Abstract
Catecholaminergic polymorphic ventricular tachycardia (CPVT) predisposes to ventricular arrhythmia due to altered Ca2+ homeostasis and can arise from ryanodine receptor (RyR2) mutations including RyR2-P2328S. Previous reports established that homozygotic murine RyR2-P2328S (RyR2S/S) hearts show an atrial arrhythmic phenotype associated with reduced action potential (AP) conduction velocity and sodium channel (Nav1.5) expression. We now relate ventricular arrhythmogenicity and slowed AP conduction in RyR2S/S hearts to connexin-43 (Cx43) and Nav1.5 expression and Na+ current (INa). Stimulation protocols applying extrasystolic S2 stimulation following 8 Hz S1 pacing at progressively decremented S1S2 intervals confirmed an arrhythmic tendency despite unchanged ventricular effective refractory periods (VERPs) in Langendorff-perfused RyR2S/S hearts. Dynamic pacing imposing S1 stimuli then demonstrated that progressive reductions of basic cycle lengths (BCLs) produced greater reductions in conduction velocity at equivalent BCLs and diastolic intervals in RyR2S/S than WT, but comparable changes in AP durations (APD90) and their alternans. Western blot analyses demonstrated that Cx43 protein expression in whole ventricles was similar, but Nav1.5 expression in both whole tissue and membrane fractions were significantly reduced in RyR2S/S compared to wild-type (WT). Loose patch-clamp studies similarly demonstrated reduced INa in RyR2S/S ventricles. We thus attribute arrhythmogenesis in RyR2S/S ventricles resulting from arrhythmic substrate produced by reduced conduction velocity to downregulated Nav1.5 reducing INa, despite normal determinants of repolarization and passive conduction. The measured changes were quantitatively compatible with earlier predictions of linear relationships between conduction velocity and the peak INa of the AP but nonlinear relationships between peak INa and maximum Na+ permeability.
Keywords: RyR2, CPVT, Nav1.5, Arrhythmogenicity, Conduction velocity, Ca2+ homeostasis
Introduction
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited arrhythmic syndrome characterized by episodic syncope and/or sudden cardiac arrest, typically triggered by adrenergic stimulation as occurs during strenuous exercise or emotional stress [1, 34, 46]. CPVT has been associated with mutations in various Ca2+ handling proteins, which lead to abnormalities in Ca2+ homeostasis; most notably, these mutations are found in the cardiac ryanodine receptor-Ca2+ release channel (RyR2) [25, 35] and the sarcoplasmic reticulum (SR) Ca2+-binding protein calsequestrin 2 (CASQ2) [13, 19]. Ca2+-dependent calmodulin missense mutations also occur in a small number of cases [18]. Some RyR2 mutations also predispose to atrial arrhythmias [4, 34, 39]. For example, the RyR2-P2328S mutation is associated with high incidences of both CPVT and atrial tachycardia (AT) [25, 37, 40]. This RyR2 variant has been associated with a normal luminal SR Ca2+ release sensitivity but an increased sensitivity to cytosolic Ca2+ [31], giving rise to lower cytosolic Ca2+ thresholds leading to Ca2+ release. If reached during increased heart rates, these could be sufficient to elicit a ‘leak’ of SR Ca2+ consequently triggering arrhythmia.
The atrial and ventricular phenotypes are replicated by the RyR2-P2328S (RyR2S/S) murine model which shows potential arrhythmic triggers in the form of delayed after-depolarizations [15, 22]. However, there remains a requirement for an electrophysiological tissue substrate in order to perpetuate and sustain arrhythmia, which has previously been typified by a reduced conduction velocity (θ) in systems showing reduced Nav1.5 expression [33], connexin 40 and/or 43 (atria) or connexin 43 (ventricle) expression [16, 23], or structural abnormalities, including fibrosis [44]. Interestingly, several reports have indicated the potential for Nav1.5 expression [6, 11, 42] and function [2, 41] to be modulated, both directly and indirectly, by alterations in cytosolic Ca2+. Rat cardiomyocytes showed reductions in Na+ channel activity following imposed increases of intracellular [Ca2+]. Additionally, the Ca2+ channel blocker verapamil and the Ca2+ ionophore calcimycin increased and decreased Nav1.5 mRNA and Nav1.5 protein expression respectively [11, 42]. In agreement with these findings, elevations and reductions of cytosolic [Ca2+], by chronic treatment with high extracellular [Ca2+] and [K+] or BAPTA-AM, decreased and increased Na+ current densities, respectively [6]. More recently, Casini et al. [5] demonstrated that acute increases in pipette [Ca2+] were capable of reducing both Na+ current density and (dV/dt)max.
Biochemical evidence accounting for the potential mechanisms of functional modulation of Nav1.5 by cytosolic [Ca2+] identifies both direct and indirect Ca2+ binding sites on Nav1.5. Direct Ca2+ binding to Nav1.5 is mediated at an EF hand motif resident at the carboxy-terminal region of Nav1.5 [47]. This binding results in a depolarizing shift of the voltage dependence of Na+ channel inactivation with a potential increase in Na+ channel activity [47]. Indirect mechanisms of Ca2+ binding have been attributed to both the presence of an additional binding site, the ‘IQ’ domain, within the C-terminal region of Nav1.5 for Ca2+/Calmodulin (Ca2+/CaM) and multiple phosphorylatable sites (including serines 516 and 571 and threonine 594) within the IDI-II linker region of Nav1.5 targeted by Ca2+/CaM Kinase II (CaMKII). These two mechanisms occur only subsequent to Ca2+ binding to the EF hand motifs of Ca2+/Calmodulin (Ca2+/CaM) or Ca2+/CaM Kinase II (CaMKII) and thus constitutes an indirect interaction of Ca2+ with Nav1.5. Ca2+/CaM binding at the IQ domain and CaMKII-dependent phosphorylation shifts Na+ current availability to a more depolarized membrane potential [2] and enhances slow inactivation of the Na+ current [41].
Recent reports have indeed implicated reduced Nav1.5 expression and Na+ channel function in the increased arrhythmogenicity in RyR2S/S atria [21, 22, 36]. They also demonstrated a reduced conduction velocity [22], resulting from a reduced Na+ current attributable either to a reduced Nav1.5 expression or the direct inhibitory effect on Na+ channel function of altered Ca2+ homeostasis outlined previously. Slowed conduction resulting from reduced Nav1.5 expression would potentially produce arrhythmogenic substrate, which would compound the arrhythmic effect of Ca2+-mediated triggered activity in the RyR2S/S [21, 22, 36].
The present study investigates for possible roles of Cx43 expression as well as Nav1.5 expression and function in RyR2S/Sventricular, as opposed to atrial, arrhythmogenicity. First, the arrhythmogenic properties of the RyR2S/S ventricle compared to the WT was confirmed in accordance with earlier reports [15] and correlated with measurements of action potential duration (APD), conduction velocity (θ) and their alternans, as well as ventricular effective refractory period (VERP). The stimulation protocols either interposed extrasystolic, S2, stimuli at progressively decremented S1S2 intervals within 8 Hz S1 pulse trains or applied steady stimulus frequencies at progressively decremented basic cycle lengths (BCLs). Second, to assess the underlying mechanism for the slowed conduction and arrhythmic phenotype, we assessed the ventricular expression of Cx43 and Nav1.5, the latter assessed in both the whole ventricle and the membrane fraction compared between WT and RyR2S/S hearts. Third, the corresponding functional evaluation of Nav1.5 was investigated through peak INa current recordings of WT and RyR2S/S ventricular tissue. These comparisons successfully correlated Nav1.5 expression and function, particularly within the functional Nav1.5 containing membrane fraction, with the incidence of ventricular arrhythmia, and the resulting conduction changes in RyR2S/S ventricles.
Materials and methods
Experimental animals
Homozygous RyR2S/S and WT mice (aged 4 to 6 months) with an inbred 129/Sv genetic background (supplied initially by Harlan, UK) were generated as described previously [15]. Mice were kept in plastic cages at room temperature in an animal facility, given free access to sterile rodent chow and water and subjected to 12 h light/dark cycles. Mice were killed by cervical dislocation for experimentation. All procedures conformed to the UK Animals (Scientific Procedures) Act 1986, approved by a university ethics review board. Hearts were rapidly excised and submerged in ice-cold Krebs-Henseleit (KH) buffer solution (containing, in mM, NaCl 119, NaHCO3 25, KCl 4, KH2PO4 1.2, MgCl2 1, CaCl2 1.8, glucose 10 and Na-pyruvate 2, pH 7.4, 95 % O2/ 5 % CO2; British Oxygen Company, Manchester, UK) for whole heart electrophysiological studies and Western blot analyses. All chemicals were purchased from Sigma-Aldrich (Poole, Dorset, UK), unless otherwise stated. Six WT and seven RyR2S/S mice were used in whole heart electrophysiological investigations. Four WT and four RyR2S/S hearts were used for Western blot studies of Cx43 expression. Seven WT and six RyR2S/S mice were used for Western blot studies of Nav1.5 expression in the whole tissue and membrane fraction. Four WT and three RyR2S/S mice were used to give n = 6 and 12 patches, respectively, for loose patch-clamp studies of Na+ currents. Male and female mice were used in statistically equal numbers in each group.
Electrophysiological studies in whole heart
Excised hearts were cannulated and retrograde perfused on a Langendorff system as previously described [28, 29, 38, 48]. Prior to electrophysiological testing, hearts were perfused with KH solution for at least 5 min to achieve a steady state. Monophasic action potentials (MAPs) were recorded by microMAP electrodes (HugoSachs, Harvard Apparatus, UK) placed upon the epicardial surface. Recordings were amplified (Neurolog NL100 preamplifier; NL104 amplifier, Digitimer, Welwyn Garden City, UK), band-pass filtered (NL125/126 filter; 0.5 Hz to 1.0 kHz) and sampled at 5 kHz (micro1401 interface) for display using Spike2 software (Cambridge Electronic Design, Cambridge, UK).
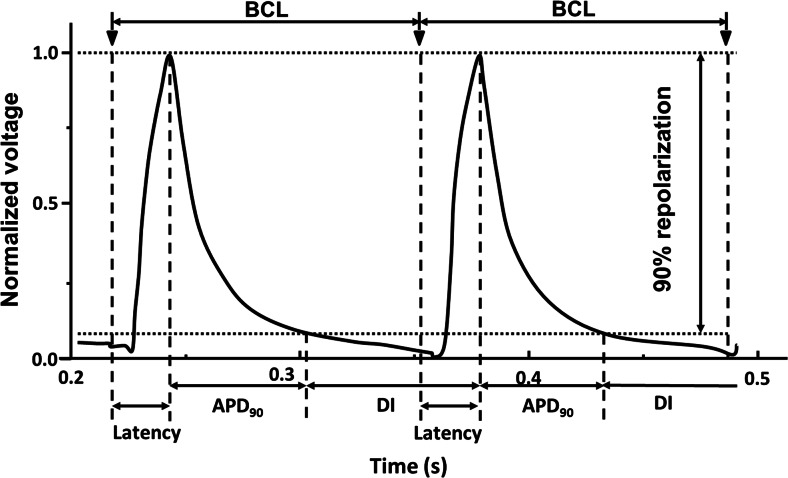
Hearts were paced at twice their excitation threshold voltage using a bipolar platinum-coated stimulating electrode placed on the ventricular septum connected to a DS2A-Mk.II stimulator (Digitimer). After pacing at 8 Hz for at least 5 min to attain and confirm a steady state, two types of pacing protocols were applied. A S1S2 protocol first stimulated hearts at frequencies of 10 Hz for 20 s; this was followed by cycles of drive trains of eight S1 beats delivered at 8 Hz followed by an S2 extra-stimulus, at S1-S2 coupling interval successively reduced by 1 ms with each subsequent cycle until either 2:1 block or sustained arrhythmia occurred. A dynamic pacing protocol [24, 28, 29] first assessed action potential (AP) properties at a BCLs of 134 ms duration for 100 stimulations. The BCL was then decremented by 5 ms, and the pacing sequence repeated until the hearts showed either entry into 2:1 block or sustained arrhythmia. Both stimulation protocols yielded incidences of arrhythmia. The S1S2 protocol additionally provided VERPs. The dynamic pacing protocol yielded APDs, and an indication of conduction velocity θ’ (1/latency, which was measured as the time from the stimuli to the peak of the MAP) as a function of BCL, measured as the recovery time from AP peak to 90 % full repolarization, APD90 (Fig. 1). The corresponding diastolic intervals (DIs) were calculated from the BCL and APD90 values using the relationship:
Fig. 1.
Two typical successive monophasic action potential (MAP) recordings at the LV epicardium of a WT heart obtained during dynamic pacing to highlight the derivation of the various parameters used for analysis; BCL, latency, APD90 and DI. BCL was the time interval between the adjacent stimuli, thus the pacing rate. Latency was measured as the time elapsed from the stimuli to the peak of MAP. APD90 is the time course over which 90 % repolarization of the MAPs obtained. DI was measured as BCL-APD90, thus comprising the final 10 % of MAP repolarization and up to the start of the next stimuli
Protein extraction and Western blot analysis
The method of protein extraction was optimized for the protein of interest. For connexin proteins, which are primarily found in the surface membrane in hexameric clusters, a well-established high content sodium dodecyl sulphate (SDS) buffer [7, 12, 43] was used in order to fully solubilize the membrane and maximize release of the connexin proteins from the gap junction channels in the plasma membrane. For Nav1.5 channels, we chose to use a milder buffer, followed by a centrifugation step and solubilization in order to separate out the membrane and non-membrane fractions based on the different distribution and abundance of Nav1.5 channels and their contribution to conduction.
Ventricular tissues of the excised hearts were snap-frozen and crushed into powder by a clamp pre-cooled with liquid N2. The powdered tissue was homogenized in either SB20 (20 % SDS, 2 mM EDTA, 150 mM Tris) [7, 12, 43] and diluted to an appropriate gel loading concentration in sample loading buffer (90 % SB20, 5 % 2-mercaptoethanol, 5 % w/v bromophenol blue) for connexin 43 analysis, or the re-suspension buffer (containing, in mM, Tris–HCl 50, NaCl 10, Sucrose 320, EDTA 5, EGTA 2.5 and Protease inhibitors (1 tablet/20 ml; Roche, West Sussex, UK), pH 7.4) and lysis buffer (containing, in mM, Tris–HCl 20, EDTA 2, NaCl 137 and Triton X-100 1 %, glycerol 10 %, pH 7.4) and then centrifuged for 15 min at 13,000g and 4 °C for Nav1.5 analysis. The supernatant was divided into two parts. One was stored at −80 °C as the whole tissue fraction and the rest was centrifuged at 100,000g at 4 °C for an hour to extract the membrane proteins. The pellet was re-suspended in radioimmunoprecipitation assay (RIPA) lysis buffer and then vortexed and placed on ice for 30 min to harvest the membrane protein.
For Western blot analysis, the protein extracts from whole tissue and the membrane fraction were separated on premade 4–12 % Bis-Tris Gels (Invitrogen, Paisley, UK) and then transferred onto PVDF membranes (Immobilon-P, Millipore, Hertfordshire, UK). Blots were blocked in 5 % skimmed milk in TBST (Tris-buffered saline; Invitrogen, Paisley, UK and Tween 20) and then probed with either anti-Nav1.5 (rabbit anti-mouse IgG, 1:1000, Alomone, Jeruselam, Israel) or anti-Cx43 (rabbit anti-mouse, 1:10,000, C6219 Sigma-Aldrich) and anti-GAPDH (Abcam, Cambridge, UK) or anti-α-tubulin (Cell Signaling Technology, Danvers, MA, USA) antibodies overnight at 4 °C. Horseradish peroxidase (HRP)-conjugated secondary antibody (Abcam, 1:10000 to 1:50000) were detected using an enhanced chemiluminescent system (GE Healthcare, Little Chalfont, Bucks, UK). Specific protein bands were quantified using Image J (National Institutes of Health, Wash., USA).
Loose patch clamp recordings of INa in ventricular tissue
Loose patch clamp experiments previously described for atrial tissue [21, 36] were performed in a right ventricular tissue preparation. This technique allows for measurement of Na+ currents in whole, perfused ventricular tissue preparations where intracellular Ca2+ homeostasis is not disrupted by cell isolation. Furthermore, the tissue preparation allows a more reliable comparison with APD90 and CV measurements of this study. Activation properties were assessed in order to determine peak INa. The activation protocol utilized a series of 75 ms duration depolarizing pulses, incremented by 10 mv steps ranging from 20 to 120 mV excursions applied 5 ms after the beginning of the sampling period using a P/4 pulse protocol [3].
Statistical analysis
Statistical analysis for differences between experimental groups was performed using Graphpad Prism software (La Jolla, CA 92037 USA), applying unpaired Student’s t tests. A value of P < 0.05 was considered statistically significant. Data are presented as means ± SEM.
Results
Comparison of ventricular arrhythmogenicity in S1S2 protocols and dynamic protocols
We initially confirmed the arrhythmogenic phenotype of the RyR2S/S murine heart as previously reported [15]. An S1S2 stimulation protocol was used to determine the incidence, frequency and duration of ventricular arrhythmia in isolated Langendorff-perfused hearts.
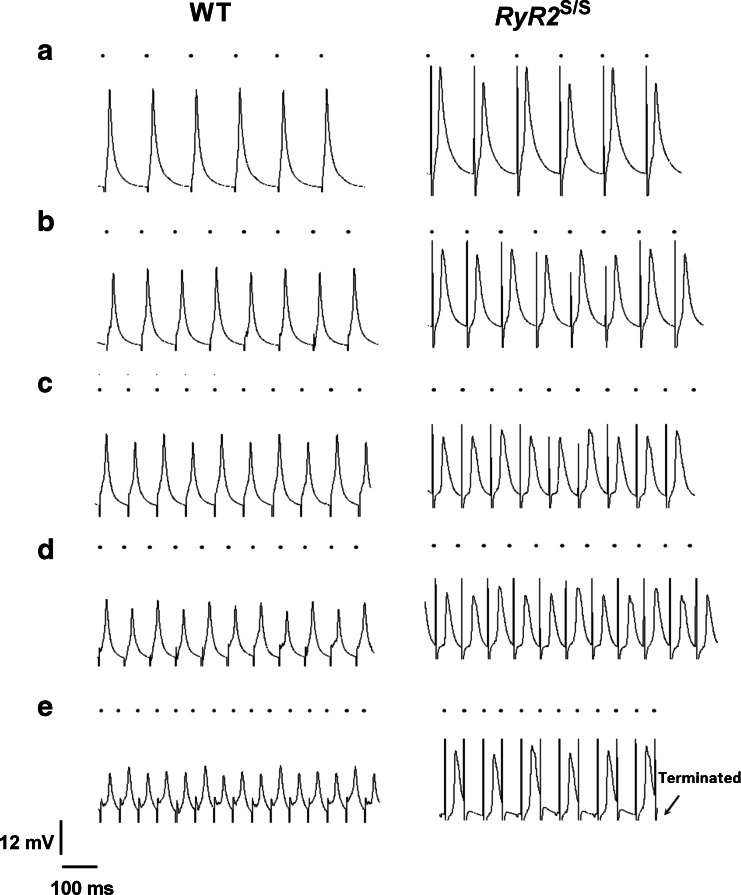
The occurrence of ventricular tachycardia (VT) was defined as an occurrence of two or more sequential spontaneous APs, as in previous work [36] in the course of programmed electrical stimulation. Figure 2 illustrates representative left ventricular epicardial traces from WT (A, B) and RyR2S/S (C-F) hearts, displaying regular activity (A), ectopic (B) and VT (C-E) and ventricular fibrillation (VF) (F) episodes during the S1S2 protocol. Six WT and seven RyR2S/S hearts were subject to the S1S2 protocol described in methods. None of the WT hearts showed VT, although one heart showed three separate ectopic events (at S1S2 intervals of 33, 31 and 30 ms). In contrast, the RyRS/S hearts showed 30 episodes of arrhythmia, taking the form of either polymorphic or monomorphic VT in three of the hearts. Of these hearts, the first showed 7 episodes of VT, lasting approximately 2.8 s, with an additional VT episode that degenerated into VF lasting approximately 22.5 s. The second heart showed one episode of VT lasting approximately 11.2 s. The third heart showed 21 episodes of VT accounting for a total time of approximately 14 s.
Fig. 2.
MAP traces obtained from the epicardium of the left ventricle in WT and RyR2 S/S hearts during S1S2 pacing highlighting typical traces of either normal activity or arrhythmogenesis. All WT hearts entered the refractory period without displaying any episodes of arrhythmia, as defined by two or more non-stimulated APs (a); however, one heart displayed the occurrence of a singular ectopic (one non-stimulated AP) (b). Multiple arrhythmic events were observed in RyR2 S/S hearts including short non-sustained ventricular tachycardia (NSVT) (c), polymorphic tachycardia following a previously imposed S2 extrastimulus (d), monomorphic ventricular tachycardia (VT) (e) and episodes of VT which deteriorated into ventricular fibrillation (VF) (F). The small black circles indicate the timing of stimuli
Ventricular effective refractory periods (VERPs) were defined as the time period during which the myocardium is incapable of re-excitation in response to the twice-threshold stimulus employed in the S1S2 protocols [10, 32]. It was thus the S1S2 interval at which loss of S2 capture first occurred in an absence of VT. WT and RyR2S/S hearts typically became refractory at similar S1S2 pacing intervals (VERP: WT: 38.2 ± 1.55 ms (n = 6); RyR2S/S: 37.5 ± 5.04 ms (n = 5); P = 0.9057). VERP could not be ascertained from all the mice studied due to arrhythmogenesis: sustained arrhythmias occurred in two of the seven RyR2S/S hearts during the S1S2 protocol.
The differing arrhythmic properties of WT and RyR2S/S were further confirmed in the dynamic pacing protocol, which subjected hearts to systematically decreasing BCLs. Two of the six WT hearts showed VT at BCLs of 39 and 44 ms. However, these correspond to BCLs which are substantially lower, thus a much higher heart rate than those experienced during normal physiological maximal exercise [8]. RyR2S/S hearts not only demonstrated higher incidences of VT and VF but they occurred also at higher BCLs than WT (54, 64, 54 and 74 ms in four RyR2S/S hearts respectively; these necessitated termination of the protocol), suggesting a reduced capacity to tolerate increased heart rates as may be observed during emotional or physical stress such as exercise [8].
Action potential properties and conduction at varying pacing rates
AP propagation and recovery properties at different BCLs were then investigated using the dynamic pacing protocol. Figure 3 illustrates typical APs thus obtained from the LV epicardium of WT (left column) and RyR2S/S hearts (right column). In both cases, AP amplitude decreased with increasing pacing rate in every heart, independent of genotype (Fig. 3a–e). At lower pacing rates, RyR2S/S hearts showed a higher incidence of alternans (Fig. 3a, right column) compared with WT hearts (Fig. 3c, left column). Around half of both the WT and the RyR2S/S hearts had shown either a loss of capture or arrhythmogenesis when the BCL reached 54 ms (Fig. 3e).
Fig. 3.
Typical MAP recordings obtained from the left ventricular epicardium of WT and RyR2 S/S during dynamic pacing. Traces from WT (left) and RyR2 S/S at progressively decreasing BCLs: 124 (a), 99 (b), 84 (c), 74 (d) and 54 ms (e). If a heart entered 2:1 block, the protocol was terminated (E). Traces are displayed along a common horizontal timescale. The vertical scale was normalized to a standard AP deflection at a BCL of 134 ms. Small black circles above each trace indicate the timing of stimuli
Figure 4 plots averaged (mean ± SEM) APD90 (A, C) and θ’ (=1/latency) (B, D) values in WT (filled symbols) and RyR2S/S (open symbols) hearts against BCL (A, B) and DI (C, D). At BCLs, where significant differences between readings at the two genotypes were obtained, this is indicated (*P < 0.05; **P < 0.01). Both WT and RyR2S/S showed similar (P > 0.05) values of APD90 at each BCL and DI. These both declined with decreasing BCL and DI. RyR2S/S and WT hearts additionally showed 2:1 block at similar values of BCL (WT 56.5 ± 5.95 ms, n = 4; RyR2S/S: 54 ± 2.5 ms, n = 3; P = 0.751). As with VERP for the S1S2 protocol, 2:1 block was not obtained from all the mice studied; this was due to arrhythmogenesis warranting termination of the dynamic pacing protocol in four of the seven RyR2S/S hearts and two of six WT hearts. In contrast to similar APD90, at equivalent BCLs, RyR2S/S hearts showed consistently lower θ ’ than WT hearts at equivalent BCLs. Indeed, the highest mean θ’ showed by the RyR2S/S (0.043 ± 0.003 m s−1), which was observed at the highest BCL, was similar to the lowest θ’ (0.042 ± 0.006 m s−1) shown by the WT, which was observed at the shortest BCLs. These findings were corroborated when the APD90 and θ’ values were plotted against their preceding DIs reflecting recovery times from the preceding APs (C, D). The present findings demonstrate normal AP repolarization characteristics, but compromised AP conduction in the RyR2S/S arrhythmic phenotype, which could arise from abnormalities in gap junction channels and/or Nav1.5.
Fig. 4.
Plots of APD90 and θ’ at different BCLs and DIs in WT and RyR2 S/S hearts. a, b Mean (± SEM) values for APD90 and θ’ respectively at different BCLs (134, 129, 124, 119, 114, 109, 104, 99, 94, 89, 84, 79, 74 and 69 ms) for WT (n = 6, filled symbols) and RyR2 S/S (n = 7, open symbols) hearts. c, d Mean (± SEM) values for APD90 and θ’, respectively, at different DIs for WT (n = 6, filled symbols) and RyR2 S/S (n = 7, open symbols) hearts. APD90 is virtually superimposable at all BCLs between WT and RyR2 S/S hearts and thus shows no statistically significant variation. However, θ’ is consistently lower in RyR2 S/S hearts as compared to WT hearts with significant differences between the genotypes denoted by asterisks; *P < 0.05 and **P < 0.01
Alternans of electrophysiological parameters, reflecting temporal variability, often presages arrhythmic activity. Figure 5 assesses the average (mean ± SEM) degree of alternans in AP amplitude (A, D) [30], APD90 (B, E) and θ’ (C, F) at different BCLs (A-C) and DIs (D-F) in WT (filled symbols) and RyR2S/S (open symbols) hearts. Alternans reflects system instability through the mean difference between alternating high and low values of a given parameter normalized to the mean value of the parameter. Both the RyR2S/S and the WT demonstrated similarly increasing AP amplitude instabilities with either decreasing BCL or decreasing DI. RyR2S/S and WT showed similar APD90 and θ’ instabilities which similarly varied with decreasing BCL or DI. θ’ instabilities were relatively small in contrast to the large changes in their mean values described.
Fig. 5.
Plots of alternans at different BCLs and DIs. The mean (± SEM) alternans characteristics of AP amplitude (a, d), APD90 (b, e) and θ’ (c, f) for WT (filled symbols) and RyR2 S/S (open symbols) hearts have been plotted as percentage variation between each beat as a function of BCL (a–c) and DI (d, e) AP magnitude displays an increasing degree of alternans with decreasing BCL and DI; however, this does not vary between genotypes. Similarly, a small degree of alternans is observed in the APD90 and less so the θ’ with decreasing BCL and DI, but these do not vary significantly between the WT and RyR2 S/S hearts
These findings implicate abnormal conduction as opposed to abnormal repolarization in the RyR2S/S ventricular arrhythmic phenotype. The underlying mechanism/cause of this abnormal conduction is thus investigated in the next sections.
Cx43 expression is comparable between the ventricles of WT and RyR2S/S murine hearts
Abnormal cardiac conduction can arise from one of three factors: abnormal connexin expression/gap junction formation, impaired Na channel function and/or structural abnormalities such as with fibrosis or hypertrophy. Due to the structural similarity of WT and RyR2S/S hearts [49], we pursued the remaining two factors.
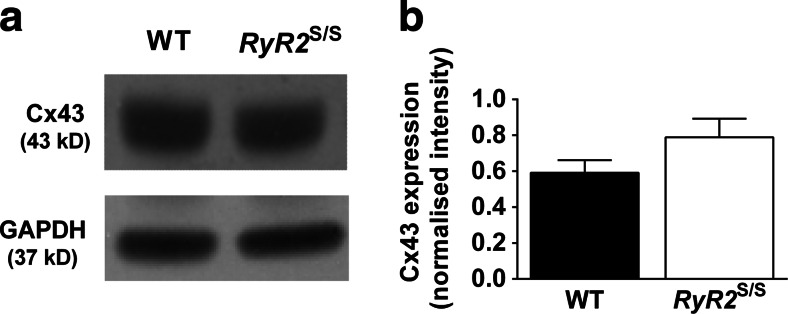
We first assessed the expression of the ventricular gap junction protein, Cx43. Western blots of whole tissue ventricular lysates from WT and RyR2S/S hearts demonstrate that the overall expression of Cx43, normalized to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH), was not significantly different between WT and RyR2S/S ventricles (0.59 ± 0.07; n = 4 and 0.79 ± 0.1; n = 4, respectively; P > 0.05, Fig. 6). This suggests that a loss of Cx43 expression is not a contributory factor to the slowed ventricular conduction and increased arrhythmogenesis observed in the ventricles of RyR2S/S hearts, in parallel to findings in the atria of the same model [21].
Fig. 6.
Total Cx43 expression in WT and RyR2 S/S ventricles. a Representative blots of Cx43 and GAPDH expression in WT and RyR2 S/S ventricular tissue and b the mean (± SEM) Cx43 expression normalized to GAPDH expression. Cx43 expression was similar between WT and RyR2 S/S (0.59 ± 0.07 and 0.79 ± 0.1, respectively; P = 0.167, n = 4) ventricles
Decreased Nav1.5 expression in the ventricles of RyR2S/S murine hearts
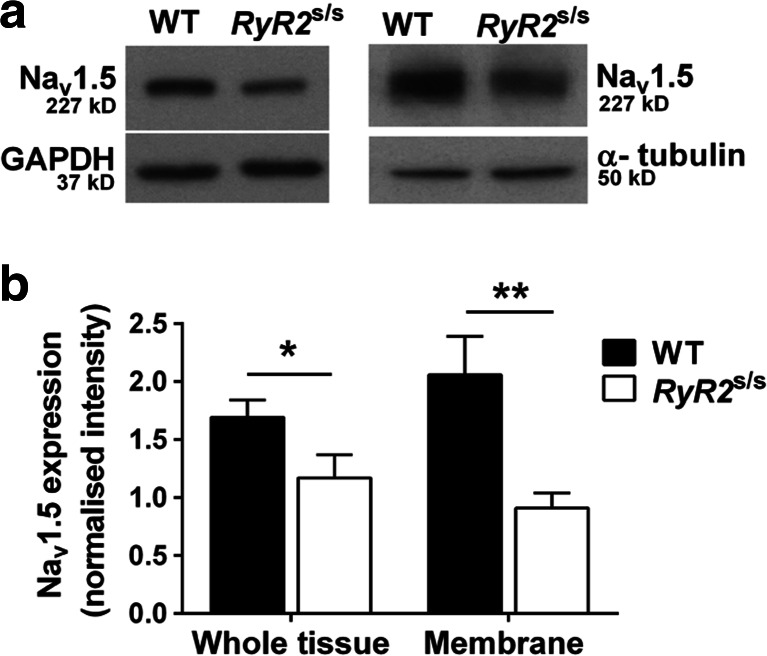
Western blots of WT and RyR2S/S ventricular homogenates (Fig. 7) illustrate a decreased Nav1.5 expression in the RyR2S/S relative to the WT in both the whole tissue fraction (Fig. 7a; left panel) (1.17 ± 0.2; n = 6, and 1.69 ± 0.15; n = 7, respectively; P < 0.05) and within the membrane fraction (Fig. 7a; right panel) (2.06 ± 0.33; n = 4, and 0.91 ± 0.13; n = 4, respectively; P < 0.01). Thus, Nav1.5 expression in RyR2S/S ventricles was approximately 69 % of that seen in the WT whole tissue fraction and down to only 44 % of WT in the membrane fraction (Fig. 7b). This significant reduction of Nav1.5 expression in the ventricular membrane where the function of Nav1.5 channels is crucial to cardiac excitability, and conduction would be expected to lead to a significant reduction in INa in the RyR2S/S heart compared to the WT.
Fig. 7.
Western blots of Nav1.5 expression in whole tissue and membrane fraction samples from WT and RyR2 S/S ventricles. Ventricular Nav1.5 expression was decreased in RyR2 S/S compared to WT, both in the whole tissue (1.17 ± 0.20; n = 6, vs 1.69 ± 0.15 n = 7, respectively, P = 0.048) and in the membrane fraction (0.91 ± 0.13; n = 4, vs 2.06 ± 0.33; n = 4, respectively, P = 0.006). This suggested a greater proportional reduction in membrane relative to total Nav1.5 expression in RyR2 S/S. Symbols denote significant differences between genotypes *P < 0.05, **P < 0.01
Decreased INa in the ventricles of RyR2S/S murine hearts
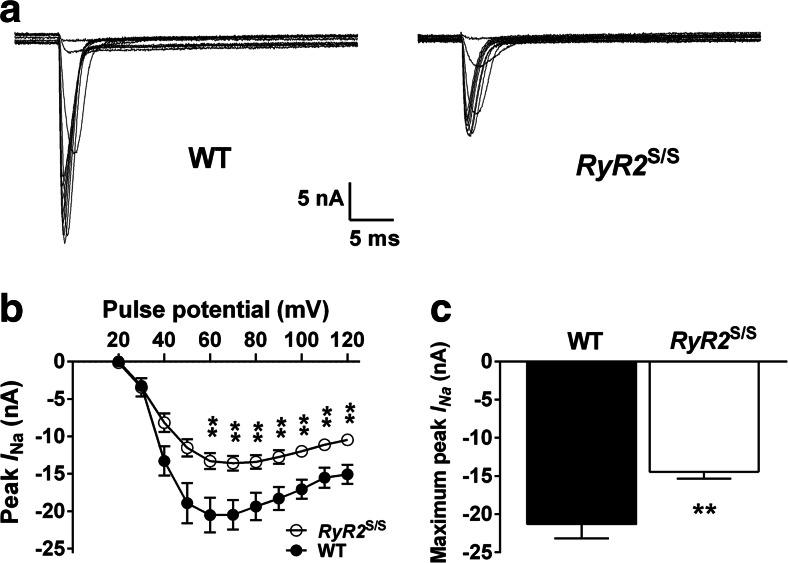
To assess whether the reduced expression of Nav1.5 in RyR2S/S ventricles correlated with a functional alteration of Nav1.5, we measured INa in both WT and RyR2S/S ventricles using the loose patch clamp technique. Figure 8a illustrates representative currents elicited by WT and RyR2S/S ventricles following a series (20–120 mV voltage excursions) of depolarizing test pulses. The peak current elicited at each voltage excursion and the overall peak current for both WT and RyR2S/S ventricles are shown in Fig. 8b, c, respectively. Currents recorded from the WT ventricle were significantly larger than those recorded in the RyR2S/S ventricle at depolarizing pulses of 60 mV or greater (P < 0.01). The overall peak current in the RyR2S/S was −14.45 nA ± 0.88 nA while in the WT it was −21.3 nA ± 1.87 nA (P < 0.01); this equates to a 32 % reduction in peak INa in the RyR2S/S.
Fig. 8.
Loose patch clamp recordings of I Na activation in WT and RyR2 S/S ventricles. a Representative currents in response to depolarizing steps increased from 20 to 120 mV in voltage-clamped WT and RyR2 S/S ventricular tissue. b Peak inward current (mean ± SEM) elicited at each voltage step for WT (n = 6) and RyR2 S/S (n = 12) ventricles. c The maximum current recorded during each complete voltage step protocol (mean ± SEM) was larger in the WT than the RyR2 S/S ventricles, P < 0.0047. The asterisks denote significant differences between genotypes of P < 0.01
Discussion
The present experiments demonstrate that reduced Nav1.5 expression and Na current is associated with the reduced conduction velocity and consequent arrhythmic substrate and ventricular arrhythmogenesis in homozygotic murine RyR2-P2328S (RyR2S/S) hearts. The quantitative changes were compatible with earlier reports of linear relationships predicted between the conduction velocity and the peak INa of the AP, but a nonlinear (logarithmic) relationship between peak INa and maximum Na+ permeability [20]. Thus, increased arrhythmogenicity was associated with a reduced conduction velocity of ∼22 % during steady 8 Hz pacing and in the region of a ∼33 % reduction during dynamic pacing, which would correspond to comparable reductions in AP wavelength given an absence of significant changes in repolarization characteristics (VERP and APD90), and determinants of passive conduction reflected in Cx43 expression. These in turn accompanied reductions in membrane Nav1.5 expression of ∼56 % and peak INa of ∼32 %.
The murine RyR2S/S heart has proven a useful experimental model for CPVT in reproducing a particular clinically observed human CPVT genotype [25, 40]. RyR2S/S ventricular myocytes show features of altered Ca2+ homeostasis [15] thought to result from an increased RyR2-mediated Ca2+ leak reflecting an increased sensitivity of Ca2+ release to cytosolic though not to SR levels [Ca2+] [31]. The consequent increase in cytosolic [Ca2+] would result in increased sodium-calcium exchanger (NCX) activity whose electrogenic actions would result in triggering events including delayed after-depolarizations leading to ectopic APs that could potentially initiate ventricular arrhythmia. However, these initial studies did not explore for the presence or otherwise for arrhythmic substrate that could sustain the resulting arrhythmia.
Genetic modifications in RyR2 are also associated with AF phenotypes [17, 31, 37]. This has also been modeled by the RyR2S/S system which demonstrates abnormal atrial Ca2+ homeostasis, delayed triggering events and atrial arrhythmia [22, 48]. However, they also demonstrated reductions in conduction velocity that could provide an arrhythmic substrate [22]. This was attributed to a reduced Na+ current which could be either attributed to a reduced Nav1.5 expression or a direct inhibitory effect on Na+ channel function of altered Ca2+ homeostasis [21]. This could arise from either increased leak of SR Ca2+ or the consequently elevated diastolic Ca2+. Indeed, recent evidence suggests that altered Ca2+ homeostasis can acutely affect cardiac excitability due to both direct [47] and indirect actions on the Na+ channel [2, 5, 41]. CaMKII has been shown to directly interact with Nav1.5, shifting Na+ current availability to a more depolarized membrane potential, thus enhancing the accumulation of Na+ channels into an intermediate inactivated state [2]. Increases in CaMKII activity additionally is known to phosphorylate RyR2 which itself increases SR Ca2+ leak [45]. Intracellular Ca2+ concentration can also acutely modulate Na+ current density in ventricular myocytes [5]. Atrial conduction slowing has also been observed in further models of RyR2-mediated Ca2+ leak including a CSQ2 mutant [14, 26].
These findings suggest that altered Ca2+ homeostasis following the chronic atrial alterations in SR Ca2+ release in the RyR2S/S system could compromise Nav1.5 expression or function as a result of the elevated diastolic Ca2+. The present study now demonstrates that RyR2S/Sventricles similarly displayed a reduced Nav1.5 expression and consequently reduced peak INa, that could explain similar reductions in their conduction velocities [49]. It further extends these findings in localizing this altered expression to the membrane, as well as the whole tissue, fraction (Fig. 7), leading to a reduced maximum rate of AP depolarization, which would be expected to reduce AP conduction velocity, thus creating an arrhythmic substrate. These findings accompanied a greater arrhythmogenicity of RyR2S/S murine ventricles, which showed arrhythmic events on extrasystolic (S2) stimulation unlike WT and more frequent arrhythmias that occurred at higher BCLs during dynamic stimulation. These findings took place despite indistinguishable AP recovery characteristics in WT and RyR2S/S ventricles, as reflected in VERP and APD90 readings, thereby excluding re-entrant mechanisms involving recovery phases of the AP [27]. In contrast, RyR2S/S showed reduced indices of conduction velocity, θ’ through all BCLs examined compared to WT, despite indistinguishable AP amplitude, APD90 and θ’ alternans and their variation with BCL or DI, particularly at low BCLs.
Our findings therefore suggest that the arrhythmic substrate results from reduced expression of Nav1.5 in the membrane, where a reduced INa leads to slowed AP conduction velocity, in the ventricles of RyR2S/S mice. This would be consistent with a situation in which abnormalities in cytosolic Ca2+ exert both short- and long-term effects. In the short term, ectopic activity can follow transient elevations in cytosolic [Ca2+]. In the long term, chronic elevations in cytosolic [Ca2+] can result in a downregulation of either Nav1.5 expression or activity, thereby reducing action potential conduction and resulting in arrhythmic substrate. In such a situation, short-term triggering events could potentially form a means for initiating electrical events then perpetuated by the pre-existing arrhythmic substrate. These findings may have broader implications for the mode of therapeutic intervention in a variety of Ca2+ dependent, and potentially some Nav1.5 dependent, arrhythmia.
Acknowledgments
This work was supported by Royal Society / National Science Foundation of China International Joint Project Grant (JP100994/ No.81211130599) (JAF and AM), Issac Newton Trust/ Wellcome Trust ISSF/ University of Cambridge Joint Research Grants Scheme (JAF) and by the Wellcome Trust and Medical Research Council (CLH).
Footnotes
Feifei Ning and Ling Luo contributed equally as the first authors.
Christopher L.-H. Huang, Aiqun Ma and Samantha C. Salvage contributed equally as the senior authors.
Contributor Information
Aiqun Ma, Phone: 86-29-85323524, Email: maaiqun@medmail.com.cn.
Samantha C. Salvage, Phone: + 44 1223 333883, Email: ss2148@cam.ac.uk
References
- 1.Abriel H, Zaklyazminskaya EV. Cardiac channelopathies: genetic and molecular mechanisms. Gene. 2013;517:1–11. doi: 10.1016/j.gene.2012.12.061. [DOI] [PubMed] [Google Scholar]
- 2.Ashpole NM, Herren AW, Ginsburg KS, Brogan JD, Johnson DE, Cummins TR, Bers DM, Hudmon A. Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates cardiac sodium channel NaV1.5 gating by multiple phosphorylation sites. J Biol Chem. 2012;287:19856–19869. doi: 10.1074/jbc.M111.322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bezanilla F, Armstrong CM. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977;70:549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhuiyan ZA, van den Berg MP, van Tintelen JP, Bink-Boelkens MTE, Wiesfeld ACP, Alders M, Postma AV, van Langen I, Mannens MMAM, Wilde AAM. Expanding spectrum of human RYR2-related disease: new electrocardiographic, structural, and genetic features. Circulation. 2007;116:1569–1576. doi: 10.1161/CIRCULATIONAHA.107.711606. [DOI] [PubMed] [Google Scholar]
- 5.Casini S, Verkerk AO, van Borren MMGJ, van Ginneken ACG, Veldkamp MW, de Bakker JMT, Tan HL. Intracellular calcium modulation of voltage-gated sodium channels in ventricular myocytes. Cardiovasc Res. 2009;81:72–81. doi: 10.1093/cvr/cvn274. [DOI] [PubMed] [Google Scholar]
- 6.Chiamvimonvat N, Kargacin ME, Clark RB, Duff HJ. Effects of intracellular calcium on sodium current density in cultured neonatal rat cardiac myocytes. J Physiol. 1995;483(Pt 2):307–318. doi: 10.1113/jphysiol.1995.sp020587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coppen SR, Dupont E, Rothery S, Severs NJ. Connexin45 expression is preferentially associated with the ventricular conduction system in mouse and rat heart. Circ Res. 1998;82:232–243. doi: 10.1161/01.RES.82.2.232. [DOI] [PubMed] [Google Scholar]
- 8.Desai KH, Sato R, Schauble E, Barsh GS, Kobilka BK, Bernstein D. Cardiovascular indexes in the mouse at rest and with exercise: new tools to study models of cardiac disease. Am J Physiol. 1997;272:H1053–H1061. doi: 10.1152/ajpheart.1997.272.2.H1053. [DOI] [PubMed] [Google Scholar]
- 9.Di Pino A, Caruso E, Costanzo L, Guccione P (2014) A novel RyR2 mutation in a 2-year-old baby presenting with atrial fibrillation, atrial flutter, and atrial ectopic tachycardia. Heart Rhythm 11:1480–1483. doi:10.1016/j.hrthm.2014.04.037 [DOI] [PubMed]
- 10.Dobrev D. Atrial Ca2+ signaling in atrial fibrillation as an antiarrhythmic drug target. Naunyn Schmiedeberg’s Arch Pharmacol. 2010;381:195–206. doi: 10.1007/s00210-009-0457-1. [DOI] [PubMed] [Google Scholar]
- 11.Duff HJ, Offord J, West J, Catterall WA. Class I and IV antiarrhythmic drugs and cytosolic calcium regulate mRNA encoding the sodium channel alpha subunit in rat cardiac muscle. Mol Pharmacol. 1992;42:570–574. [PubMed] [Google Scholar]
- 12.Dupont E, el Aoumari A, Briand JP, Fromaget C, Gros D. Cross-linking of cardiac gap junction connexons by thiol/disulfide exchanges. J Membr Biol. 1989;108:247–252. doi: 10.1007/BF01871739. [DOI] [PubMed] [Google Scholar]
- 13.Faggioni M, Kryshtal DO, Knollmann BC. Calsequestrin mutations and catecholaminergic polymorphic ventricular tachycardia. Pediatr Cardiol. 2012;33:959–967. doi: 10.1007/s00246-012-0256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glukhov AV, Kalyanasundaram A, Lou Q, Hage LT, Hansen BJ, Belevych AE, Mohler PJ, Knollmann BC, Periasamy M, Györke S, Fedorov VV. Calsequestrin 2 deletion causes sinoatrial node dysfunction and atrial arrhythmias associated with altered sarcoplasmic reticulum calcium cycling and degenerative fibrosis within the mouse atrial pacemaker complex1. Eur Heart J. 2015;36:686–697. doi: 10.1093/eurheartj/eht452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goddard CA, Ghais NS, Zhang Y, Williams AJ, Colledge WH, Grace AA, Huang CL-H. Physiological consequences of the P2328S mutation in the ryanodine receptor (RyR2) gene in genetically modified murine hearts. Acta Physiol (Oxf) 2008;194:123–140. doi: 10.1111/j.1748-1716.2008.01865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.RES.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herron TJ, Milstein ML, Anumonwo J, Priori SG, Jalife J. Purkinje cell calcium dysregulation is the cellular mechanism that underlies catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2010;7:1122–1128. doi: 10.1016/j.hrthm.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang HS, Nitu FR, Yang Y, Walweel K, Pereira L, Johnson CN, Faggioni M, Chazin WJ, Laver D, George AL, Cornea RL, Bers DM, Knollmann BC. Divergent regulation of ryanodine receptor 2 calcium release channels by arrhythmogenic human calmodulin missense mutants. Circ Res. 2014;114:1114–1124. doi: 10.1161/CIRCRESAHA.114.303391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz G, Shainberg A, Hochhauser E, Kurtzwald-Josefson E, Issac A, El-Ani D, Aravot D, Afek A, Seidman JG, Seidman CE, Eldar M, Arad M. The role of mutant protein level in autosomal recessive catecholamine dependent polymorphic ventricular tachycardia (CPVT2) Biochem Pharmacol. 2013;86:1576–1583. doi: 10.1016/j.bcp.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King JH, Huang CLH, Fraser JA. Determinants of myocardial conduction velocity: Implications for arrhythmogenesis. Front Physiol. 2013;4:154. doi: 10.3389/fphys.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King JH, Wickramarachchi C, Kua K, Du Y, Jeevaratnam K, Matthews HR, Grace AA, Huang CL-H, Fraser JA. Loss of Nav1.5 expression and function in murine atria containing the RyR2-P2328S gain-of-function mutation. Cardiovasc Res. 2013;99:751–759. doi: 10.1093/cvr/cvt141. [DOI] [PubMed] [Google Scholar]
- 22.King JH, Zhang Y, Lei M, Grace AA, Huang CL-H, Fraser JA. Atrial arrhythmia, triggering events and conduction abnormalities in isolated murine RyR2-P2328S hearts. Acta Physiol (Oxf) 2013;207:308–323. doi: 10.1111/apha.12006. [DOI] [PubMed] [Google Scholar]
- 23.Kirchhoff S, Nelles E, Hagendorff A, Krüger O, Traub O, Willecke K. Reduced cardiac conduction velocity and predisposition to arrhythmias in connexin40-deficient mice. Curr Biol. 1998;8:299–302. doi: 10.1016/S0960-9822(98)70114-9. [DOI] [PubMed] [Google Scholar]
- 24.Koller ML, Riccio ML, Gilmour RF. Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am J Physiol. 1998;275:H1635–H1642. doi: 10.1152/ajpheart.1998.275.5.H1635. [DOI] [PubMed] [Google Scholar]
- 25.Laitinen PJ, Brown KM, Piippo K, Swan H, Devaney JM, Brahmbhatt B, Donarum EA, Marino M, Tiso N, Viitasalo M, Toivonen L, Stephan DA, Kontula K. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–490. doi: 10.1161/01.CIR.103.4.485. [DOI] [PubMed] [Google Scholar]
- 26.Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, Respress JL, Ather S, Skapura DG, Jordan VK, Horrigan FT, Schmitz W, Müller FU, Valderrabano M, Nattel S, Dobrev D, Wehrens XHT. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation. 2014;129:1276–1285. doi: 10.1161/CIRCULATIONAHA.113.006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin CA, Grace AA, Huang CL-H. Refractory dispersion promotes conduction disturbance and arrhythmias in a Scn5a (+/−) mouse model. Pflugers Arch. 2011;462:495–504. doi: 10.1007/s00424-011-0989-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews GDK, Guzadhur L, Grace A, Huang CL-H. Nonlinearity between action potential alternans and restitution, which both predict ventricular arrhythmic properties in Scn5a+/− and wild-type murine hearts. J Appl Physiol. 2012;112:1847–1863. doi: 10.1152/japplphysiol.00039.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews GDK, Guzadhur L, Sabir IN, Grace AA, Huang CL. Action potential wavelength restitution predicts alternans and arrhythmia in murine Scn5a+/− hearts. J Physiol. 2013 doi: 10.1113/jphysiol.2013.254938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews GDK, Martin CA, Grace AA, Zhang Y, Huang CL-H. Regional variations in action potential alternans in isolated murine Scn5a (+/−) hearts during dynamic pacing. Acta Physiol (Oxf) 2010;200:129–146. doi: 10.1111/j.1748-1716.2010.02138.x. [DOI] [PubMed] [Google Scholar]
- 31.Meli AC, Refaat MM, Dura M, Reiken S, Wronska A, Wojciak J, Carroll J, Scheinman MM, Marks AR. A novel ryanodine receptor mutation linked to sudden death increases sensitivity to cytosolic calcium. Circ Res. 2011;109:281–290. doi: 10.1161/CIRCRESAHA.111.244970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nattel S, Shiroshita-Takeshita A, Brundel BJJM, Rivard L Mechanisms of atrial fibrillation: lessons from animal models. Prog Cardiovasc Dis 48:9–28. doi: 10.1016/j.pcad.2005.06.002 [DOI] [PubMed]
- 33.Papadatos GA, Wallerstein PMR, Head CEG, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AEO, Huang CL-H, Vandenberg JI, Colledge WH, Grace AA. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci U S A. 2002;99:6210–6215. doi: 10.1073/pnas.082121299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Priori SG, Chen SRW. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.CIR.103.2.196. [DOI] [PubMed] [Google Scholar]
- 36.Salvage SC, King JH, Chandrasekharan KH, Jafferji DIG, Guzadhur L, Matthews HR, Huang CL-H, Fraser JA. Flecainide exerts paradoxical effects on sodium currents and atrial arrhythmia in murine RyR2-P2328S hearts. Acta Physiol. 2015;214:361–375. doi: 10.1111/apha.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shan J, Xie W, Betzenhauser M, Reiken S, Chen B-X, Wronska A, Marks AR. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2012;111:708–717. doi: 10.1161/CIRCRESAHA.112.273342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stokoe KS, Balasubramaniam R, Goddard CA, Colledge WH, Grace AA, Huang CL-H. Effects of flecainide and quinidine on arrhythmogenic properties of Scn5a+/− murine hearts modelling the Brugada syndrome. J Physiol. 2007;581:255–275. doi: 10.1113/jphysiol.2007.128785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sumitomo N, Sakurada H, Taniguchi K, Matsumura M, Abe O, Miyashita M, Kanamaru H, Karasawa K, Ayusawa M, Fukamizu S, Nagaoka I, Horie M, Harada K, Hiraoka M. Association of atrial arrhythmia and sinus node dysfunction in patients with catecholaminergic polymorphic ventricular tachycardia. Circ J. 2007;71:1606–1609. doi: 10.1253/circj.71.1606. [DOI] [PubMed] [Google Scholar]
- 40.Swan H, Piippo K, Viitasalo M, Heikkilä P, Paavonen T, Kainulainen K, Kere J, Keto P, Kontula K, Toivonen L. Arrhythmic disorder mapped to chromosome 1q42–q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts. J Am Coll Cardiol. 1999;34:2035–2042. doi: 10.1016/S0735-1097(99)00461-1. [DOI] [PubMed] [Google Scholar]
- 41.Tan HL, Kupershmidt S, Zhang R, Stepanovic S, Roden DM, Wilde AAM, Anderson ME, Balser JR. A calcium sensor in the sodium channel modulates cardiac excitability. Nature. 2002;415:442–447. doi: 10.1038/415442a. [DOI] [PubMed] [Google Scholar]
- 42.Taouis M, Sheldon RS, Duff HJ. Upregulation of the rat cardiac sodium channel by in vivo treatment with a class I antiarrhythmic drug. J Clin Invest. 1991;88:375–378. doi: 10.1172/JCI115313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tribulová N, Dupont E, Soukup T, Okruhlicová L, Severs NJ. Sex differences in connexin-43 expression in left ventricles of aging rats. Physiol Res. 2005;54:705–708. [PubMed] [Google Scholar]
- 44.Van Veen TAB, Stein M, Royer A, Le Quang K, Charpentier F, Colledge WH, Huang CL-H, Wilders R, Grace AA, Escande D, de Bakker JMT, van Rijen HVM. Impaired impulse propagation in Scn5a-knockout mice: combined contribution of excitability, connexin expression, and tissue architecture in relation to aging. Circulation. 2005;112:1927–1935. doi: 10.1161/CIRCULATIONAHA.105.539072. [DOI] [PubMed] [Google Scholar]
- 45.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XHT, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na + −Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webster G, Berul CI. An update on channelopathies: from mechanisms to management. Circulation. 2013;127:126–140. doi: 10.1161/CIRCULATIONAHA.111.060343. [DOI] [PubMed] [Google Scholar]
- 47.Wingo TL, Shah VN, Anderson ME, Lybrand TP, Chazin WJ, Balser JR. An EF-hand in the sodium channel couples intracellular calcium to cardiac excitability. Nat Struct Mol Biol. 2004;11:219–225. doi: 10.1038/nsmb737. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Fraser JA, Jeevaratnam K, Hao X, Hothi SS, Grace AA, Lei M, Huang CL-H. Acute atrial arrhythmogenicity and altered Ca(2+) homeostasis in murine RyR2-P2328S hearts. Cardiovasc Res. 2011;89:794–804. doi: 10.1093/cvr/cvq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Wu J, Jeevaratnam K, King JH, Guzadhur L, Ren X, Grace AA, Lei M, Huang CL-H, Fraser JA. Conduction Slowing Contributes to Spontaneous Ventricular Arrhythmias in Intrinsically Active Murine RyR2-P2328S Hearts. J Cardiovasc Electrophysiol. 2013;24:210–218. doi: 10.1111/jce.12015. [DOI] [PubMed] [Google Scholar]