Abstract
Lyme disease is a tick-borne zoonotic infectious disease caused by Borrelia burgdorferi. The present study assessed the infection status of B. burgdorferi among horses reared in Korea using ELISA and PCR. Between 2009 and 2013, blood samples were collected from 727 horses throughout Korea. Data for each animal including age, gender, breed, and region of sample collection were used for epidemiological analysis. Overall, 38 (5.2%; true prevalence: 5.5%) of 727 horses were seropositive by ELISA. There were statistically significant differences according to breed and region (P<0.001) whose differences might be attributed to the ecology of vector ticks and climate conditions. Using 2 nested PCR, none of the samples tested positive for B. burgdorferi. Thus, a positive ELISA result can indicate only that the tested horse was previously exposed to B. burgdorferi, with no certainty over the time of exposure. Since global warming is likely to increase the abundance of ticks in Korea, continuous monitoring of tick-borne diseases in Korean horses is needed.
Keywords: Borrelia burgdorferi, equine, ELISA, PCR, serology
The spirochete Borrelia burgdorferi is an agent of Lyme disease, which is an important tick-borne disease in humans and various animals [1]. B. burgdorferi is transmitted by the bite of Ixodes spp., and mammals and birds are reservoirs [2,3]. B. burgdorferi was isolated in New York, USA [4], and was at first thought to be homogenous, but is now known to comprise of at least 19 species, including Borrelia afzelii, Borrelia garinii, and Borrelia valaisiana [5]. Each species is characterized by their antigen structure and geographical distribution [6].
Most horses infected with Lyme disease are asymptomatic [7], but some show encephalitis [8], meningitis, cranial neuritis, radiculoneuritis [9], lameness [10], arthritis, panuveitis [11], and pseudolymphoma [12]. In infected humans, erythematous rash, neurological signs, and arthritis have been reported, but as with horses, some patients do not experience any specific symptoms [13]. According to the Centers for Disease Control and Prevention, 300,000 people are infected with Lyme disease every year in the United States alone, and this number is increasing.
Lyme disease has been reported in other countries of Asia, including China [14], Malaysia [15], and Japan [16]. In Korea, Park et al. [17] first reported B. burgdorferi infection from Ixodes ticks and mice, and until now, serological and molecular investigations have been performed in humans, dogs, and ticks [2,18,19]. According to Kim et al. [20], Haemaphysalis longicornis is the most prevalent tick species in Korea, but the existence of other species of Haemaphysalis and Ixodes has also been documented.
Recently, the equine industry in Korea has expanded, and the number of horses is increasing. However, only a few studies exist about disease distribution in horses, and none is concerned with tick-borne diseases [21,22]. Because of global warming and climatic changes, it is expected that tick-borne disease will become more prevalent in Korea [23]. Thus, this study was initiated to assess the infection status of B. burgdorferi, using ELISA and PCR, in horses reared in Korea.
Blood samples were collected from the jugular vein of 727 horses, from 2009 to 2013 throughout different localities of Korea. The overall number of horses in Korea is approximately 30,000. The sample size in this study was determined using the following formula, with an expected prevalence of 5%, and a desired absolute precision 5% with a simple random sampling design [24]:
, where n=required sample size, pexp =expected prevalence, and d=desired absolute precision. According to the formula, a minimum of 292 samples were needed, and the samples were collected from various regions of Korea. Data for each animal including age, gender, breed, and region of sampling were collected for epidemiological analysis. Cases with insufficient data were grouped as ‘unknown’. The studied areas were divided into 4 regions: northern, central, southern, and Jeju-do (Fig. 1).
Fig. 1.
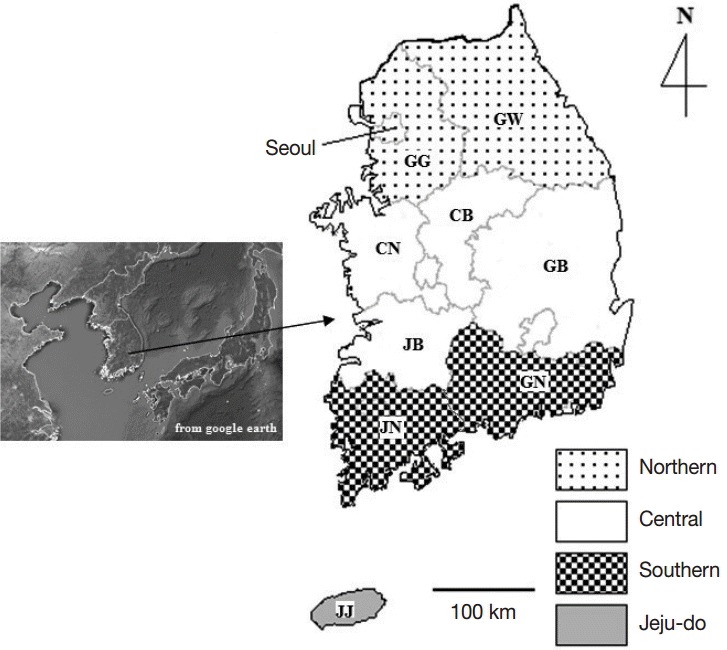
Map of Korea showing the studied regions where horse blood samples were collected. The provinces of Gyeonggi (GG) and Gangwon (GW) are classified as northern; the provinces of Chungbuk (CB), Chungnam (CN), Jeonbuk (JB), and Gyeongbuk (GB) as central; the provinces of Jeonnam (JN) and Gyeongnam (GN) as southern; and the province of Jeju island as Jeju-do (JJ).
To detect anti-B. burgdorferi antibodies in sera, the SNAP® 4Dx test (IDEXX Laboratories, Westbrook, Maine, USA) was used according to the manufacturer’s instructions. Although the SNAP® 4Dx test was originally manufactured to detect antibodies in dogs or cats, it also showed useful results to detect antibodies in horses [25,26]. In horses, the sensitivity and specificity of the SNAP® 4Dx test are 95% and 100% when compared with immunofluorescence assay [25].
For PCR, DNA was extracted from horse blood using the DNeasy® Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. To detect a specific gene of B. burgdorferi and increase the possibility of detection, nested PCR (nPCR) was carried out using 2 sets of primers; Bb23S3/Bb23Sa and Bb23S3nF/Bb23SanR which amplify a 226-266 bp fragment of B. burgdorferi 5S-23S rRNA intergenic spacer [27], and BbospF/BbospR and BbospnF/BbospR which amplify a 314 bp fragment of the B. burgdorferi ospC gene [4]. For each nPCR analyses, B. garinii DNA isolated from a dog-attached tick was included as a positive control. The amplicons were determined on 1.0% agarose gel stained with ethidium bromide.
The observed prevalence (OP) was calculated as the number of positive samples/total number of samples. The true prevalence (TP) was estimated using the following formula: TP=(OP+Sp-1)/(Se+Sp-1), where Se and Sp are sensitivity and specificity, respectively [28]. The chi-square or Fisher’s exact tests were used for statistical analysis among variables. The ‘unknown’ group was excluded from the statistical analysis. Statistical analyses were carried out using SPSS 21.0 (IBM Corporation, New York, USA), and P values <0.05 were regarded as significant. The 95% confidence interval for the adjusted prevalence of each estimate was calculated using Blaker’s method [28].
Out of 727 horse blood samples, 38 (5.2%; TP: 5.5%) tested positive for B. burgdorferi using ELISA (Table 1). None of the tested samples showed positivity for B. burgdorferi by 2 different nPCR experiments. The seroprevalence was 5.6% (11/197; TP: 5.9%), 1.7% (4/236; TP: 1.8%), 3.0% (6/200; TP: 3.2%), and 18.1% (17/94; TP: 19.0%) for horses aged <5 years, 5-10 years, >10 years, and of unknown age, respectively. With respect to gender, 3.2% (4/124; TP: 3.4%), 4.0% (10/250; TP: 4.2%), 2.7% (7/259; TP: 2.8%), and 18.1% (17/94; TP: 19.0%) of male, female, castrated, and unknown gender horses were seropositive, respectively. In terms of breed, 3.6% (17/477; TP: 3.8%), 15.6% (17/109; TP: 16.4%), 6.6% (4/61; TP: 6.9%), and 0% (0/80; TP: 0%) of serology results were positive for thoroughbreds, Korean native ponies, warm-bloods, and mixed breeds, respectively. Regarding regions, 2.4% (5/211; TP: 2.5%), 2.2% (4/179; TP: 2.4%), 4.9% (12/243; TP: 5.2%), and 18.1% (17/94; TP: 19.0%) were seropositive in the northern, central, southern, and Jeju-do regions, respectively. There were statistically significant differences according to breed and region (P <0.001).
Table 1.
Seroprevalence of Borrelia burgdorferi, determined using ELISA, in 727 horses according to age, gender, breed, and region of sample collection
| Group | No. tested | No. positive | Observed prevalence (%) | True prevalence (%) | 95% CI* | P-value | |
|---|---|---|---|---|---|---|---|
| Age (yr) | < 5 | 197 | 11 | 5.6 | 5.9 | 3.0-10.2 | 0.075 |
| 5-10 | 236 | 4 | 1.7 | 1.8 | 0.6-4.5 | ||
| > 10 | 200 | 6 | 3.0 | 3.2 | 1.4-6.6 | ||
| Unknown | 94 | 17 | 18.1 | 19.0 | 12.0-28.3 | ||
| Gender | Male | 124 | 4 | 3.2 | 3.4 | 1.2-8.2 | 0.710 |
| Female | 250 | 10 | 4.0 | 4.2 | 2.2-7.5 | ||
| Castrated | 259 | 7 | 2.7 | 2.8 | 1.3-5.7 | ||
| Unknown | 94 | 17 | 18.1 | 19.0 | 12.0-28.3 | ||
| Breed | Thoroughbred | 477 | 17 | 3.6 | 3.8 | 2.2-5.9 | < 0.001 |
| Korean native pony | 109 | 17 | 15.6 | 16.4 | 10.2-24.9 | ||
| Warmblood | 61 | 4 | 6.6 | 6.9 | 2.4-16.7 | ||
| Mixed | 80 | 0 | 0.0 | 0.0 | 0-4.7 | ||
| Region | Northern | 211 | 5 | 2.4 | 2.5 | 1.0-5.6 | < 0.001 |
| Central | 179 | 4 | 2.2 | 2.4 | 0.8-5.7 | ||
| Southern | 243 | 12 | 4.9 | 5.2 | 2.9-8.7 | ||
| Jeju-do | 94 | 17 | 18.1 | 19.0 | 12.0-28.3 | ||
| Total | 727 | 38 | 5.2 | 5.5 | 4.0-7.5 |
CI: confidence interval, calculated by Blaker’s method.
In this study, statistically significant differences were observed between our ELISA results in terms of region and breed. Climate conditions influence the distribution and abundance of ticks that transmit B. burgdorferi [29]. In Korea, lower latitude regions (the southern and Jeju-do regions) are warmer and receive more precipitation than the higher latitude regions (the northern and central regions); these factors are important in tick ecology. Therefore, the more favorable climate conditions in the southern and Jeju-do regions may increase tick abundance and result in greater exposure of horses to B. burgdorferi.
Although a statistically significant difference according to breed was observed in the ELISA results, the authors do not think that the seroprevalence was affected by breed. Korean native ponies and Jeju-do were the breed and region with the highest seroprevalence, respectively. Among the 109 Korean native pony samples, 94 (86.2%) were collected in Jeju-do, and all the seropositive samples were collected from Jeju-do (data not shown). Therefore, the higher seroprevalence in Korean native ponies is likely due to the higher seroprevalence in the Jeju-do region.
There were no statistically significant differences in the seroprevalence according to age and gender. Older horses might be expected to show higher seroprevalence than younger horses, as older horses are more likely to have been exposed to vector ticks with a reservoir of B. burgdorferi, and antibodies against B. burgdorferi can exist for long periods of time [30]. From our ELISA data and the fact that Lyme borreliosis is a tick-borne disease, the authors cautiously suggest that regional factors related to climate are more important than age and gender in rates of equine B. burgdorferi infection.
In contrast to the ELISA results, when tested using 2 different nPCR experiments, none of the samples tested positive. The authors attribute this to the properties of the PCR and ELISA used, which detect antigens and antibodies, respectively. Borchers et al. [13] reported that several weeks are needed for humans to develop antibodies against B. burgdorferi. In the same study, IgM was not detected until 1-2 weeks after infection; IgG required 4-6 weeks to develop, but then persisted for several years. Although there are no known data on B. burgdorferi antibody duration in horses, antibodies could persist for long periods of time, influenced by the timing and frequency of exposure to B. burgdorferi [30]. Therefore, a positive ELISA result can indicate only that the tested horse was previously exposed to B. burgdorferi, with no certainty over the time of exposure. Comparing the PCR and ELISA results from this study, the authors can conclude that most of the horses tested were exposed to B. burgdorferi at a previous time, but not at the current time.
To our knowledge, this study describes the nationwide prevalence of B. burgdorferi in horses reared in Korea for the first time. Conclusively, this study indicates that B. burgdorferi exists in Korean horses, and that the prevalence is greatest in Jeju-do region. Since global warming is likely to increase the abundance of ticks in Korea, continuous monitoring of tick-borne diseases in Korean horses is needed.
Acknowledgments
This research was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2013R1A1A2013102).
Footnotes
The authors report no conflict of interest related to this study.
REFERENCES
- 1.Skotarczak B. Why are there several species of Borrelia burgdorferi sensu lato detected in dogs and humans? Infect Genet Evol. 2014;23:182–188. doi: 10.1016/j.meegid.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Moon S, Gwack J, Hwang KJ, Kwon D, Kim S, Noh Y, Roh J, Shin EH, Jeong K, Seok WS. Autochthonous lyme borreliosis in humans and ticks in Korea. Osong Public Health Res Perspect. 2013;4:52–56. doi: 10.1016/j.phrp.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler CM, Houwers DJ, Jongejan F, Van Der Kolk JH. Borrelia burgdorferi infections with special reference to horses. A review. Vet Q. 2005;27:146–156. [PubMed] [Google Scholar]
- 4.Wang IN, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM, Luft BJ. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151:15–30. doi: 10.1093/genetics/151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannelli A, Bertolotti L, Gern L, Gray J. Ecology of Borrelia burgdorferi sensu lato in Europe: transmission dynamics in multi-host systems, influence of molecular processes and effects of climate change. FEMS Microbiol Rev. 2012;36:837–861. doi: 10.1111/j.1574-6976.2011.00312.x. [DOI] [PubMed] [Google Scholar]
- 6.Skotarczak B, Wodecka B. Identification of Borrelia burgdorferi genospecies inducing Lyme disease in dogs from western Poland. Acta Vet Hung. 2005;53:13–21. doi: 10.1556/AVet.53.2005.1.2. [DOI] [PubMed] [Google Scholar]
- 7.Burbelo PD, Bren KE, Ching KH, Coleman A, Yang X, Kariu T, Iadarola MJ, Pal U. Antibody profiling of Borrelia burgdorferi infection in horses. Clin Vaccine Immunol. 2011;18:1562–1567. doi: 10.1128/CVI.05123-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgess EC, Mattison M. Encephalitis associated with Borrelia burgdorferi infection in a horse. J Am Vet Med Assoc. 1987;191:1457–1458. [PubMed] [Google Scholar]
- 9.James FM, Engiles JB, Beech J. Meningitis, cranial neuritis, and radiculoneuritis associated with Borrelia burgdorferi infection in a horse. J Am Vet Med Assoc. 2010;237:1180–1185. doi: 10.2460/javma.237.10.1180. [DOI] [PubMed] [Google Scholar]
- 10.Browning A, Carter SD, Barnes A, May C, Bennett D. Lameness associated with Borrelia burgdorferi infection in the horse. Vet Rec. 1993;132:610–611. doi: 10.1136/vr.132.24.610. [DOI] [PubMed] [Google Scholar]
- 11.Burgess EC, Gillette D, Pickett JP. Arthritis and panuveitis as manifestations of Borrelia burgdorferi infection in a Wisconsin pony. J Am Vet Med Assoc. 1986;189:1340–1342. [PubMed] [Google Scholar]
- 12.Sears KP, Divers TJ, Neff RT, Miller WH, Jr, McDonough SP. A case of Borrelia-associated cutaneous pseudolymphoma in a horse. Vet Dermatol. 2012;23:153–156. doi: 10.1111/j.1365-3164.2011.01013.x. [DOI] [PubMed] [Google Scholar]
- 13.Borchers AT, Keen CL, Huntley AC, Gershwin ME. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82–115. doi: 10.1016/j.jaut.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Chengxu A, Yuxin W, Yongguo Z, Shaoshan W, Quicheng Q, Zhixue S, Deyou L, Dongquan C, Xiaodong L, Jienhua Z. Clinical manifestations and epidemiological characteristics of lyme disease in Hailin county, Heilongjiang Province, China. Ann NewYork Acad Sci. 1988;539:302–313. doi: 10.1111/j.1749-6632.1988.tb31864.x. [DOI] [PubMed] [Google Scholar]
- 15.Tay ST, Kamalanathan M, Rohani MY. Borrelia burgdorferi (strain B. afzelii) antibodies among Malaysian blood donors and patients. Southeast Asian J Trop Med Public Health. 2002;33:787–793. [PubMed] [Google Scholar]
- 16.Kawabata M, Baba S, Iguchi K, Yamaguti N, Russell H. Lyme disease in Japan and its possible incriminated tick vector, Ixodes persulcatus. J Infect Dis. 1987;156:854. doi: 10.1093/infdis/156.5.854. [DOI] [PubMed] [Google Scholar]
- 17.Park KH, Chang WH, Schwan TG. Identification and characterization of lyme disease spirochetes, Borrelia burgdorferi sensu lato, isolated in Korea. J Clin Microbiol. 1993;31:1831–1837. doi: 10.1128/jcm.31.7.1831-1837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung BY, Gebeyehu EB, Seo MG, Byun JW, Kim HY, Kwak D. Prevalence of vector-borne diseases in shelter dogs in Korea. Vet Rec. 2012;171:249. doi: 10.1136/vr.100650. [DOI] [PubMed] [Google Scholar]
- 19.Lim S, Irwin PJ, Lee S, Oh M, Ahn K, Myung B, Shin S. Comparison of selected canine vector-borne diseases between urban animal shelter and rural hunting dogs in Korea. Parasit Vectors. 2010;3:32. doi: 10.1186/1756-3305-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HC, Han SH, Chong ST, Klein TA, Choi CY, Nam HY, Chae HY, Lee H, Ko S, Kang JG, Chae JS. Ticks collected from selected mammalian hosts surveyed in the Republic of Korea during 2008-2009. Korean J Parasitol. 2011;49:331–335. doi: 10.3347/kjp.2011.49.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, Lee SE, Seo MG, Goo YK, Cho KH, Cho GJ, Kwon OD, Kwak D, Lee WJ. Evidence of Toxoplasma gondii exposure among horses in Korea. J Vet Med Sci. 2014;76:1663–1665. doi: 10.1292/jvms.14-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung BY, Lee KW, Ha TY. Seroprevalence of Leptospira spp. in clinically healthy racing horses in Korea. J Vet Med Sci. 2010;72:197–201. doi: 10.1292/jvms.09-0273. [DOI] [PubMed] [Google Scholar]
- 23.Chae JS, Adjemian JZ, Kim HC, Ko S, Klein TA, Foley J. Predicting the emergence of tick-borne infections based on climatic changes in Korea. Vector Borne Zoonotic Dis. 2008;8:265–276. doi: 10.1089/vbz.2007.0190. [DOI] [PubMed] [Google Scholar]
- 24.Thrusfield M. Veterinary Epidemiology. 3rd ed. Oxford, UK: Blackwell Publishing; 2005. pp. 228–246. [Google Scholar]
- 25.Chandrashekar R, Daniluk D, Moffitt S, Lorentzen L, Williams J. Serologic diagnosis of equine borreliosis: evaluation of an inclinic enzyme-linked immunosorbent assay (SNAP 4Dx) Intern J Appl Res Vet Med. 2008;6:145–150. [Google Scholar]
- 26.Hansen MG, Christoffersen M, Thuesen LR, Petersen MR, Bojesen AM. Seroprevalence of Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in Danish horses. Acta Vet Scand. 2010;52:3. doi: 10.1186/1751-0147-52-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu CY, Jiang BG, Liu W, Zhao QM, Wu XM, Zhang PH, Zhan L, Yang H, Cao WC. Presence of pathogenic Borrelia burgdorferi sensu lato in ticks and rodents in Zhejiang, south-east China. J Med Microbiol. 2008;57:980–985. doi: 10.1099/jmm.0.47663-0. [DOI] [PubMed] [Google Scholar]
- 28.Reiczigel J, Földi J, Ózsvári L. Exact confidence limits for prevalence of a disease with an imperfect diagnostic test. Epidemiol Infect. 2010;138:1674–1678. doi: 10.1017/S0950268810000385. [DOI] [PubMed] [Google Scholar]
- 29.Léger E, Vourc’h G, Vial L, Chevillon C, McCoy KD. Changing distributions of ticks: causes and consequences. Exp Appl Acarol. 2013;59:219–244. doi: 10.1007/s10493-012-9615-0. [DOI] [PubMed] [Google Scholar]
- 30.Divers TJ, Grice AL, Mohammed HO, Glaser AL, Wagner B. Changes in Borrelia burgdorferi ELISA antibody over time in both antibiotic treated and untreated horses. Acta Vet Hung. 2012;60:421–429. doi: 10.1556/AVet.2012.036. [DOI] [PubMed] [Google Scholar]


