Abstract
This study investigated the relationship between education and physical activity and the difference between a physiological prediction of age and chronological age. Cortical and subcortical grey matter regional volumes were calculated from 331 healthy adults (range: 19-79 years). Multivariate analyses identified a covariance pattern of brain volumes best predicting chronological age (CA)(R2 = 47%). Individual expression of this brain pattern served as a physiologic measure of brain age (BA). The difference between CA and BA was predicted by education and self-report measures of physical activity. Education and the daily number of flights of stairs climbed were the only two significant predictors of decreased brain age. Effect sizes demonstrated that brain age decreased by 0.95 years for each year of education and by 0.58 years for one additional daily FOSC. Effects of education and FOSC on regional brain volume were largely driven by temporal and subcortical volumes. These results demonstrate that higher levels of education and daily FOSC are related to larger brain volume than predicted by chronological age which supports the utility of regional grey matter volume as a biomarker of healthy brain aging.
Keywords: healthy aging, lifestyle, grey matter volume, brain age, education, physical activity
1 Introduction
Grey matter volume decline is a highly visible aspect of the chronological aging process resulting from neural shrinkage and neuronal loss (Terry et al., 1987). These neural changes are detectable with magnetic resonance imaging (MRI) as volumetric declines in subcortical regions and throughout the cortical mantel (Dale et al., 1999; Sowell et al., 2004). Although volumetric decline is a common aspect of aging, the rate and degree of decline is highly variable across regions of the brain and between individuals (Raz et al., 2010). Furthermore, differences in lifetime exposures, such as years of education or physical activity, have been associated with differential amounts of grey matter volumetric decline with advancing age (Ahlskog et al., 2011; Erickson et al., 2010; Nithianantharajah and Hannan, 2009).
Interindividual variability in genetics and development along with positive and negative effects of lifetime exposure will result in different quantities of brain volume loss. Several investigators have suggested the concept of physiological brain age where the difference between chronological age and predicted age, based on brain measures, serves as a more informative marker of brain health than chronological age alone (Franke et al., 2010; Irimia et al., 2014). Regional brain volume measures would be useful for calculating a physiological brain age measurement.
In this study we used regional measures of grey matter volume from 331 healthy adults across the lifespan to derive a biomarker of brain age. We defined the difference between chronological age and brain age as a marker of whether the brain is younger or older than expected. We then investigated whether this difference was related to lifetime exposures including years of education and self-reported assessments of physical activity. Such relationships would suggest that certain lifetime exposures help maintain the brain in a more “youthful” state.
2 Materials and Methods
2.1 Participants
Data from 331 healthy adults between the ages of 19 and 79 were included in this study. Participants were drawn from three different studies from our laboratory using the same testing apparatus, procedures and MRI. Table 1 lists the number of participants by decade, sex and study. Participants were recruited using market-mailing procedures from within 10 miles of our northern Manhattan, New York USA site to equalize the recruitment approaches across the lifespan. Participants who responded to the mailing were telephone screened to ensure that they met basic inclusion criteria (right handed, English speaking, no psychiatric or neurological disorders, normal or corrected-to-normal vision). All participants found eligible via the initial telephone screen were further screened in person with structured medical, neurological, psychiatric, and neuropsychological evaluations to ensure that they had no neurological or psychiatric disease or cognitive impairment. The screening procedure included a detailed interview that excluded individuals with a self-reported history of major or unstable medical illness, significant neurological history (e.g. epilepsy, brain tumor, stroke), history of head trauma with loss of consciousness for greater than five minutes or history of Axis I psychiatric disorder (Association, 1994). Individuals taking psychotropic medications were also excluded. Global cognitive functioning was assessed with the Mattis Dementia Rating Scale, on which a score of at least 133 was required for retention in the study (Mattis, 1988). This study was approved by the Internal Review Board of the College of Physicians and Surgeons of Columbia University and written informed consent was obtained from all participants prior to study participation, and after the purpose and risks of the study were explained. Participants were compensated for their participation in the study.
Table 1. Sample sizes split by decade, sex and study.
| Age Group | Total N F/M |
Study 1 N F/M |
Study 2 N F/M |
Study 2&3 N F/M |
Study 3 N F/M |
|---|---|---|---|---|---|
| > 30 | 43/21 | 9/4 | 10/5 | 10/3 | 14/9 |
| 30-39 | 32/19 | 3/1 | 2/1 | 4/1 | 23/16 |
| 40-49 | 14/19 | 0/0 | 0/0 | 0/0 | 14/19 |
| 50-59 | 20/23 | 0/0 | 1/0 | 0/1 | 19/22 |
| 60-69 | 46/45 | 7/9 | 16/10 | 15/19 | 8/7 |
| 70 and older | 27/22 | 1/0 | 4/2 | 2/2 | 20/18 |
| Total | 182/149 | 20/14 | 33/18 | 31/26 | 98/91 |
2.2 Measures of Lifetime Exposures
Lifetime exposures included measures of education and physical activity. Education was assessed as the number of years engaged in formal education. Physical activity was assessed using a questionnaire containing nine questions about the amount of time spent doing various physical activities. These activities included: walking/hiking, jogging, running, bicycling, aerobic exercise, lap swimming, tennis/squash/racquetball, low intensity exercise and flights of stairs climbed daily (FOSC). The flights of stairs climbed daily was coded as: None, 1-2, 3-4, 5-9, 10-14 and more than 15. These values were coded as 0, 1.5, 3.5, 7, 12 and 16. The other questions referred to the amount of time spent within a week and were coded as: None, 1-19 minutes, 20-50 minutes, 1 hour, 1.5 hours, 2-3 hours, 4-6 hours and over 7 hours. This questionnaire is similar to that used in other assessments of physical activity (Chao et al., 2004; Thacker et al., 2008). For all physical activities these measures were converted to Metabolic Equivalent of Task (MET) (Ainsworth et al., 2000; Bassett et al., 1997) and a total MET score was calculated.
2.3 Image Acquisition Procedure
MRI images were acquired in a 3.0T Philips Achieva Magnet using a standard quadrature head coil. A T1-weighted scout image was acquired to determine the participant's position. One hundred sixty five contiguous 1 mm coronal T1-weighted images of the whole brain were acquired for each participant with an MPRAGE sequence using the following parameters: TR 6.5 ms, TE 3 ms; flip angle 8°, acquisition matrix 256 × 256 and 240 mm field of view. A neuroradiologist reviewed the anatomical scans to identify any potentially clinically significant findings.
2.4 FreeSurfer Methods
Each participant's structural T1 scans were reconstructed using FreeSurfer (Fischl, 2012) (http://surfer.nmr.mgh.harvard.edu/). The accuracy of Freesurfer's subcortical segmentation and cortical parcellation (Fischl et al., 2004, 2002) has been reported to be comparable to manual labeling. Each participant's white and grey matter boundaries as well as grey matter and cerebrospinal fluid boundaries were visually inspected slice by slice by an experienced user, manual control points were added in the case of any visible discrepancy, and reconstruction was repeated until we reached satisfactory results within every participant. The subcortical structure borders were plotted by Freeview visualization tools and compared against the actual brain regions. In case of discrepancy, they were corrected manually. In total this procedure quantified 84 regions, 16 subcortical and 68 cortical.
2.5 Scaled subprofile modeling
We used the scaled subprofile modeling (SSM) (Moeller et al., 1987; Spetsieris and Eidelberg, 2010) approach to calculate physiological brain age (BA) based on regional grey matter volumes from cortical and subcortical locations using the principal components analysis (PCA) toolbox (http://groups.google.-com/group/gcva) (Habeck et al., 2005; Habeck and Stern, 2007). Briefly, the eighty-four grey matter volume regions of interest were subjected to a principal component analysis. This produced a series of principal component images, vi, and their respective subject scaling factors (SSFi), which are each individual's expression of that respective principal component. The number of components comprising approximately 95% of the variance of the structural data were retained. Predicted Age (pAge) was calculated by regressing the SSF's taken from this subset of principal components against chronological age (CA) controlling for total intracranial volume, study (which of the studies they were recruited for) and sex:
Both sex and study were dummy-coded, with female and study 1 as the references. The weights from the SSF variables were used to combine the respective eigenimages, vi, to produce an age-related brain covariance pattern. This was then projected back into the original data to calculate the expression of this pattern for each participant. This value served as the Brain Age (BA) measure. The stability of the regions within the resultant pattern was assessed using 1,000 bootstrap resamples and tested with bias-corrected, accelerated confidence intervals.
For each individual, CA-BA indicated whether their brain age was older or younger than their chronological age. Positive values indicate their brain age was younger than their chronological age would predict, while a negative value indicates the brain age is older than their chronological age. We then tested whether the measures of education and physical activity were related to this difference.
Similar to assessing regional contributions to the BA map, regional contributions to the CA-BA relationship with education and physical activity were also calculated. The stability of the regions was assessed using 1,000 bootstrap resamples and tested with bias-corrected, accelerated confidence intervals. Regions were identified as being significantly related to the lifetime exposure measures if they had a Z score magnitude greater than 2 and had effect sizes greater than 1/84 of the total effect. An effect size of 1/84 of the total effect for each brain region signifies that each brain region is contributing equally to the overall covariance pattern and there is no regional concentration of the effect.
3 Results
3.1 Global Results
The first thirty-eight principal components comprised 95% of the total variance in the regional grey matter volume structural data. In total 64% of chronological age related variance was accounted for, 47% by the individual expression of the covariance pattern and an additional 17% by the nuisance regressors of total intracranial volume, study and sex. Statistics for the nuisance regressors were: Total intracranial volume (B = 58.7, t(324) = 5.52, p < 0.001), sex (B = 9.81, t(324) = 6.89, p < 0.001) and study (R2 = 0.007, F(3,324) = 2.10, p = 0.10). Study was dummy coded according to which study the participant was part of or whether they were part of two studies, see Table 1. A scatter plot of chronological age versus brain age is shown in Figure 1.
Figure 1.
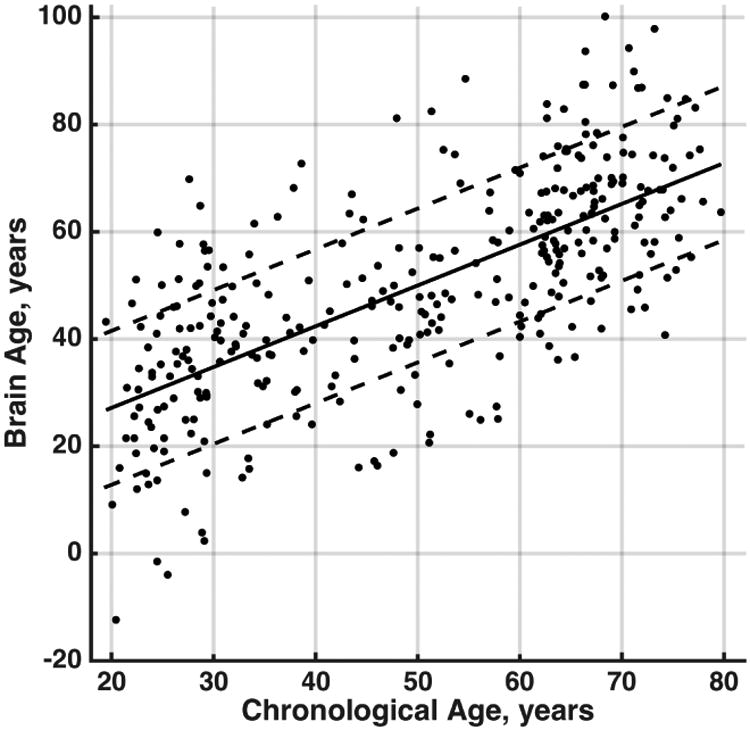
Scatter plot of chronological age in years versus brain age in years after adjusting for nuisance variables. The solid line is the regression fit between the two variables and the dashed lines are one standard deviation away from the regression fit.
Education and MET scores for each of the nine physical activities were regressed against the difference between chronological age and brain age (CA - BA). The overall F-test was significant for this model (F(10, 320) = 2.14, p = 0.021). CA-BA was significantly related to education (B = 0.95, t(320) = 2.84, p = 0.0048) and FOSC (B = 0.58, t(320) = 2.84, p = 0.0048). All other measures were non-significant: walking/hiking (B = 0.041, t(320) = 0.40, p = 0.69), jogging (B = 0.16, t(320) = 1.32, p = 0.19), running (B = -0.042, t(320) = -0.36, p = 0.72), bicycling (B = 0.055, t(320) = 0.56, p = 0.57), aerobic exercise (B = -0.063, t(320) = -0.82, p = 0.41), lap swimming (B = -0.072, t(320) = -0.55, p = 0.58), tennis/squash/racquetball (B = 0.004, t(320) = 0.027, p = 0.98) and low intensity exercise (B = -0.031, t(320) = -0.13, p = 0.90). The combined effect of one additional year of education combined with one additional flight of stairs climbed per day was related to a 1.53 year younger brain than chronological age would predict.
The non-significant regressors were removed and the regression analysis redone. The overall F-test was still significant for this model (F(2, 328) = 9.45, p < 0.001). CA-BA was significantly related to education (B = 0.99, t(328) = 3.014, p = 0.0028) and FOSC (B = 0.60, t(328) = 3.06, p = 0.0024). The reduction in variance accounted for was not significant (difference in R2 = 0.0083, F(8, 320) = 0.36, p = 0.94).
The correlation table between the nine physical activity measures and age is shown in Table 2. With increasing age there is a decrease in the amount of time engaged in jogging, running and aerobic exercise. Also included in Table 2 is the proportion of participants engaged in each activity after a median split (age 52) into young and old adults. This reiterates the age effect on the activities, but it also demonstrates that walking and stair climbing are activities engaged in by the vast majority of participants regardless of age. The significant relationships between FOSC and BA-CA demonstrate a dose effect of stair climbing.
Table 2. Correlations and group engagement proportions for the markers of physical activity.
| Age | Walk/hike | Jog | Run | Bicycle | Aerobic | Swim | Tennis | Low Intensity | FOSC | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1.00 | |||||||||
| Walk/hike | -0.01 | |||||||||
| Jog | -0.16** | 0.09 | ||||||||
| Run | -0.26** | 0.14* | 0.41** | |||||||
| Bicycle | 0.07 | 0.08 | 0.05 | 0.04 | ||||||
| Aerobic | -0.11* | 0.13* | 0.28** | 0.17** | 0.06 | |||||
| Swim | 0.03 | 0.01 | 0.04 | -0.04 | -0.03 | -0.06 | ||||
| Tennis | -0.01 | -0.08 | 0.07 | 0.04 | 0.01 | -0.04 | 0 | |||
| Low Intensity | 0.00 | 0.21** | 0.12* | 0.17** | 0.16** | 0.36** | -0.08 | -0.01 | ||
| FOSC | 0.00 | 0.22** | -0.03 | -0.01 | 0.15** | 0.06 | 0.02 | 0.08 | -0.01 | |
| Engagement | ||||||||||
| Young (<52) | 0.98 | 0.39 | 0.39 | 0.23 | 0.54 | 0.07 | 0.07 | 0.63 | 0.93 | |
| Old (>52) | 0.98 | 0.15 | 0.07 | 0.21 | 0.42 | 0.1 | 0.05 | 0.53 | 0.88 | |
Notes: FOSC = Flights of stairs climbed;
p < 0.05,
p < 0.01
3.2 Regional Results
Regional contributions to the CA-BA relationship with education and FOSC are shown in Table 3 and overlays in Figure 2. For education the effects included bilateral putamen, right amygdala, left lateral orbitofrontal gyrus, right superior frontal gyrus, right frontal pole, left precuneus, right precentral gyrus, bilateral superior temporal gyri, left middle temporal gyrus, left supramarginal gyrus and right lateral occipital gyrus.
Table 3. Regional relationships between the CA-BA difference and education and FOSC.
| Measure | Region | Hemi. | Z | B | pTotal |
|---|---|---|---|---|---|
| Education | Putamen | L | 2.10 | 0.045 | 7.6 |
| Putamen | R | 3.34 | 0.071 | 12.2 | |
| Amygdala | R | 2.55 | 0.083 | 14.3 | |
| Lateral OrbitoFrontal | L | 2.35 | 0.106 | 18.3 | |
| Superior Frontal | R | 2.39 | 0.035 | 6.1 | |
| Frontal Pole | R | 4.14 | 0.035 | 6.0 | |
| Precuneus | L | 2.00 | 0.059 | 10.1 | |
| Precentral | R | 3.26 | 0.026 | 4.5 | |
| Superior Temporal | L | 3.08 | 0.083 | 14.3 | |
| Superior Temporal | R | 2.66 | 0.075 | 12.9 | |
| Middle Temporal | L | 2.02 | 0.059 | 10.1 | |
| Supramarginal | L | 2.27 | 0.041 | 7.0 | |
| Lateral Occipital | R | 2.30 | 0.082 | 14.1 | |
| FOSC | Amygdala | R | 2.35 | 0.120 | 12.4 |
| Accumbens | R | 2.11 | 0.013 | 1.4 | |
| Rostral Middle Frontal | R | 2.48 | 0.024 | 2.5 | |
| Caudal Anterior Cingulate | L | 2.08 | 0.013 | 1.4 | |
| Caudal Anterior Cingulate | R | 2.24 | 0.014 | 1.4 | |
| Posterior Cingulate | R | 2.03 | 0.013 | 1.3 | |
| Rostral Anterior Cingulate | R | 2.29 | 0.012 | 1.2 | |
| Precuneus | L | 2.07 | 0.040 | 4.2 | |
| Paracentral | R | 2.97 | 0.020 | 2.1 | |
| Inferior Temporal | R | 2.13 | 0.024 | 2.5 | |
| Middle Temporal | L | 2.45 | 0.087 | 9.0 | |
| Transverse Temporal | R | 2.53 | 0.012 | 1.2 | |
| Insula | R | 2.24 | 0.018 | 1.9 |
Notes: Hemi. = Hemisphere; FOSC = Flights of stairs climbed; Z is calculated using 1,000 bootstrap resamples and bias-corrected, accelerated confidence intervals, beta is the effect with units of years and pTotal is the percent of the total effect each region represents. The total education effect is 0.97 and for FOSC is 0.58.
Figure 2.
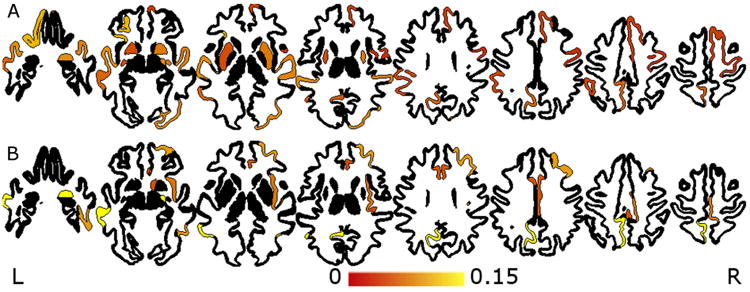
Overlay of effect sizes for significant regions for education (A) and flights of stairs climbed (B).
For FOSC, the effects were observed in the right amygdala, right accumbens, right rostral middle frontal gyrus, bilateral caudal anterior cingulate, right rostral anterior and posterior cingulate, left precuneus, right paracentral gyrus, right inferior and transverse temporal gyrus, left middle temporal gyrus and the right insula.
4 Discussion
Brain aging can be conceptualized as comprising two mechanisms, the inevitable and universal effects of advancing age and the effects resulting from a lifetime of exposures. Lifetime exposures comprise both negative effects related to unhealthy lifestyle and injuries and positive effects resulting from a healthy lifestyle and enriched environments. The culmination of a lifetime of genetic, developmental and lifetime exposures produces large variation in the physiological age of our brains. Having a physiological measurement of the age of the brain provides a means for assessing the effect of a lifetime of exposures on the brain. Not only does the CA-BA measure provide a more individualistic assessment, it could also provide a means of identifying exposures associated with maintenance of a more youthful brain in late life.
The current work used grey matter regional brain volumes as measures to compute a physiological age, (i.e. brain age). This approach may be generalized to include other brain-based measures (e.g. white matter volumes, white matter hyperintensity burden, amyloid buildup) as well as non-brain based measures such as blood-based markers (e.g., complete blood count (CBC) panels). Inclusion of additional measures would provide a more global estimation of physiological age and will likely account for a larger amount of the variance in chronological age.
The results of this study suggest that individual levels of education and FOSC have a positive effect on the brain. Increased values on either of these lifetime exposure measures were associated with significant increases in the CA-BA measure. Maintaining the structure of the brain in a younger state may have profound effects on delaying the onset of age-related neurodegenerative diseases.
Education and FOSC are associated with larger brain volume than predicted by chronological age supporting the idea of brain maintenance (Nyberg et al., 2012). Brain maintenance describes the preservation of brain measures (including cellular, neurochemical, volumetric and systems-level activation patterns) in the face of advancing age and is argued to be a primary indicator of successful cognitive aging. Therefore, higher levels of education and FOSC daily are related to larger regional brain volumes, with respect to others with lower education and FOSC. Thus suggesting that education and FOSC lead to the maintenance of brain volumes at a more youthful state.
It is important to stress that the current data are from a cross-sectional study; therefore, causality cannot be tested. The theoretical framework underlying the present study is based on literature showing that certain lifetime exposures, such as education and physical activity, have a positive effect on the aging brain. The findings from the present study support this theory. However, it is also possible that larger brain volumes may predispose individuals to obtain greater levels of education and to seek out physical activity. Longitudinal and intervention studies may address the causal nature of the current findings and tease apart the concepts of predisposition and maintenance. For instance, brain age, calculated using measurements obtained early in life, compared to lifetime educational attainment and levels of physical activity, obtained later in life, could be used to address the question of predisposition. A natural experiment comparing brain age measures at the start of adulthood to brain age measures calculated after completion of educational attainment, would be useful in understanding whether educational attainment altered brain age. This would test the theory that education contributes to brain maintenance. Likewise, an intervention study over a shorter time scale, using a physical activity protocol, could identify whether or not the intervention altered the rate of brain age decline, helping to further our understanding of brain maintenance.
The pattern of brain regions showing a relationship with lifetime exposures is similar to the pattern of brain regions demonstrating the most substantial age-related declines (Raz et al., 1997). This suggests that these brain regions, in particular the temporal cortex, are more sensitive to the effects of aging, but also sensitive to the lifetime exposures which may counteract some of the aging effects. The temporal cortex had significantly large contributions to the education and FOSC related increases in the CA-BA measure. A previous aerobic walking study with older adults showed that increased functional connectivity was found within the temporal gyri and was associated with increased levels of brain-derived neurotrophic factor (Voss et al., 2012). Atrophy within these regions has also been implicated as an early indicator of Alzheimer's disease (Convit et al., 2000). Exercise is noted to moderate the amount of age-related atrophy within the temporal gyri (Bugg and Head, 2011). The volume of these regions also mediate age-related cognitive performance on memory strategies (Kirchhoff et al., 2014). Furthermore, greater walking distance predicted greater amounts of grey matter volume in regions of the occipital, temporal and frontal lobes (Erickson et al., 2010).
Identification of biomarkers of healthy normal aging is an important step in understanding neurodegenerative decline (Jack et al., 2010). Using biomarkers of healthy aging could provide a measurement scale to identify deviation from normal. Their use may be particularly informative with respect to intervention studies. A biomarker of healthy aging could also provide a metric for identifying the effects of intervention programs.
Years of education and daily number of flights of stairs climbed are both straightforward measures. The ease of collecting these measurements is to their advantage; however, they also each have drawbacks. Years of education does not assess quality of education (Manly et al., 2002). It also does not include age of acquisition of education. While a large bolus of education is typically acquired early in life, the acquisition of higher education is not mandated to the young. Individuals that acquire 16 continuous years of education may differ from those that acquired their higher education later in life. Unfortunately, our measure does not make such distinctions.
The number of flights of stairs climbed daily represents a proxy of a type of physical activity that has been used as a health-related fitness tool (Kennedy et al., 2007). These authors demonstrate that stair climbing enhances cardiovascular fitness. Stair climbing as an exercise also meets the minimum requirements for cardiorespiratory benefits and serves as a viable exercise, suitable for promoting physical activity (Teh and Aziz, 2002). In assessments of the validity of stair climbing, it was shown to not be significantly related to VO2 max assessed using a treadmill test in 138 participants (Resnicow et al., 2003). In another study of 76 post-menopausal, sedentary women using the P-PAQ, sweat index was a better marker of measured kilocalories of energy expenditure than stair climbing (Laporte et al., 1983). The greatest support for the FOSC measure comes from the Harvard Alumni Health Study. This study demonstrated that in 13,485 participants, the number of flights of stairs climbed and distance walked independently and significantly were related to decreased mortality rates by up to 25 percent. Physical activity at light, moderate or vigorous levels had no significant effects (Lee and Paffenbarger, 2000). Despite the validity of stair climbing as a physical activity, we measured it as a self-report which is inherently problematic (Prince et al., 2008). Despite this limitation, the other measures of physical activity were also self-report and were not significantly related to our difference between chronological age and brain age.
The assessment of flights of stairs climbed is an item from the Paffenbarger Physical Activity Questionnaire (P-PAQ) (Paffenbarger et al., 1993). Overall this is reliable and valid assessment of physical activity; however, there is limited evidence comparing the self-reported number of flights of stairs climbed against measured flights climbed. Future research will need to validate self reported stair climbing against actual measurements, when accuracy of pedometers increases for free-living conditions (De Cocker et al., 2012; Zhu and Lee, 2010).
Our results demonstrate that years of education and stair climbing, not other measures of physical activity, were related to the difference between chronological age and brain age. This raises the question of what makes stair climbing different from other forms of physical activity in its relationship with brain health. One difference between stair climbing and other activities may be motivational. Many physical activities, e.g. running or swimming, are done out of personal desire. Climbing a flight of stairs may also be motivated from personal desire, e.g. for health reasons, and may be considered engagement in optional physical activity. On the other hand, climbing the stairs may be a physical activity engaged in out of necessity. In our cohorts, climbing stairs may reflect housing or transportation situations. The cohorts for this study were recruited from northern Manhattan, with a predominance of apartment buildings, and residential northern New Jersey, with a predominance of single family homes. Someone may live in a multi-level home and going up and down stairs is simply a fact of daily life. Someone may also live in a multi-level apartment building, with no elevator, where again climbing flights of stairs is a fact of daily life. Public transportation in this region often involves ascending and descending flights of stairs to reach train platforms. A potentially complicating factor is that differences in housing may represent socio-economic differences. In an observational study, pedestrians in higher socio-economic regions had a significantly higher rate of optionally taking the stairs than pedestrians in lower socio-economic regions (Ryan et al., 2011).
The nature of stair climbing also differs from many other forms of physical activity. Climbing stairs is often done repeatedly throughout the day, on most if not all days of the week, and represents small bouts of moderately intense physical activity. Engagement of the other activities we assessed typically occur less frequently throughout a typical week and for longer durations. Future work with measures of stair-climbing will attempt to tease apart the motivation and socio-economical differences behind taking the stairs. This will be useful in determining which aspect has the greatest impact on brain age.
Stair-climbing is also used as a marker of mobility. In a large sample of older adults, education was significantly related to stair-climbing (Sainio et al., 2007). The authors discussed that the pathway of this effect appeared to be common chronic diseases, such as smoking and obesity. The lower educated participants had greater levels of these common diseases which limited their stair climbing ability. In our sample there was no significant relationship between education and FOSC (r = 0.02), likely due to our eligibility screening.
Even with the limitations of the education and FOSC measures, they are both significantly related to the difference between chronological and biological age. Future work will improve the quantification of education and physical activity to more deeply probe the roles of these lifetime exposures, as well as exploring dosage effects of education and physical activity (Erickson et al., 2010).
Interestingly, engagement differs across the various physical activities. There are also correlations between the various measures and age. The more vigorous activities have lower numbers of participants engaging in them with advancing age. However, energy expenditure due to FOSC is unrelated to age. Engagement in FOSC, regardless of duration, is also unaffected by age. Therefore, FOSC is a physical activity that is engaged in by a majority of adults. Current results suggest there may be a dose response with respect to estimated brain age. Many people are already climbing the stairs at least once per day, our results suggest that climbing more flights of stairs per day may offer even greater benefit. This is encouraging because it demonstrates that FOSC has great potential as an intervention tool to promote brain health. There already exist many “Take the stairs” campaigns in office environments and public transportation centers. The current results demonstrate that these campaigns should also be expanded for older adults.
Prediction of chronological age using physiological measurements is gaining interest and is demonstrating its usefulness in the literature. Currently, there are multiple methods of deriving a brain age estimate which are applicable for identifying differential rates of aging (Bunge and Whitaker, 2012; Franke et al., 2010; Wachinger et al., 2015), tracking recovery from traumatic brain injuries (Cole et al., 2015) and in measuring brain development in patients with multiple sclerosis (Aubert-Broche et al., 2014). When predicting chronological age, other methods have explained more variance in their data then in the methods employed in this work (Cole et al., 2015; Franke et al., 2010; Wang et al., 2014). This is likely due to the voxel/vertex wise nature of their approaches employing hundreds of thousands of brain measurements per participant. These techniques are quite powerful; however, they suffer from high sensitivity to accurate registrations between data and templates and processing steps. Franke et al. (2010) discuss and demonstrate the sensitivity of their measure to preprocessing choices (Franke et al., 2010).
The current approach used 84 brain measurements per participant using FreeSurfer extraction of regional volumes. This attempts to circumvent error due to preprocessing sensitivity using a robust extraction method that preserves the data in the original native collected space without excessive transformations. The current work also used the scaled subprofile modeling approach (Moeller et al., 1987). This method is straightforward and well established in the imaging field with demonstrated reproducibility (Moeller et al., 1999). Derived covariance patterns, like those produced here, can serve as biomarkers of the healthy aging brain and may be forward applied to new datasets (Brickman et al., 2008). This is in common with the other described brain age calculations.
Using 95% of the variance in the brain data, this approach accounted for 47% of the variance in chronological age. While other methods may account for larger amounts of variance in their data, our preference was to minimize sensitivity to processing choices and to use established methods from the literature. It is also anticipated that the greatest increase in variance accounted for in chronological age will come from the combination of multiple physiological measurements.
5 Conclusions
Differences between chronological and brain age were significantly related to years of education and the daily number of flights of stairs climbed. These results support the idea that more education and climbing a greater number of stairs are healthy lifestyle habits as reflected by regional brain volumes being larger than expected by chronological age. The identified full brain pattern of brain age also provides a biomarker which may be useful for predicting whether brain age is consistent or inconsistent with chronological age.
Highlights.
Four years of education is related to reduced predicted age of the brain by nearly four years.
One additional flight of stairs climbed per day is related to a decreased predicted brain age by 7 months.
Education and physical activity appear to help maintain the brain at a more youthful state.
Acknowledgments
This research was supported by grants from the National Institute on Aging (AG035061, PI Dr. Steffener, AG044467, PI Dr. Razlighi, and AG026158, AG038465, PI Dr. Stern). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The sponsors had no role in the study design, data collection, analysis or interpretation, writing of the report, or decision to submit the article for publication.
Footnotes
Disclosure Statement: All authors verify that they have no conflict of interest related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86:876–884. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press; 1994. [Google Scholar]
- Aubert-Broche B, Fonov V, Narayanan S, Arnold DL, Araujo D, Fetco D, Till C, Sled JG, Banwell B, Collins DL Canadian Pediatric Demyelinating Disease Network. Onset of multiple sclerosis before adulthood leads to failure of age-expected brain growth. Neurology. 2014;83:2140–2146. doi: 10.1212/WNL.0000000000001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DR, Vachon JA, Kirkland AO, Howley ET, Duncan GE, Johnson KR. Energy cost of stair climbing and descending on the college alumnus questionnaire. Med Sci Sports Exerc. 1997;29:1250–1254. doi: 10.1097/00005768-199709000-00019. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Habeck C, Ramos MA, Scarmeas N, Stern Y. A forward application of age associated gray and white matter networks. Hum Brain Mapp. 2008;29:1139–1146. doi: 10.1002/hbm.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg JM, Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol Aging. 2011;32:506–514. doi: 10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Whitaker KJ. Brain imaging: your brain scan doesn't lie about your age. Curr Biol. 2012;22:R800–1. doi: 10.1016/j.cub.2012.07.032. [DOI] [PubMed] [Google Scholar]
- Chao A, Connell CJ, Jacobs EJ, McCullough ML, Patel AV, Calle EE, Cokkinides VE, Thun MJ. Amount, type, and timing of recreational physical activity in relation to colon and rectal cancer in older adults: the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2004;13:2187–2195. [PubMed] [Google Scholar]
- Cole JH, Leech R, Sharp DJ For the Alzheimer's Disease Neuroimaging Initiative. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol. 2015 doi: 10.1002/ana.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convit A, de Asis J, de Leon MJ, Tarshish CY, De Santi S, Rusinek H. Atrophy of the medial occipitotemporal, inferior, and middle temporal gyri in non-demented elderly predict decline to Alzheimer's disease<. Neurobiol Aging. 2000;21:19–26. doi: 10.1016/s0197-4580(99)00107-4. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- De Cocker KA, De Meyer J, De Bourdeaudhuij IM, Cardon GM. Non-traditional wearing positions of pedometers: validity and reliability of the Omron HJ-203-ED pedometer under controlled and free-living conditions. J Sci Med Sport. 2012;15:418–424. doi: 10.1016/j.jsams.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Gach HM, Thompson PM, Ho AJ, Kuller LH. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology. 2010;75:1415–1422. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Franke K, Ziegler G, Klöppel S, Gaser C Alzheimer's Disease Neuroimaging Initiative. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage. 2010;50:883–892. doi: 10.1016/j.neuroimage.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Habeck C, Krakauer JW, Ghez C, Sackeim HA, Eidelberg D, Stern Y, Moeller JR. A new approach to spatial covariance modeling of functional brain imaging data: ordinal trend analysis. Neural Comput. 2005;17:1602–1645. doi: 10.1162/0899766053723023. [DOI] [PubMed] [Google Scholar]
- Habeck C, Stern Y. Neural network approaches and their reproducibility in the study of verbal working memory and Alzheimer's disease. Clin Neurosci Res. 2007;6:381–390. doi: 10.1016/j.cnr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia A, Torgerson CM, Goh SYM, Van Horn JD. Statistical estimation of physiological brain age as a descriptor of senescence rate during adulthood. Brain Imaging Behav. 2014 doi: 10.1007/s11682-014-9321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RA, Boreham CAG, Murphy MH, Young IS, Mutrie N. Evaluating the effects of a low volume stairclimbing programme on measures of health-related fitness in sedentary office workers. J Sports Sci Med. 2007;6:448–454. [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff BA, Gordon BA, Head D. Prefrontal gray matter volume mediates age effects on memory strategies. Neuroimage. 2014;90:326–334. doi: 10.1016/j.neuroimage.2013.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte RE, Black-sandler R, Cauley JA, Link M, Bayles C, Marks B. The Assessment of Physical Activity in Older Women: Analysis of the Interrelationship and Reliability of Activity Monitoring, Activity Surveys, and Caloric Intake. J Gerontol. 1983;38:394–397. doi: 10.1093/geronj/38.4.394. [DOI] [PubMed] [Google Scholar]
- Lee IM, Paffenbarger RS. Associations of Light, Moderate, and Vigorous Intensity Physical Activity with Longevity: The Harvard Alumni Health Study. Am J Epidemiol. 2000;151:293–299. doi: 10.1093/oxfordjournals.aje.a010205. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y. Reading level attenuates differences in neuropsychological test performance between African American and White elders. J Int Neuropsychol Soc. 2002;8:341–348. doi: 10.1017/s1355617702813157. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia rating scale. Psychological assessment resources; 1988. [Google Scholar]
- Moeller JR, Nakamura T, Mentis MJ, Dhawan V, Spetsieres P, Antonini A, Missimer J, Leenders KL, Eidelberg D. Reproducibility of regional metabolic covariance patterns: comparison of four populations. J Nucl Med. 1999;40:1264–1269. [PubMed] [Google Scholar]
- Moeller JR, Strother SC, Sidtis JJ, Rottenberg DA. Scaled subprofile model: a statistical approach to the analysis of functional patterns in positron emission tomographic data. J Cereb Blood Flow Metab. 1987;7:649–658. doi: 10.1038/jcbfm.1987.118. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. The neurobiology of brain and cognitive reserve: mental and physical activity as modulators of brain disorders. Prog Neurobiol. 2009;89:369–382. doi: 10.1016/j.pneurobio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L. Memory aging and brain maintenance. Trends Cogn Sci. 2012;16:292–305. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Jr, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Resnicow K, McCarty F, Blissett D, Wang T, Heitzler C, Lee RE. Validity of a modified CHAMPS physical activity questionnaire among African-Americans. Med Sci Sports Exerc. 2003;35:1537–1545. doi: 10.1249/01.MSS.0000084419.64044.2B. [DOI] [PubMed] [Google Scholar]
- Ryan J, Lyon K, Webb OJ, Eves FF, Ryan CG. Promoting physical activity in a low socioeconomic area: results from an intervention targeting stair climbing. Prev Med. 2011;52:352–354. doi: 10.1016/j.ypmed.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Sainio P, Martelin T, Koskinen S, Heliövaara M. Educational differences in mobility: the contribution of physical workload, obesity, smoking and chronic conditions. J Epidemiol Community Health. 2007;61:401–408. doi: 10.1136/jech.2006.048306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Spetsieris PG, Eidelberg D. Scaled subprofile modeling of resting state imaging data in Parkinson's disease: methodological issues. Neuroimage. 2010;54:2899–2914. doi: 10.1016/j.neuroimage.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh KC, Aziz AR. Heart rate, oxygen uptake, and energy cost of ascending and descending the stairs. Med Sci Sports Exerc. 2002;34:695–699. doi: 10.1097/00005768-200204000-00021. [DOI] [PubMed] [Google Scholar]
- Terry RD, DeTeresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Ann Neurol. 1987;21:530–539. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- Thacker EL, Chen H, Patel AV, McCullough ML, Calle EE, Thun MJ, Schwarzschild MA, Ascherio A. Recreational physical activity and risk of Parkinson's disease. Mov Disord. 2008;23:69–74. doi: 10.1002/mds.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS, Alves H, Szabo A, Phillips SM, Wójcicki TR, Mailey EL, Olson EA, Gothe N, Vieira-Potter VJ, Martin SA, Pence BD, Cook MD, Woods JA, McAuley E, Kramer AF. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun. 2012;28:90–99. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachinger C, Golland P, Kremen W, Fischl B, Reuter M Alzheimer's Disease Neuroimaging Initiative. BrainPrint: A discriminative characterization of brain morphology. Neuroimage. 2015;109:232–248. doi: 10.1016/j.neuroimage.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li W, Miao W, Dai D, Hua J, He H. Age estimation using cortical surface pattern combining thickness with curvatures. Med Biol Eng Comput. 2014;52:331–341. doi: 10.1007/s11517-013-1131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Lee M. Invariance of wearing location of Omron-BI pedometers: a validation study. J Phys Act Health. 2010;7:706–717. doi: 10.1123/jpah.7.6.706. [DOI] [PubMed] [Google Scholar]


