Abstract
The objective of this study was to evaluate the effects of several different commercial disinfectants on the embryogenic development of Ascaris suum eggs. A 1-ml aliquot of each disinfectant was mixed with approximately 40,000 decorticated or intact A. suum eggs in sterile tubes. After each treatment time (at 0.5, 1, 5, 10, 30, and 60 min), disinfectants were washed away, and egg suspensions were incubated at 25˚C in distilled water for development of larvae inside. At 3 weeks of incubation after exposure, ethanol, methanol, and chlorohexidin treatments did not affect the larval development of A. suum eggs, regardless of their concentration and treatment time. Among disinfectants tested in this study, 3% cresol, 0.2% sodium hypochlorite and 0.02% sodium hypochlorite delayed but not inactivated the embryonation of decorticated eggs at 3 weeks of incubation, because at 6 weeks of incubation, undeveloped eggs completed embryonation regardless of exposure time, except for 10% povidone iodine. When the albumin layer of A. suum eggs remained intact, however, even the 10% povidone iodine solution took at least 5 min to reasonably inactivate most eggs, but never completely kill them with even 60 min of exposure. This study demonstrated that the treatment of A. suum eggs with many commercially available disinfectants does not affect the embryonation. Although some disinfectants may delay or stop the embryonation of A. suum eggs, they can hardly kill them completely.
Keywords: Ascaris suum, egg, disinfectant, larval development
A number of zoonotic pathogens, in particular intestinal parasites, can be transmitted from livestock and pets to humans [1]. Among them, Ascaris lumbricoides and Ascaris suum are now considered to be a single species that can complete life cycle both in pigs and humans [2]. Humans can get infected with these parasites through several ways, but the most common route of transmission is ingestion of the eggs in contaminated food, water, or soil, which has been contaminated with feces of infected animals [3].
Contamination of the environment with feces of livestock and companion animals that contain parasite eggs or oocysts poses a serious risk to public health, which is widely documented worldwide [1,4-6]. A single roundworm can lay up to 200,000 eggs per day creating significant environmental contamination very quickly [7]. In addition, some worm eggs are highly resistant to adverse environmental conditions and may persist in the soil for years [8]. Consequently, once an environment is contaminated with these parasitic eggs, it is very difficult to resolve it. To reduce the spread of these parasites, it is most important to prevent initial environmental contamination through appropriate sanitation and disinfection.
Eggs of the genus Ascaris have the highest resistance and survive under numerous treatment conditions such as a variety of strong acids, strong bases, oxidants, reductants, protein-disrupting agents, and surface-active agents [9]. Due to its resistance to biocontrol mechanisms, A. suum eggs have been chosen as model eggs to measure treatment efficiency [10]. Several studies have been conducted to inactivate A. suum larvae using fatty acids [11], high hydrostatic pressure [12], low-pressure UV radiation [10], and ammonia [13], which are less practical or not readily available for home use. However, adequate information is not available regarding the effect of disinfectants commonly used in house or laboratory work such as alcohols, cresol, and bleach. Furthermore, in previous reports, some disinfectants such as povidone-iodine showed discrepancy in efficacy against Ascaris eggs [14-16]. In this study, therefore, we evaluated the efficacy of some disinfectants commonly used in veterinary clinics, medical laboratories, and for general housekeeping purposes on the inactivation of A. suum egg development.
Female gravid adult worms of A. suum were collected from the small intestines of naturally infected pigs at a slaughter-house in Gwangju, Korea. Eggs isolated from the uterine tubule of female worms were incubated in 4% sodium hypochlorite (Clorax, Youhanyanghaeng, Seoul, Korea) for 3 min to remove the albumin layer which causes ascarid eggs to adhere to each other. Then, sodium hypochlorite was washed away from the medium by centrifugation in distilled water 3 times, and the eggs were stored at 5˚C in distilled water for further use. Eggs of A. suum were also used intact without removing the albumin layer to assess the influence of decortication with 4% sodium hypochlorite. For this experiment, eggs isolated from the uterus of gravid female A. suum worms were washed 3 times in distilled water and stored at 5˚C in distilled water for further use.
Commercial disinfectants were prepared as follows: 99% ethanol (ethyl alcohol anhydrous, Carlo ERRA, Spain), 70% ethanol (diluted with distilled water), 99% methanol (Carlo ERRA), 70% methanol (diluted with distilled water), 10% povidone iodine (Povidin, SungKawangPharm, Seoul, Korea), 3% cresol (Saponated cresol solution, Green Pharmaceutical Co., Seoul, Korea), 0.2% sodium hypochlorite (Clorax), 0.02% sodium hypochlorite (Clorax), 5% chlorohexidin (α-hexidin 5%, SungKawangPharm). Tap water was also included in disinfectants group, and saline and distilled water were used for control group. Povidone iodine solution (10%) was diluted in distilled water to 1% and 0.1% to use in a separate experiment in which intact A. suum eggs were treated with 4 different levels (10%, 1%, 0.1%, and 0%) of povidone iodine concentration.
A 1-ml aliquot of each disinfectants and tap water (hereafter referred to as samples), and also saline and distilled water (hereafter referred to as controls) were each mixed with approximately 40,000 either intact or decorticated A. suum eggs in sterile polypropylene conical centrifuge tubes (Sewonyanghaeng, Seoul, Korea). For assessing the effect of different treatment duration, 6 sets of the mixture were prepared for each sample and control. After each treatment time (at 0.5, 1, 5, 10, 30, and 60 min) at room temperature (25˚C), disinfectants were washed away by centrifugation (10 min, at 930 g) in 50 ml distilled water 3 times. Then, the egg suspensions in each tube were distributed to 2 tubes filled with 1-ml distilled water and stored at 25˚C for development of larvae.
Larval development of decorticated A. suum eggs were evaluated at 2 different incubation times during the experimental period (3 and 6 weeks after incubation) by microscopic observations. At each time point, a total of 100 eggs per each sample were observed microscopically and the number of only fully embryonated eggs was counted. This counting procedure was repeated 5 times on each sample. The larval development rate was calculated using the following equation: larval development rate (%)=mean no. of eggs with fully developed larvae/no. of eggs counted ×100. Differences in larval development among treatment groups were tested by repeated measures ANOVA.
The results of disinfectant exposure on decorticated A. suum egg embryogenesis are shown in Table 1. At 3 weeks of incubation after exposure, ethanol and methanol did not affect the larval development of decorticated A. suum eggs, regardless of their concentration and exposure time. Chlorohexidin (5%) and tap water also had almost no effect on embryogenesis of A. suum eggs (Fig. 1). Among disinfectants tested in this study, 10% povidone iodine, 3% cresol, 0.2% sodium hypochlorite, and 0.02% sodium hypochlorite inhibited embryonation at 3 weeks of incubation, because almost no eggs showed larval development from 5 min exposure to these disinfectants. At 6 weeks of incubation, however, eggs exposed to all disinfectants tested showed embryonation regardless of exposure time, except for 10% povidone iodine. Only 10% povidone iodine completely inhibited the embryonation of decorticated A. suum eggs at 6 weeks of incubation.
Table 1.
Embryonic development of decorticated Ascaris suum eggs for 3 and 6 weeks of incubation at 25˚C after exposure to different disinfectants
| Types of disinfectants | Duration of treatment time (min) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 |
1 |
5 |
10 |
30 |
60 |
|||||||
| 3w PIa | 6w PIb | 3w PI | 6w PI | 3w PI | 6w PI | 3w PI | 6w PI | 3w PI | 6w PI | 3w PI | 6w PI | |
| Ethanol 70% | 100 ± 0c | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Ethanol 99% | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Methanol 70% | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Methanol 99% | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Povidone iodine 10% | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Cresol 3% | 100 ± 0 | 100 ± 0 | 3 ± 0.9 | 100 ± 0 | 0 ± 0 | 100 ± 0 | 0 ± 0 | 100 ± 0 | 0 ± 0 | 100 ± 0 | 0 ± 0 | 100 ± 0 |
| Sodium hypochlorite 0.02% | 100 ± 0 | 100 ± 0 | 82 ± 1.3 | 100 ± 0 | 2 ± 0.6 | 100 ± 0 | 0 ± 0 | 100 ± 0 | 0 ± 0 | 100 ± 0 | 0 ± 0 | 100 ± 0 |
| Sodium hypochlorite 0.2% | 100 ± 0 | 100 ± 0 | 15 ± 1.6 | 100 ± 0 | 0 ± 0 | 100 ± 0 | 0 ± 0 | 100 ± 0 | 0 ± 0 | 100 ± 0 | 0 ± 0 | 100 ± 0 |
| Chlorohexidin 5% | 100 ± 0 | 100 ± 0 | 97 ± 0.5 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 82 ± 0.4 | 100 ± 0 | 98 ± 0.4 | 100 ± 0 |
| Tap water | 100 ± 0 | 100 ± 0 | 87 ± 1.2 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Distilled water | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Saline | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
3 weeks after treatment with disinfectants.
6 weeks after treatment with disinfectants.
Percentages of larval development.
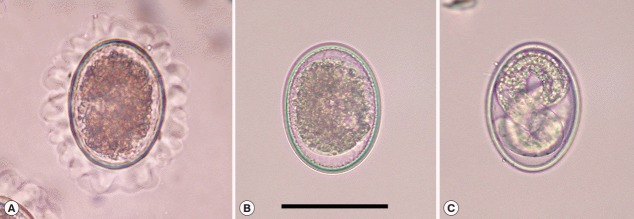
Fig. 1.
Ascaris suum eggs in various conditions. (A) Ascaris suum egg freshly isolated from the uterus of an adult female. Note that the egg has conspicuous mamillation on its surface. (B) An unembryonated egg of A. suum after the albumin layer of the egg surface was decorticated by incubating in 4% sodium hypochlorite for 3 min. Eggs that failed to go through larval development affected by the treatment of disinfectants remain unembryonated. (C) A fully embryonated egg of A. suum after 3 weeks of incubation at 25˚C in distilled water. Bar=50 μm.
Results of the embryogenesis of intact, undecorticated A. suum eggs exposed to povidone iodine are shown in Table 2. At 3 weeks of incubation, the number of embryonated eggs exposed to 10% povidone iodine solution for 30 sec was 94.3±0.8 which was similar to non-treated control group (av. 95.6±0.7). However, a significant reduction in embryogenesis from over 90% to below 5% was observed from 5 min exposure to 10% and 10 min exposure to 1% povidone iodine solutions (P<0.001, by repeated measures ANOVA). However, even the strongest 10% concentration of povidone iodine did not completely inactivate A. suum eggs (0.1±0.1%). Exposure of intact A. suum eggs to 0.1% povidone iodine solution did not affect the embryogenesis regardless of the incubation time.
Table 2.
Embryonic development of intact Ascaris suum eggs after exposure to various levels of povidone iodine concentration
| Conc. of Povidone (%) | Duration of treatment time (min) |
|||||
|---|---|---|---|---|---|---|
| 0.5 | 1 | 5 | 10 | 30 | 60 | |
| 10 | 94.3 ± 0.8* | 87.0 ± 0.7 | 3.3 ± 1.0 | 4.4 ± 0.8 | 0.2 ± 0.1 | 0.1 ± 0.1 |
| 1 | 94.2 ± 0.9 | 92.8 ± 1.3 | 93.6 ± 0.8 | 2.7 ± 0.8 | 1.4 ± 0.4 | 1.0 ± 0.2 |
| 0.1 | 93.8 ± 0.6 | 93.5 ± 0.7 | 93.1 ± 0.6 | 95.5 ± 0.4 | 95.4 ± 0.6 | 95.0 ± 0.6 |
| 0 | 95.5 ± 0.6 | 94.9 ± 0.9 | 95.3 ± 0.8 | 94.8 ± 0.6 | 95.9 ± 0.5 | 97.6 ± 0.7 |
Percentages of larval development.
Results of this study demonstrated that most commercial disinfectants widely used in hospitals and homes were unsuccessful to kill A. suum eggs in that even the most effective disinfectant, 10% povidone iodine, took at least 5 min to kill most eggs but never to completely eliminate live eggs. Since most people including doctors and nurses would feel safe after disinfecting hospital facilities and their hands with disinfectants used in this study, it is noteworthy to inform them that ascarid eggs are tough to kill by such measure. Dogs and cats are the hosts for zoonotic parasites such as Toxocara spp. and Toxascaris leonina, and infected animals pass unembryonated eggs in their feces [1,4] as infected pigs do with A. suum eggs. Human infections with ascarid eggs occur most commonly through ingestion of embryonated eggs, so contamination by them is a public health problem [1]. In particular, veterinary clinics or laboratories can be contaminated with various harmful pathogens, thus proper cleaning and disinfection is essential to reduce the risk of infection from microbial contamination.
Although Ascaris eggs can be inactivated in minutes by temperatures above 60˚C, they can survive for more than 1 year at 40˚C [17]. It is well known that many helminth eggs are very resistant to unfavorable conditions and various kinds of chemical agents. In particular, Ascaris eggs are known to be the most resistant to most types of inactivation including low-pressure UV, chlorine, phenol, cresol, sodium or potassium hydroxide, quaternary ammonium compounds, glutaraldehydes, or paraformaldehyde [10,13,18]. However, contradictory results have been reported on the inactivation of Ascaris eggs by iodine. Zaman and Visuvalingam [14], for instance, reported 250 ppm iodine at a 10-min exposure completely inactivated Ascaris larvae [14]. Similar results were obtained by others [16]. However, povidone-iodine at 100%, 50%, 10%, and 1% had no effect on A. suum eggs at exposures of 5, 15, 30, 60, or 120 min (22˚C) compared with water controls [15]. This result by Labare et al. [15] showed distinct discrepancy compared to our results, because embryogenesis of A. suum was significantly decreased in our study by both 10% and 1% povidone-iodine solutions at 5 and 10 min of incubation times, respectively. We repeated the experiment with intact A. suum eggs shown in Table 2 twice using 2 other commercial brands of povidone-iodine (Green Povidone, Green Pharmaceutical Co. and Gumi Povidone iodine solution, Gumi Pharm Co., Korea, data not shown) from which we obtained the same results. Although not conclusive, the discrepancy might have originated from using either different A. suum egg source or different brands of povidone-iodine. Also, Labare et al. [15] collected eggs from the intestinal content of pigs natureally infected with A. suum, while we collected eggs directly from the uterus of gravid female worms. Additional coating of A. suum eggs with intestinal content of pigs might have provided eggs with extra protection from iodine toxicity. Povidone-iodine solution contains 1% available iodine and has been shown to have a maximum free iodine concentration of approximately 20 ppm in a 1% dilution of povidone-iodine [19].
Povidone iodine has been reported as an effective anti-infective agent against a variety of pathogens [20,21]. It was also reported that this agent have virucidal activity against avian influenza A viruses and that it could be useful in the prevention and control of human infection by avian influenza A [22]. Povidone-iodine, generally made in 10% solution, is known to be a powerful broad spectrum germicidal agent effective against a wide range of bacteria, viruses, fungi, protozoa, and spores. It is mostly used for skin disinfection, disinfection of mucocutaneous trauma, chronic pharyngitis, and oral ulcers. Commercial povidone iodine solution is also available for disinfection of the environment of poultry house and pig house.
Ethanol, methanol, and sodium hypochlorite solutions are easy and inexpensive for veterinary clinics, laboratories, and regular households to acquire. In previous studies, ethanol and sodium hypochlorite were shown to have full efficacy against infective T. canis eggs and were strongly recommended as disinfectants of kennels, animal shelters, cages, and veterinary to eliminate Toxocara canis eggs and to avoid contamination [23,24]. In this study, ethanol and methanol could not inhibit the embryonation of decorticated A. suum eggs even after 1-hr exposure, regardless of their concentration. Also, although sodium hypochlorite could delay the development of A. suum eggs after 5 min exposure, they could not completely inactivate the eggs. This is in agreement with Krishnaswami and Post [25], who also reported that disinfection with chlorine at commonly applied doses was ineffectual on inactivation of ascarid eggs.
In conclusion, this study clearly demonstrates that most commercially available disinfectants do not completely inactivate A. suum eggs and even the highly effective povidone iodine takes at least 5 min to reasonably disinfect intact, undecorticated ascarid eggs. Although some disinfectants may delay the embryonation of A. suum eggs, most can hardly kill them completely.
Acknowledgments
This work was supported in part by the Ministry of Education and Human Resources Development through the Brain Korea 21 Project in Korea.
Footnotes
The authors have no conflicts of interest concerning the work reported in this paper.
REFERENCES
- 1.Overgaauw PA, van Zutphen L, Hoek D, Yaya FO, Roelfsema J, Pinelli E, van Knapen F, Kortbeek LM. Zoonotic parasites in fecal samples and fur from dogs and cats in The Netherlands. Vet Parasitol. 2009;163:115–122. doi: 10.1016/j.vetpar.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 2.Leles D, Gardner SL, Reinhard K, Iñiguez A, Araujo A. Are Ascaris lumbricoides and Ascaris suum a single species? Parasit Vectors. 2012;5:42. doi: 10.1186/1756-3305-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodruff A, De Savigny D, Jacobs D. Study of toxocaral infection in dog breeders. Br Med J. 1978;2:1747–1748. doi: 10.1136/bmj.2.6154.1747-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson ID, Thompson R. Enteric parasitic zoonoses of domesticated dogs and cats. Microbes Infect. 2002;4:867–873. doi: 10.1016/s1286-4579(02)01607-6. [DOI] [PubMed] [Google Scholar]
- 5.Shin SS. Parasitic diseases of companion animals. Hanyang Med Rev. 2010;30:246–264. [Google Scholar]
- 6.Overgaauw PA, van Knapen F. Veterinary and public health aspects of Toxocara spp. Vet Parasitol. 2013;193:398–403. doi: 10.1016/j.vetpar.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 7.Sinniah B. Daily egg production of Ascaris lumbricoides: the distribution of eggs in the faeces and the variability of egg counts. Parasitology. 1982;84:167–175. doi: 10.1017/s0031182000051763. [DOI] [PubMed] [Google Scholar]
- 8.O'lorcain P, Holland C. The public health importance of Ascaris lumbricoides. Parasitology. 2000;121:S51–S71. doi: 10.1017/s0031182000006442. [DOI] [PubMed] [Google Scholar]
- 9.Barrett J. Studies on the induction of permeability in Ascaris lumbricoides eggs. Parasitology. 1976;73:109–121. doi: 10.1017/s0031182000051374. [DOI] [PubMed] [Google Scholar]
- 10.Brownell SA, Nelson KL. Inactivation of single-celled Ascaris suum eggs by low-pressure UV radiation. Appl Environ Microbiol. 2006;72:2178–2184. doi: 10.1128/AEM.72.3.2178-2184.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butkus MA, Hughes KT, Bowman DD, Liotta JL, Jenkins MB, Labare MP. Inactivation of Ascaris suum by short-chain fatty acids. Appl Environ Microbiol. 2011;77:363–366. doi: 10.1128/AEM.01675-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosypal AC, Bowman DD, Holliman D, Flick GJ, Lindsay DS. Effects of high hydrostatic pressure on embryonation of Ascaris suum eggs. Vet Parasitol. 2007;145:86–89. doi: 10.1016/j.vetpar.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Pecson BM, Nelson KL. Inactivation of Ascaris suum eggs by ammonia. Environ Sci Technol. 2005;39:7909–7914. doi: 10.1021/es050659a. [DOI] [PubMed] [Google Scholar]
- 14.Zaman V, Visuvalingam N. Action of aqueous iodine on ova of Ascaris lumbricoides and Ascaris suum. Trans R Soc Trop Med Hyg. 1967;61:443–444. [Google Scholar]
- 15.Labare MP, Soohoo H, Kim D, yan Tsoi K, Liotta JL, Bowman DD. Ineffectiveness of a quaternary ammonium salt and povidone-iodine for the inactivation of Ascaris suum eggs. Am J Infect Control. 2013;41:360–361. doi: 10.1016/j.ajic.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Wattal C, Malla N, Khan I, Agarwal S. Reversible inhibition of development and hatching of infective eggs of Ascaris lumbricoides var. hominis. J Parasitol. 1985;71:518. [PubMed] [Google Scholar]
- 17.Feachem R, Bradley D, Garelick H, Mara D. Ascaris and ascariasis. Sanitation and disease: health aspects of excreta and wastewater management. Washington DC, USA: World Bank; 1983. p. 391. [Google Scholar]
- 18.Borgsteede F. Effects of various disinfectants on the development and survival possibilities of the pre-parasitic stages of Ostertagia ostertagi, Cooperia oncophora and Ascaris suum. Tijdschr Diergeneesk. 1987;112:769–778. [PubMed] [Google Scholar]
- 19.Zamora JL. Chemical and microbiologic characteristics and toxicity of povidone-iodine solutions. Am J Surg. 1986;151:400–406. doi: 10.1016/0002-9610(86)90477-0. [DOI] [PubMed] [Google Scholar]
- 20.Lindquist TD, Maxwell AJ, Miller TD, Win'E TL, Novicki T, Fritsche TR, Iliakis B, Montoya M. Preparation of corneal donor eyes comparing 1% versus 5% povidone iodine. Cornea. 2011;30:333–337. doi: 10.1097/ICO.0b013e3181eeb5d2. [DOI] [PubMed] [Google Scholar]
- 21.Pelletier JS, Miller D, Liang B, Capriotti JA. In vitro efficacy of a povidone–iodine 0.4% and dexamethasone 0.1% suspension against ocular pathogens. J Cataract Refract Surg. 2011;37:763–766. doi: 10.1016/j.jcrs.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 22.Ito H, Ito T, Hikida M, Yashiro J, Otsuka A, Kida H, Otsuki K. Outbreak of highly pathogenic avian influenza in Japan and anti-influenza virus activity of povidone-iodine products. Dermatology. 2006;212:115–118. doi: 10.1159/000089210. [DOI] [PubMed] [Google Scholar]
- 23.Morrondo P, Díez-Morrondo C, Pedreira J, Díez-Baños N, Sánchez-Andrade R, Paz-Silva A, Díez-Baños P. Toxocara canis larvae viability after disinfectant-exposition. Parasitol Res. 2006;99:558–561. doi: 10.1007/s00436-006-0200-5. [DOI] [PubMed] [Google Scholar]
- 24.Verocai G, Tavares P, Ribeiro DA, Correia T, Scott F. Effects of disinfectants on Toxocara canis embryogenesis and larval establishment in mice tissues. Zoonoses Public Health. 2010;57:e213–e216. doi: 10.1111/j.1863-2378.2010.01330.x. [DOI] [PubMed] [Google Scholar]
- 25.Krishnaswami S, Post F. Effect of chlorine on Ascaris (Nematoda) eggs. Health Lab Sci. 1968;5:225. [PubMed] [Google Scholar]