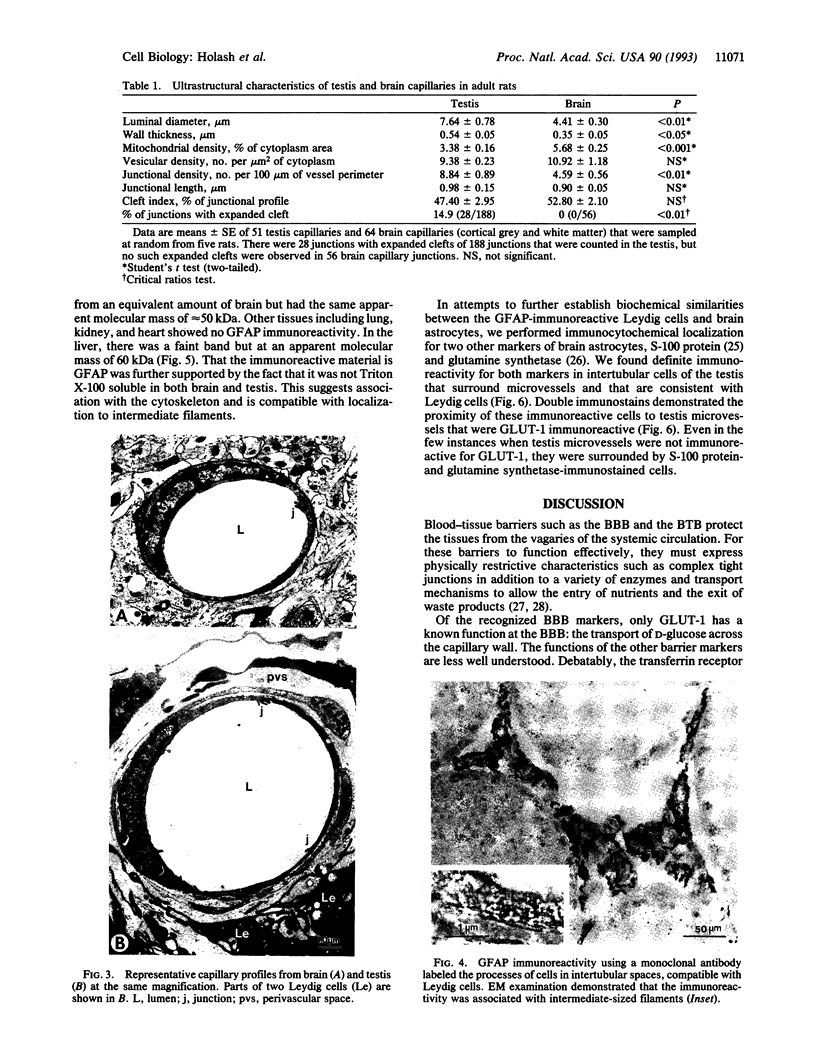
Abstract
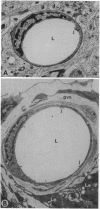
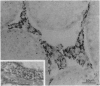
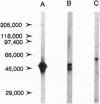
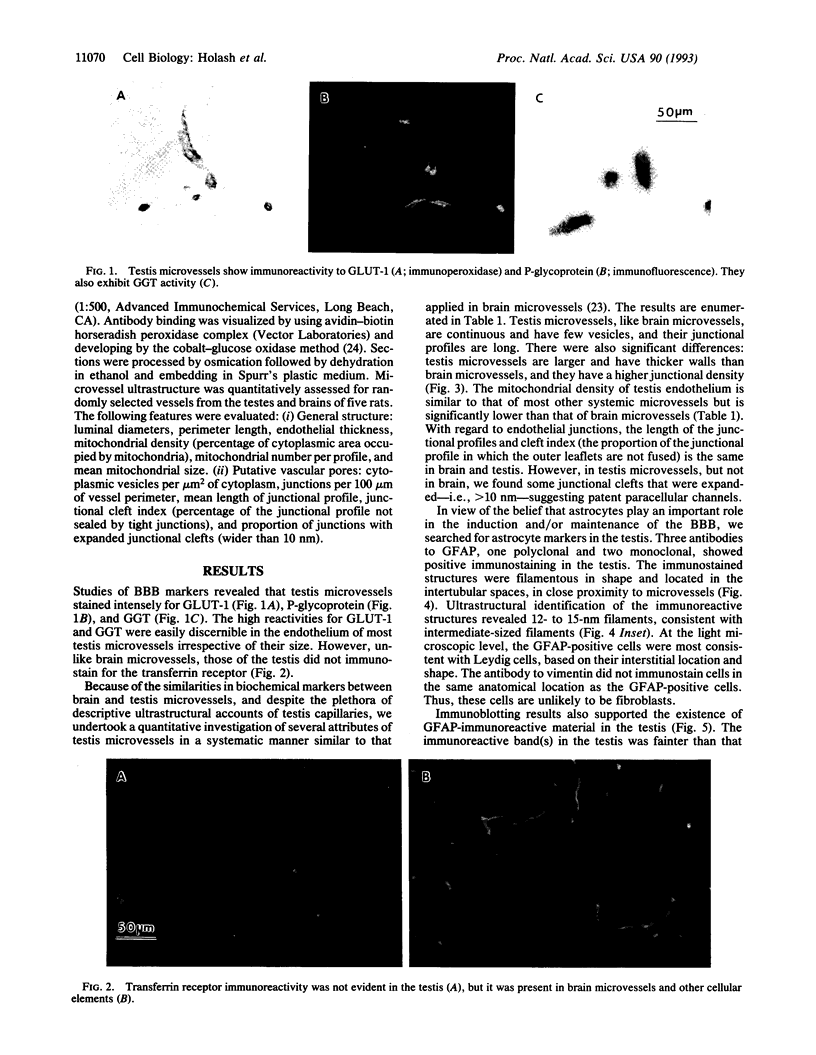
The blood-testis barrier is believed to be constituted by tight junctions between Sertoli cells in seminiferous tubules and possibly by myoid cells that encircle these tubules. We now show that testis microvessels are endowed with several markers of barrier properties of brain microvessels, such as the glucose transporter, P-glycoprotein, and gamma-glutamyl transpeptidase. Quantitative EM studies show that the endothelium in testis, as in brain, is continuous and has long junctional profiles and few vesicles. However, a small proportion of testis capillaries have expansions in their junctional clefts suggestive of patent paracellular channels, which may explain their higher permeability. Because barrier features are thought to be induced and/or maintained in brain microvessels by astrocytes, we assessed whether astrocyte-like cells exist in the testis. We found that the intertubular Leydig cells, adjacent to microvessels, express the astrocyte markers: glial fibrillary acidic protein, glutamine synthetase, and S-100 protein. We suggest that the testis endothelium contributes to the blood-testis barrier and that these endothelial barrier features are influenced by Leydig cells. We believe that the endothelial and the epithelial (Sertoli) components of the blood-testis barrier are "in series" and complement each other in achieving a stable milieu for spermatogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972 Aug 25;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard M. The three-dimensional organization of tight junctions in a capillary endothelium revealed by serial-section electron microscopy. J Ultrastruct Res. 1984 Jul;88(1):1–17. doi: 10.1016/s0022-5320(84)90177-1. [DOI] [PubMed] [Google Scholar]
- Coomber B. L., Stewart P. A., Hayakawa E. M., Farrell C. L., Del Maestro R. F. A quantitative assessment of microvessel ultrastructure in C6 astrocytoma spheroids transplanted to brain and to muscle. J Neuropathol Exp Neurol. 1988 Jan;47(1):29–40. doi: 10.1097/00005072-198801000-00004. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., O'Brien J. P., Boccia J., Casals D., Bertino J. R., Melamed M. R. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem. 1990 Sep;38(9):1277–1287. doi: 10.1177/38.9.1974900. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., O'Brien J. P., Casals D., Rittman-Grauer L., Biedler J. L., Melamed M. R., Bertino J. R. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989 Jan;86(2):695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C. Facilitated transfer of glucose from blood into brain tissue. J Physiol. 1965 Nov;181(1):103–113. doi: 10.1113/jphysiol.1965.sp007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C. Permeability of single capillaries. Clin Invest Med. 1985;8(1):89–95. [PubMed] [Google Scholar]
- Dermietzel R., Krause D. Molecular anatomy of the blood-brain barrier as defined by immunocytochemistry. Int Rev Cytol. 1991;127:57–109. doi: 10.1016/s0074-7696(08)60692-0. [DOI] [PubMed] [Google Scholar]
- Dick A. P., Harik S. I., Klip A., Walker D. M. Identification and characterization of the glucose transporter of the blood-brain barrier by cytochalasin B binding and immunological reactivity. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7233–7237. doi: 10.1073/pnas.81.22.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dym M., Cavicchia J. C. Further observations on the blood-testis barrier in monkeys. Biol Reprod. 1977 Oct;17(3):390–403. doi: 10.1095/biolreprod17.3.390. [DOI] [PubMed] [Google Scholar]
- Dym M., Fawcett D. W. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970 Dec;3(3):308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- Dym M. The fine structure of the monkey (Macaca) Sertoli cell and its role in maintaining the blood-testis barrier. Anat Rec. 1973 Apr;175(4):639–656. doi: 10.1002/ar.1091750402. [DOI] [PubMed] [Google Scholar]
- EVERETT N. B., SIMMONS Measurement and radioautographic localization of albumin in rat tissues after intravenous administration. Circ Res. 1958 May;6(3):307–313. doi: 10.1161/01.res.6.3.307. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., Heidger P. M., Leak L. V. Lymph vascular system of the interstitial tissue of the testis as revealed by electron microscopy. J Reprod Fertil. 1969 Jun;19(1):109–119. doi: 10.1530/jrf.0.0190109. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., Leak L. V., Heidger P. M., Jr Electron microscopic observations on the structural components of the blood-testis barrier. J Reprod Fertil Suppl. 1970;10:105–122. [PubMed] [Google Scholar]
- Flickinger C., Fawcett D. W. The junctional specializations of Sertoli cells in the seminiferous epithelium. Anat Rec. 1967 Jun;158(2):207–221. doi: 10.1002/ar.1091580210. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Barnes G. W., Skelton F. R. Light-microscopic and immunopathologic observations on cadmium chloride-induced injury in mature rat testis. Am J Pathol. 1967 Aug;51(2):191–205. [PMC free article] [PubMed] [Google Scholar]
- Harik S. I., Kalaria R. N., Andersson L., Lundahl P., Perry G. Immunocytochemical localization of the erythroid glucose transporter: abundance in tissues with barrier functions. J Neurosci. 1990 Dec;10(12):3862–3872. doi: 10.1523/JNEUROSCI.10-12-03862.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harik S. I., Kalaria R. N., Whitney P. M., Andersson L., Lundahl P., Ledbetter S. R., Perry G. Glucose transporters are abundant in cells with "occluding" junctions at the blood-eye barriers. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4261–4264. doi: 10.1073/pnas.87.11.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Konishi A., Nomura S., Mizuno N., Nakamura Y., Sugimoto T. Application of coupled oxidation reaction to electron microscopic demonstration of horseradish peroxidase: cobalt-glucose oxidase method. Brain Res. 1979 Oct 19;175(2):341–346. doi: 10.1016/0006-8993(79)91013-8. [DOI] [PubMed] [Google Scholar]
- Janzer R. C., Raff M. C. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987 Jan 15;325(6101):253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Jefferies W. A., Brandon M. R., Hunt S. V., Williams A. F., Gatter K. C., Mason D. Y. Transferrin receptor on endothelium of brain capillaries. Nature. 1984 Nov 8;312(5990):162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- Kalaria R. N., Gravina S. A., Schmidley J. W., Perry G., Harik S. I. The glucose transporter of the human brain and blood-brain barrier. Ann Neurol. 1988 Dec;24(6):757–764. doi: 10.1002/ana.410240610. [DOI] [PubMed] [Google Scholar]
- Kormano M. Penetration of intravenous trypan blue into the rat testis and epididymis. Acta Histochem. 1968;30(1):133–136. [PubMed] [Google Scholar]
- Levick J. R., Smaje L. H. An analysis of the permeability of a fenestra. Microvasc Res. 1987 Mar;33(2):233–256. doi: 10.1016/0026-2862(87)90020-3. [DOI] [PubMed] [Google Scholar]
- Lipp W. Blood serum proteins and the mineralization of bone ground substance. Histochemie. 1967;9(4):339–353. doi: 10.1007/BF00305817. [DOI] [PubMed] [Google Scholar]
- Ludwin S. K., Kosek J. C., Eng L. F. The topographical distribution of S-100 and GFA proteins in the adult rat brain: an immunohistochemical study using horseradish peroxidase-labelled antibodies. J Comp Neurol. 1976 Jan 15;165(2):197–207. doi: 10.1002/cne.901650206. [DOI] [PubMed] [Google Scholar]
- MANCINI R. E., VILAR O., ALVAREZ B., SEIGUER A. C. EXTRAVASCULAR AND INTRATUBULAR DIFFUSION OF LABELED SERUM PROTEINS IN THE RAT TESTIS. J Histochem Cytochem. 1965 May-Jun;13:376–385. doi: 10.1177/13.5.376. [DOI] [PubMed] [Google Scholar]
- Maxwell K., Berliner J. A., Cancilla P. A. Induction of gamma-glutamyl transpeptidase in cultured cerebral endothelial cells by a product released by astrocytes. Brain Res. 1987 May 5;410(2):309–314. doi: 10.1016/0006-8993(87)90329-5. [DOI] [PubMed] [Google Scholar]
- Mayerhofer A., Bartke A. Developing testicular microvasculature in the golden hamster, Mesocricetus auratus: a model for angiogenesis under physiological conditions. Acta Anat (Basel) 1990;139(1):78–85. doi: 10.1159/000146982. [DOI] [PubMed] [Google Scholar]
- Mayerhofer A., Seidl K., Lahr G., Bitter-Suermann D., Christoph A., Barthels D., Wille W., Gratzl M. Leydig cells express neural cell adhesion molecules in vivo and in vitro. Biol Reprod. 1992 Oct;47(4):656–664. doi: 10.1095/biolreprod47.4.656. [DOI] [PubMed] [Google Scholar]
- Michetti F., Lauriola L., Rende M., Stolfi V. M., Battaglia F., Cocchia D. S-100 protein in the testis. An immunochemical and immunohistochemical study. Cell Tissue Res. 1985;240(1):137–142. doi: 10.1007/BF00217566. [DOI] [PubMed] [Google Scholar]
- Milici A. J., Watrous N. E., Stukenbrok H., Palade G. E. Transcytosis of albumin in capillary endothelium. J Cell Biol. 1987 Dec;105(6 Pt 1):2603–2612. doi: 10.1083/jcb.105.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn A., Ghersi-Egea J. F., Perrin R., Leininger B., Siest G. Drug metabolizing enzymes in the brain and cerebral microvessels. Brain Res Brain Res Rev. 1991 Jan-Apr;16(1):65–82. doi: 10.1016/0165-0173(91)90020-9. [DOI] [PubMed] [Google Scholar]
- Nicander L. An electron microscopical study of cell contacts in the seminiferous tubules of some mammals. Z Zellforsch Mikrosk Anat. 1967;83(3):375–397. doi: 10.1007/BF00336866. [DOI] [PubMed] [Google Scholar]
- Norenberg M. D. Distribution of glutamine synthetase in the rat central nervous system. J Histochem Cytochem. 1979 Mar;27(3):756–762. doi: 10.1177/27.3.39099. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Sessa G., Green J. P. Gamma-glutamyl transpeptidase in brain capillaries: possible site of a blood-brain barrier for amino acids. Science. 1974 Apr 5;184(4132):66–68. doi: 10.1126/science.184.4132.66. [DOI] [PubMed] [Google Scholar]
- Reese T. S., Karnovsky M. J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967 Jul;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. D. The blood-testis barrier and its formation relative to spermatocyte maturation in the adult rat: a lanthanum tracer study. Anat Rec. 1978 Jan;190(1):99–111. doi: 10.1002/ar.1091900109. [DOI] [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Schulze W., Davidoff M. S., Holstein A. F. Are Leydig cells of neural origin? Substance P-like immunoreactivity in human testicular tissue. Acta Endocrinol (Copenh) 1987 Jul;115(3):373–377. doi: 10.1530/acta.0.1150373. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Siminoescu M., Palade G. E. Permeability of muscle capillaries to small heme-peptides. Evidence for the existence of patent transendothelial channels. J Cell Biol. 1975 Mar;64(3):586–607. doi: 10.1083/jcb.64.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M., Palade G. E. Open junctions in the endothelium of the postcapillary venules of the diaphragm. J Cell Biol. 1978 Oct;79(1):27–44. doi: 10.1083/jcb.79.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P. A., Hayakawa E. M. Interendothelial junctional changes underlie the developmental 'tightening' of the blood-brain barrier. Brain Res. 1987 Apr;429(2):271–281. doi: 10.1016/0165-3806(87)90107-6. [DOI] [PubMed] [Google Scholar]
- Stewart P. A., Hayakawa K., Farrell C. L., Del Maestro R. F. Quantitative study of microvessel ultrastructure in human peritumoral brain tissue. Evidence for a blood-brain barrier defect. J Neurosurg. 1987 Nov;67(5):697–705. doi: 10.3171/jns.1987.67.5.0697. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng J. H., Nagy Z., Brightman M. W. Tight junctions of brain endothelium in vitro are enhanced by astroglia. J Neurosci. 1987 Oct;7(10):3293–3299. doi: 10.1523/JNEUROSCI.07-10-03293.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta T., Naito M., Oh-hara T., Sugawara I., Tsuruo T. Functional involvement of P-glycoprotein in blood-brain barrier. J Biol Chem. 1992 Oct 5;267(28):20383–20391. [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Immunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle protein. J Histochem Cytochem. 1989 Feb;37(2):159–164. doi: 10.1177/37.2.2463300. [DOI] [PubMed] [Google Scholar]
- Weinbaum S., Tsay R., Curry F. E. A three-dimensional junction-pore-matrix model for capillary permeability. Microvasc Res. 1992 Jul;44(1):85–111. doi: 10.1016/0026-2862(92)90104-w. [DOI] [PubMed] [Google Scholar]