rab1WT compared with cells infected with control lentiviruses (Fig. 6B and C). It was that lentivirus-mediated Rab1WT expression mainly enhanced β2-AR-mediated ERK1/2 activation. These data indicate that Rab1 modulates not only β2-AR trafficking but also its signal transduction indirectly.
Fig. 6.
Effect of Rab1 on β-ARs mediated ERK1/2 activation in RPMVECs by Western blotting analysis using phospho-specific ERK1/2 (ERK1/2-P) antibodies. RPMVECs cultured in six-well dishes were infected with control, Rab1WT, or Rab1N124I lentivirus at an m.o.i. of 20 for 2 days. Representative blots of ERK1/2 (top) and total ERK1/2 expression (bottom) are shown. (A) The RPMVECs were stimulated at 37°C with ISO (10 μM). (B) The cells were treated with ISO plus ICI 118,551 (10 μM). (C) The cells were treated with ISO plus atenolol (10 μM). (D) Quantitative data of ERK1/2 phosphorylation normalized to the total ERK1/2 expression. The data shown are the percentage of the mean value obtained from the RPMVECs infected with control lentivirus and presented as the means ± S.E. at three separate experiments. The groups labed ‘-’ were did not treated with ISO, ISO /ICI and ISO / Atenolol (The value of ERK1/2 were not shown). * p < 0.05 versus RPMVECs infected with control lentivirus.
3.7 Effect of over-expressing Rab1WT on the monolayer permeability of RPMVECs
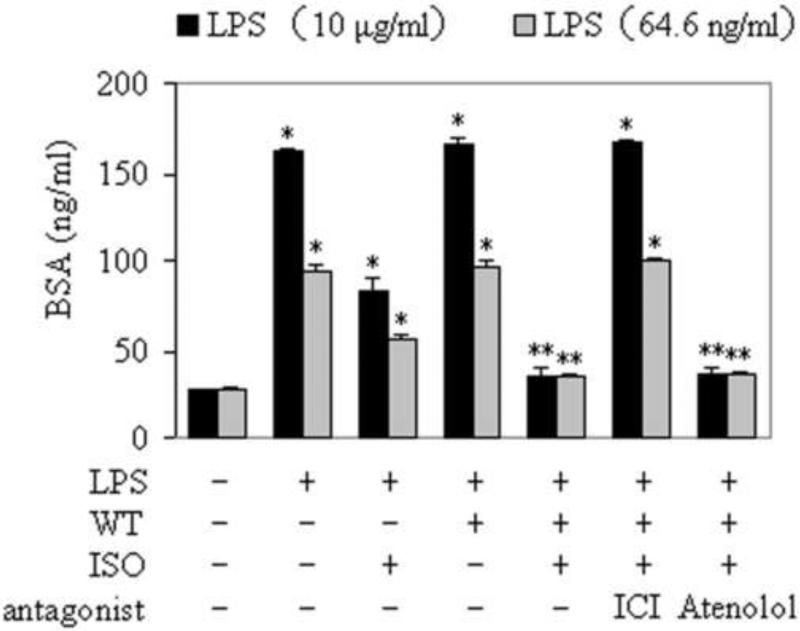
To determine the effect of Rab1 on the monolayer permeability of RPMVECs, cells infected with control or Rab1 lentivirus were treated with LPS for 2 h and then stimulated with the nonselective β-ARs agonist ISO in the absence or presence of atenolol or ICI 118,551. Although the expression of Rab1WT did not directly influence the permeability of RPMVECs, the monolayer permeability of RPMVECs infected with Rab1WT lentivirus and stimulated with ISO was significantly abated compared with the monolayer permeability of RPMVECs infected with control lentivirus (Fig. 7). Importantly, consistent with Rab1 function in modulation of β-ARs signaling, lentivirus-mediated Rab1WT expression attenuated permeability mainly through activation of β2-AR. These data indicate that manipulation of Rab1 function modifies the cell-surface expression of endogenous β-ARs in RPMVECs and subsequently regulates of the monolayer permeability of RPMVECs.
Fig. 7.
Effect of over-expressing Rab1WT on the monolayer permeability of RPMVECs. RPMVECs were grown to confluence on 0.4 μm polyester membranes in the upper chambers of coculture wells, and infected with control and Rab1WT lentivirus for 2 days. Select cultures were then subjected to LPS 10 (ng/ml), and LPS (10μg/ml), respectively for 2 h. ISO (10 μM), ISO plus ICI 118,551 (10 μM) and ISO plus atenolol (10 μM) were added to upper chamber wells simultaneously with 500 μg/mL biotin-BSA. Aliquots of lower chamber media were aspirated at 0.5 h after treatment. Biotin-BSA concentrations were determined by enzyme-linked immunosorbent assay. * p < 0.05 versus RPMVECs infected with control lentivirus. ** p < 0.05 versus RPMVECs infected with control lentivirus and treated with LPS and ISO.
4. Discussion
In this report, we determined that Rab1 controls the export traffic and function of endogenous β-ARs in RPMVECs. Endogenous Rab1 function was manipulated through lentivirus-mediated gene transfer of Rab1WT and its dominant-negative mutant Rab1N124I. We demonstrated that Rab1WT and its dominant-negative mutant have opposing effects on the ER-to-Golgi transport and cell-surface expression of β-ARs as well as β-AR-mediated signaling in RPMVECs.
Pulmonary endothelium serves as a semi-selective barrier between the plasma and interstitial cells against circulatory cells, micro-molecules, and bioactive agents. It is an an important physiological process to maintain this semi-selective barrier for vessel wall homeostasis and lung function. Injury to the endothelium results in barrier dysfunction with exudation of proteins and fluid within the interstitial tissue and alveolar space that contributes to edema in lung injury (Zhang and Sun, 2005).
β-ARs agonists have been widely reported to reduce vascular permeability both in vivo (Mizus et al., 1985; Adamski et al., 1987; Whelan and Johnson, 1992) and in vitro (Minnear et al., 1989; Gudgeon and Martin, 1989; Langeler and van Hinsbergh, 1991). While β-ARs agonists have been shown to reduce the endothelial hyper-permeability induced by a permeability-enhancing agent, these β-ARs agonists could not abolish this hyper-permeability during the early phase of inflammation (Bolton et al., 1997). A number of studies have demonstrated that there is a baseline in the system of permeability that may be associated with the number of β-ARs on the cell surface, and only at this baseline can the permeability be efficiently regulated by β-ARs agonists (Minnear et al., 1989; Langeler and van Hinsbergh, 1991; Bolton et al., 1997; Allen et al., 1995).
LPS, also known as endotoxin, is a structural component of the outer membrane of Gram-negative bacteria. It triggers a systemic inflammatory response during sepsis that includes vasomotor dysfunction, endothelial cell apoptosis, and coagulation activation with fibrin deposition (Cohen J, 2002). Administration of LPS in various models induced profound vascular leakage in vivo (Gao et al., 2001; Hirano et al., 2004; Penn and Chisolm, 1991) and increased permeability of cultured endothelial cells (Berman et al., 1993; Goldblum et al., 1993; Meyrick et al., 1986). Studies have shown that in cultured endothelial cells, an LPS-induced increase in endothelial permeability occurred through several pathways, such as endothelial contraction caused by a RhoA-dependent increase in myosin light chain kinase (MLC) phosphorylation (Essler et al., 2000), reorganization of actin filaments (Goldblum et al., 1993), protein tyrosine phosphorylation (Bannerman and Goldblum, 1997), induction of HSP27 phosphorylation in association with endothelial barrier dysfunction in rats (Hirano et al., 2004), and so on. However, it has remained unclear whether the LPS-induced increase in endothelial permeability is associated with a reduction of expression of Rab1 and the total cell-surface number of β2-AR.
In this report, we first demonstrated that addition of LPS reduced the expression of Rab1 and reduced the total cell-surface number of β2-AR in RPMVECs. In addition, lentivirus-mediated expression of wild-type Rab1 influenced export trafficking of endogenous β-ARs and significantly increased the total cell-surface number of β-ARs as measured by intact cell ligand binding. These data suggest that the level of endogenous Rab1 may be a rate-limiting factor for the ER-to-Golgi transport of β2-AR. It would be meaningful to understand the regulatory machinery of β2-AR upon the permeability of RPMVECs. However, the mechanism for the rapid decrease in detectable β-ARs from the cell surface and whether LPS treatment similar to agonist stimulation remain largely unclear. In my experiments, the expression of Rab1 was decreased in RPMVECs after LPS challenge. Rab1 is localized in the ER and Golgi, and it is known that Rab1 regulates the transport of G protein–coupled receptors (GPCRs) from the ER to the cell surface. The decreased expression of Rab1 may contribute to the decrease of β2-ARs.That may be a possible mechanism.
The results of microscopic analysis indicated that the expression of Rab1N124I induced an accumulation of β2-AR in the ER. A similar result was observed when endogenous Rab1 was depleted using siRNA. These data suggest that β2-AR trafficking from the ER to the cell surface is mediated through the Rab1-dependent pathway and that Rab1 is involved in the transport from the ER to the Golgi.
To determine whether the modification of β-AR transport from the ER to the Golgi by Rab1 influenced β-ARs function in RPMVECs, we determined the effect of Rab1 on β-AR-mediated MAPK activation. Lentivirus expression of Rab1WT significantly augmented ERK1/2 activation while Rab1N124I attenuated β2-AR-stimulated ERK1/2 activation in RPMVECs, in agreement with the effects of Rab1 on β2-AR expression at the cell surface. Therefore, we conclude that the modulation of β2-AR-mediated signaling was due to the influence of Rab1 on β2-AR transport from the ER to the Golgi apparatus and subsequently to the cell surface. However, we cannot exclude the possibility that altering Rab1 function may also modulate the intracellular trafficking of other molecules involved in β2-AR signaling systems, and these molecules may in turn contribute to the modulation of β2-AR function.
We also determined the effect of Lentivirus-mediated expression of Rab1WT on the permeability of RPMVECs. In vitro models have been developed that allow direct measurement of endothelial permeability by culturing endothelial mono-layers on filters (Cooper et al., 1987). The endothelium-coated filter is then used to separate two chambers and the passage of macromolecules between these chambers, across the filter, is measured. Albumin plays an important role in maintaining the trans-endothelial oncotic pressure, and so, in this report, we determined the permeability of the endothelium to albumin. We found that expression of Rab1WT did not directly influence the permeability of RPMVECs; however, it markedly increased the cell-surface expression of β2-AR and subsequently decreased the endothelial permeability as compared with that from RPMVECs infected with control lentivirus. These data imply that the permeability of RPMVECs can be manipulated by controlling the transport of β2-AR at the level of the ER and the Golgi compartment.
The physiological functions of GPCRs are dependent on their precise localization in the cell. Indeed, defective GPCR transport from the ER to the cell surface is associated with the pathogenesis of a variety of human diseases. The transport from the ER to the Golgi compartment of GPCRs represents the first step in intracellular trafficking of the receptors and influences the cell-surface expression and function of the receptors (Petaja-Repo et al., 2000). As β-ARs has been demonstrated to play a critical role in the regulation of the permeability and function of vascular endothelial cells, it will be interesting to determine whether Rab1 regulates the trafficking and function of endogenous β-ARs in primary cultures of RPMVECs. Our results have demonstrated that Rab1 regulates the ER-to-Golgi transport and function of β-ARs. Export from the ER and to the cell surface involves the selection of distinct transport pathways for different GPCRs. Therefore, defining the functional role of individual Rab GTPases, in regulation of the function of the respiratory system through modification and transport of selective GPCRs at distinct steps, may provide a novel foundation for the development of strategies in treating the diseases of the respiratory system.
Acknowledgments
We thank Dr. Guangyu Wu for kindly providing the Wild type Rab1-pcDNA3, Rab1N124I (dominant negative mutant)-pcDNA3, pDsRed2-ER, and human β2-AR tagged with green fluorescent protein (GFP). This work was supported by Grants 30770928 and 30971309 from National Science Foundation of China (NSFC) (to G. W.) and the PLA Grants 08G093 (to G. W) and 06G083 (to G. Qian).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamski SW, Langone JJ, Grega GJ. Modulation of macromolecular permeability by immune complexes and a beta-adrenoceptor stimulant. Am J Physiol. 1987;253:H1586–1595. doi: 10.1152/ajpheart.1987.253.6.H1586. [DOI] [PubMed] [Google Scholar]
- Allen MJ, Coleman RA. Beta 2-adrenoceptors mediate a reduction in endothelial permeability in vitro. Eur J Pharmacol. 1995;274:7–15. doi: 10.1016/0014-2999(94)00689-5. [DOI] [PubMed] [Google Scholar]
- Bannerman DD, Goldblum SE. Endotoxin induces endothelial barrier dysfunction through protein tyrosine phosphorylation. Am J Physiol Lung Cell Mol Physiol. 1997;73:217–L226. doi: 10.1152/ajplung.1997.273.1.L217. [DOI] [PubMed] [Google Scholar]
- Berman RS, Frew JD, Martin W. Endotoxin-induced arterial endothelial barrier dysfunction assessed by an in vitro model. Br J Pharmacol. 1993;110:1282–1284. doi: 10.1111/j.1476-5381.1993.tb13956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogatcheva NV, Zemskova MA, Kovlenkov Y, Poirier C, Verin AD. Molecular Mechanisms Mediating Protective Effect of cAMP on Lipopolysaccharide (LPS)-Induced Human Lung Microvascular Endothelial Cells (HLMVEC) Hyperpermeability. J Cell Physiol. 2009;221:750–759. doi: 10.1002/jcp.21913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton PB, Lefevre P, McDonald DM. Salmeterol Reduces Early-and Late-phase Plasmal Leakage and Leukocyte Adhesion in Rat Airways. Am J Respir Crit Care Med. 1997;155:1428–1435. doi: 10.1164/ajrccm.155.4.9105089. [DOI] [PubMed] [Google Scholar]
- Calls J, Cases A, Lario S, Esforzado N, Pare JC, Azqueta M, et al. β-Adrenergic receptor density and function in left ventricular hypertrophy in young essential hypertensives. J Hum Hypertens. 2000;14:17–21. doi: 10.1038/sj.jhh.1000927. [DOI] [PubMed] [Google Scholar]
- Chang YS, Munn LL, Hillsley MV, Dull RO, Yuan J, Lakshminarayanan S, et al. Effect of Vascular Endothelial Growth Factor on Cultured Endothelial Cell Monolayer Transport Properties. Microvasc Res. 2000;59:265–277. doi: 10.1006/mvre.1999.2225. [DOI] [PubMed] [Google Scholar]
- Chen SF, Fei X, Li SH. A new simple method for isolation of microvascular endothelial cells avoiding both chemical and mechanical injuries. Microvasc Res. 1995;50:119–128. doi: 10.1006/mvre.1995.1044. [DOI] [PubMed] [Google Scholar]
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Del Vecchio PJ, Minnear FL, Burhop KE, Selig WM, Garcia JG, et al. Measurement of albumin permeability across endothelial monolayers in vitro. J Appl Physiol. 1987;62:1076–1083. doi: 10.1152/jappl.1987.62.3.1076. [DOI] [PubMed] [Google Scholar]
- DE Leeuw HP, Koster PM, Calafat J, Janssen H, van Zonneveld AJ, van Mourik JA, et al. Small GTP-binding proteins in human endothelial cells. Br J Haematol. 1998;103:15–19. doi: 10.1046/j.1365-2141.1998.00965.x. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Kobilka BK, Strader DJ, Benovic JL, Dohlman HG, Frielle T, et al. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986;321:75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ Res. 2006;99:570–582. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- Duvernay MT, Filipeanu CM, Wu G. The regulatory mechanisms of export trafficking of G protein-coupled receptors. Cell Signal. 2005;17:1457–1465. doi: 10.1016/j.cellsig.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Duvernay MT, Zhou F, Wu G. A conserved motif for the transport of G protein-coupled receptors from the endoplasmic reticulum to the cell surface. J Biol Chem. 2004;279:30741–30750. doi: 10.1074/jbc.M313881200. [DOI] [PubMed] [Google Scholar]
- Emorine LJ, Marullo S, Briend-Sutren MM, Patey G, Tate K, Delavier-Klutchko C, et al. Molecular characterization of the human β3-adrenergic receptor. Science. 1989;245:1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- Essler M, Staddon JM, Weber PC, Aepfelbacher M. Cyclic AMP blocks bacterial lipopolysaccharide-induced myosinlight chain phosphorylation in endothelial cells through inhibition of Rho/Rho kinase signaling. J Immunol. 2000;164:6543–6549. doi: 10.4049/jimmunol.164.12.6543. [DOI] [PubMed] [Google Scholar]
- Filipeanu CM, Zhou F, Claycomb WC, Wu G. Regulation of the cell surface expression and function of angiotensin II type 1 receptor by Rab1-mediated endoplasmic reticulum-to-Golgi transport in cardiac myocytes. J Biol Chem. 2004;279:1077–41084. doi: 10.1074/jbc.M405988200. [DOI] [PubMed] [Google Scholar]
- Filipeanu CM, Zhou F, Fugetta EK, Wu G. Differential Regulation of the Cell-Surface Targeting and Function of β- and α1-Adrenergic Receptors by Rab1 GTPase in Cardiac Myocytes. Mol Pharmacol. 2006;69:1571–1578. doi: 10.1124/mol.105.019984. [DOI] [PubMed] [Google Scholar]
- Frielle T, Collins S, Daniel KW, Caron MG, Lefkowitz RJ, Kobilka BK. Cloning of the cDNA for the human beta 1-adrenergic receptor. Proc Natl Acad Sci USA. 1987;84:7920–7924. doi: 10.1073/pnas.84.22.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Xu N, Sekosan M, Mehta D, Ma SY, Rahman A, et al. Differential role of CD18 integrins in mediating lung neutrophil sequestration and increased microvascular permeability induced by Escherichia coli in mice. J Immunol. 2001;167:2895–2901. doi: 10.4049/jimmunol.167.5.2895. [DOI] [PubMed] [Google Scholar]
- Goldblum SE, Ding X, Brann TW, Campbell-Washington J. Bacterial lipopolysaccharide induces actin reorganization, intercellular gap formation, and endothelial barrier dysfunction in pulmonary vascular endothelial cells: concurrent F-actin deploy merization and new actin synthesis. J Cell Physiol. 1993;157:13–23. doi: 10.1002/jcp.1041570103. [DOI] [PubMed] [Google Scholar]
- Gudgeon JR, Martin W. Modulation of arterial endothelial permeability: studies on an in vitro model. Br J Pharamcol. 1989;98:1267–1274. doi: 10.1111/j.1476-5381.1989.tb12673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes S, Moura D. Vascular Adrenoceptors: An Update. Pharmacol Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- Hirano S, Rees RS, Yancy SL, Welsh MJ, Remick DG, Yamada T, Hata J, et al. Endothelial barrier dysfunction caused by LPS correlates with phosphorylation of HSP27 in vivo. Cell Biol Toxicol. 2004;20:1–14. doi: 10.1023/b:cbto.0000021019.50889.aa. [DOI] [PubMed] [Google Scholar]
- Karniguian A, Zahraoui A, Tavitian A. Identification of small GTP-binding rab proteins in human platelets: thrombininduced phosphorylation of rab3B, rab6, and rab8 proteins. Proc Natl Acad Sci USA. 1993;90:7647–7651. doi: 10.1073/pnas.90.16.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan ML, Gómez AD, Baluk P, Hashizume H, McDonald DM. Airway vasculature after mycoplasma infection: chronic leakiness and selective hypersensitivity to substance P. Am J Physiol Lung Cell Mol Physiol. 2001;280:L286–L297. doi: 10.1152/ajplung.2001.280.2.L286. [DOI] [PubMed] [Google Scholar]
- Langeler EG, van Hinsbergh VW. Norepinephrine and iloprost improve barrier function of human endothelial cell monolayers: role of cAMP. Am J Physiol. 1991;260:C1052–1059. doi: 10.1152/ajpcell.1991.260.5.C1052. [DOI] [PubMed] [Google Scholar]
- Martinez O, Goud B. Olivier Martinez, Bruno Goud. Rab proteins. Biochim Biophys Acta. 1998;1404:101–112. doi: 10.1016/s0167-4889(98)00050-0. [DOI] [PubMed] [Google Scholar]
- McDonald DM. Angiogenesis and Remodeling of Airway Vasculature in Chronic Inflammation. Am J Respir Crit Care Med. 2001;164:S39–S45. doi: 10.1164/ajrccm.164.supplement_2.2106065. [DOI] [PubMed] [Google Scholar]
- McLean AJ, Bevan N, Rees S, Milligan G. Visualizing differences in ligand regulation of wild-type and constitutively active mutant β2-adrenoceptor-green fluorescent protein fusion proteins. Mol Pharmacol. 1999;56:1182–1191. doi: 10.1124/mol.56.6.1182. [DOI] [PubMed] [Google Scholar]
- Mehta D, Bhattacharya J, Matthay MA, Malik AB. Integrated control of lung fluid balance. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1081–L1090. doi: 10.1152/ajplung.00268.2004. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik AB. Signaling Mechanisms Regulating Endothelial Permeability . Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Meyrick BO, Ryan US, Brigham KL. Direct effects of E. coli endotoxin on structure and permeability of pulmonary endothelial monolayers and the endothelial layer of intimal explants. Am J Pathol. 1986;122:140–151. [PMC free article] [PubMed] [Google Scholar]
- Minnear FL, DeMichele MA, Leonhardt S, Andersen TT, Teitler M. Isoproterenol antagonizes endothelial permeability induced by thrombin and thrombin receptor peptide. J Appl Physiol. 1993;75:1171–1179. doi: 10.1152/jappl.1993.75.3.1171. [DOI] [PubMed] [Google Scholar]
- Minnear FL, DeMichele MA, Moon DG, Rieder CL, Fenton JW., 2nd. Isoproterenol reduces thrombin-induced pulmonary endothelial permeability in vitro. Am J Physiol. 1989;257:H1613–1623. doi: 10.1152/ajpheart.1989.257.5.H1613. [DOI] [PubMed] [Google Scholar]
- Mizus I, Summer W, Farrukh I, Michael JR, Gurtner GH. Isoproterenol or aminophylline attenuate pulmonary edema after acid lung injury. Am Rev Respir Dis. 1985;131:256–259. doi: 10.1164/arrd.1985.131.2.256. [DOI] [PubMed] [Google Scholar]
- Murphy JT, Duffy SL, Hybki DL, Kamm K. Thrombin-Mediated Permeability of Human Microvascular Pulmonary Endothelial Cells Is Calcium Dependent. J Trauma. 2001;50:213–222. doi: 10.1097/00005373-200102000-00005. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Penn MS, Chisolm GM. Relation between lipopolysaccharide-induced endothelial cell injury and entry of macromolecules into the rat aorta in vivo. Circ Res. 1991;68:1259–1269. doi: 10.1161/01.res.68.5.1259. [DOI] [PubMed] [Google Scholar]
- Petaja-Repo UE, Hogue M, Laperriere A, Walker P, Bouvier MP. Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human delta opioid receptor. J Biol Chem. 2000;275:13727–13736. doi: 10.1074/jbc.275.18.13727. [DOI] [PubMed] [Google Scholar]
- Reiter E, Lefkowitz RJ. GRKs and β-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Reiser J. Production and concentration of pseudotyped HIV-1-based gene transfer vectors. Gene Ther. 2000;7:910–913. doi: 10.1038/sj.gt.3301188. [DOI] [PubMed] [Google Scholar]
- Saraste J, Goud B. Functional Symmetry of Endomembranes. Mol Biol Cell. 2007;18:1430–1436. doi: 10.1091/mbc.E06-10-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer U, Seibold S, Schneider A, Neugebauer E. Isolation and characterisation of the human rab18 gene after stimulation of endothelial cells with histamine. FEBS Lett. 2000;466:148–154. doi: 10.1016/s0014-5793(99)01778-0. [DOI] [PubMed] [Google Scholar]
- Segev N. Ypt/rab GTPases: regulators of protein trafficking. Sci STKE. 2001;100:re11. doi: 10.1126/stke.2001.100.re11. [DOI] [PubMed] [Google Scholar]
- Sena-Esteves M, Tebbets JC, Steffens S, Crombleholme T, Flake AW. Optimized large-scale production of high titer lentivirus vector pseudotypes. J Virol Methods. 2004;122:131–139. doi: 10.1016/j.jviromet.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Short B, Haas A, Barr F. Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochim Biophys Acta. 2005;1744:383–395. doi: 10.1016/j.bbamcr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1:241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongena GP, van Hinsbergh VW. Targets for pharmacological intervention of endothelial hyperpermeability and barrier function. Vascul Pharmacol. 2003;39:257–272. doi: 10.1016/s1537-1891(03)00014-4. [DOI] [PubMed] [Google Scholar]
- Wang G, Qian P, Jacksonb FR, Qian G, Wu G. Sequential activation of JAKs, STATs and xanthine dehydrogenase/oxidase by hypoxia in lung microvascular endothelial cells. Int J Biochem Cell Biol. 2008;40:461–470. doi: 10.1016/j.biocel.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan CJ, Johnson M. Inhibition by salmeterol of increased vascular permeability and granulocyte accumulation in guinea-pig lung and skin. Br J Pharamcol. 1992;10:831–838. doi: 10.1111/j.1476-5381.1992.tb09065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DB, Wilson MP. Identification and subcellular localization of human rab5b, a new member of the ras-related superfamily of GTPases. J Clin Invest. 1992;89:996–1005. doi: 10.1172/JCI115683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittinghofer A. Phosphoryl transfer in Ras proteins, conclusive orelusive? Trends Biochem Sci. 2006;31:21–23. doi: 10.1016/j.tibs.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Wu G, Yussman MG, Barrett TJ, Hahn HS, Osinska H, Hilliard GM, et al. Increased myocardial Rab GTPase expression: a consequence and cause of cardiomyopathy. Circ Res. 2001;89:1130–1137. doi: 10.1161/hh2401.100427. [DOI] [PubMed] [Google Scholar]
- Wu G, Zhao G, He Y. Distinct pathways for the trafficking of angiotensin II and adrenergic receptors from the endoplasmic reticulum to the cell surface: Rab1 independent transport of a G protein-coupled receptor. J Bio Chem. 2003;278:47062–47069. doi: 10.1074/jbc.M305707200. [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sun GY. LPS induces permeability injury in lung microvascular endothelium via AT (1) receptor. Arch Biochem Biophys. 2005;441:75–83. doi: 10.1016/j.abb.2005.06.022. [DOI] [PubMed] [Google Scholar]