Abstract
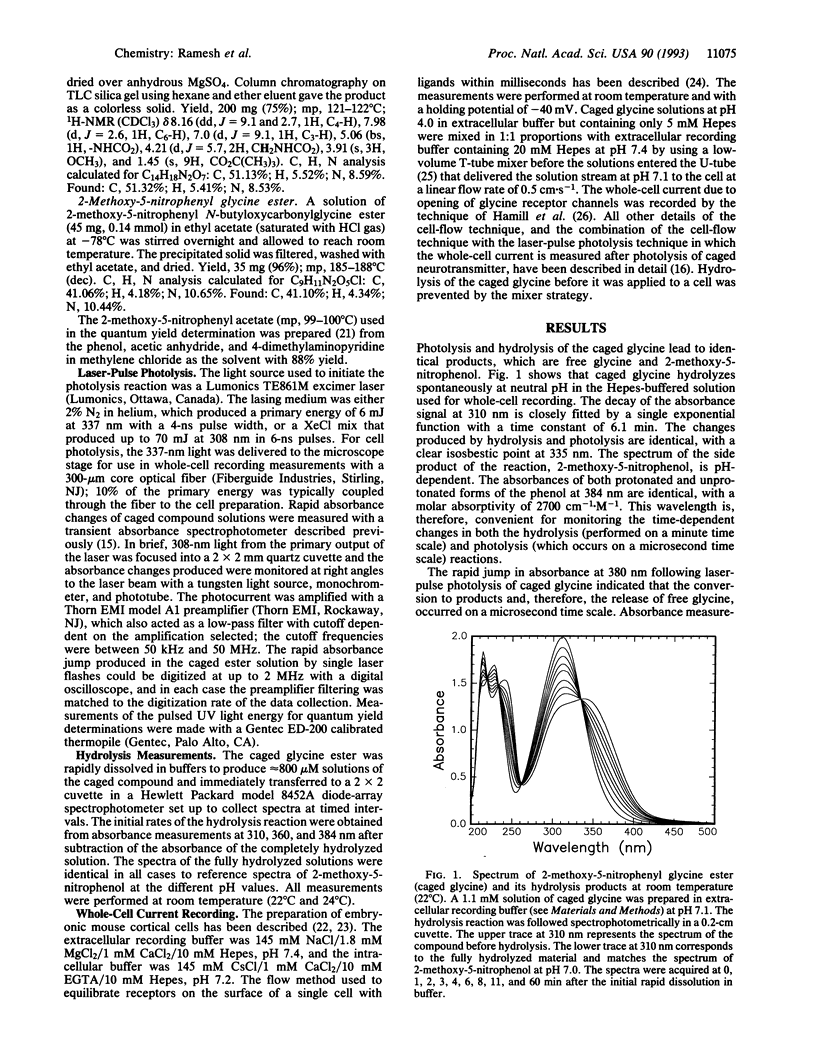
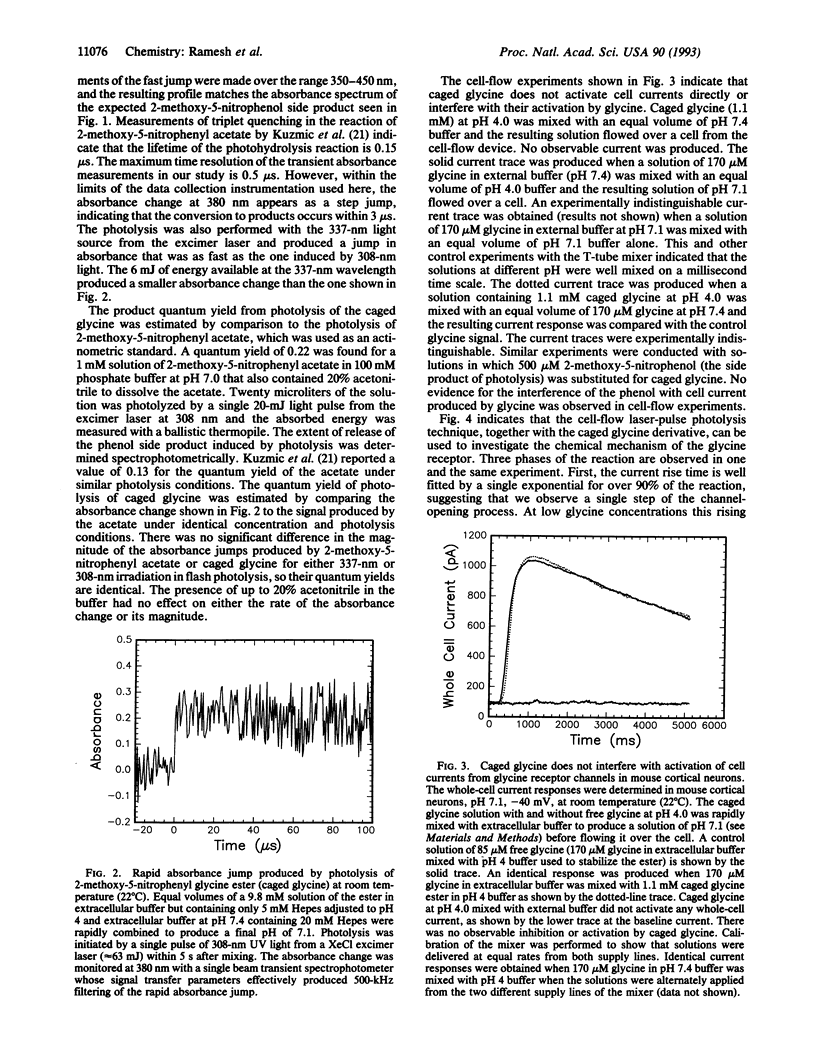
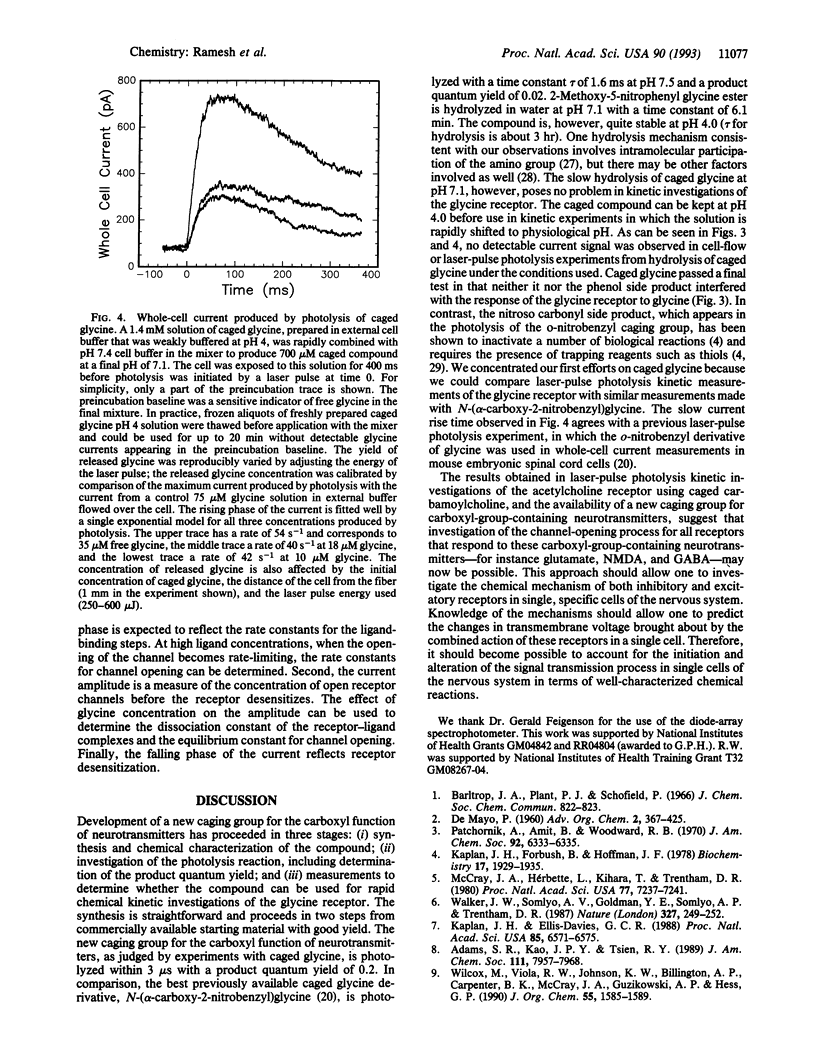
The synthesis of a photosensitive blocking group for the carboxyl function of neurotransmitters, in this case glycine, is reported. The compound, 2-methoxy-5-nitrophenyl glycine ester (caged glycine), is photolyzed by a laser pulse at 308 or 337 nm within 3 microseconds and with a product quantum yield of 0.2. The compound is hydrolyzed in water with a time constant tau of 6.1 min at pH 7.1 and 3 hr at pH 4.0. Mouse cerebral cortical neurons containing glycine receptors were used in biological assays. A cell-flow device, in which solutions of caged glycine at pH 4.0 were mixed with buffer to give a final pH of 7.1, was used to equilibrate the compound with receptors on the cell surface. Neither the caged compound nor the 2-methoxy-5-nitrophenol photolysis product affected the glycine receptors or modified their response to glycine. When cells equilibrated with caged glycine are irradiated by a laser pulse at 337 nm, glycine receptor channels are opened, as detected in whole-cell current recordings. The approach described may be used in the synthesis and characterization of photolabile precursors of neurotransmitters and other compounds that contain carboxyl groups and for kinetic investigations of neurotransmitter receptors in central nervous system cells in the microsecond time domain.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billington A. P., Walstrom K. M., Ramesh D., Guzikowski A. P., Carpenter B. K., Hess G. P. Synthesis and photochemistry of photolabile N-glycine derivatives and effects of one on the glycine receptor. Biochemistry. 1992 Jun 23;31(24):5500–5507. doi: 10.1021/bi00139a011. [DOI] [PubMed] [Google Scholar]
- Farkas D. L., Baxter G., DeBiasio R. L., Gough A., Nederlof M. A., Pane D., Pane J., Patek D. R., Ryan K. W., Taylor D. L. Multimode light microscopy and the dynamics of molecules, cells, and tissues. Annu Rev Physiol. 1993;55:785–817. doi: 10.1146/annurev.ph.55.030193.004033. [DOI] [PubMed] [Google Scholar]
- Geetha N., Hess G. P. On the mechanism of the gamma-aminobutyric acid receptor in the mammalian (mouse) cerebral cortex. Chemical kinetic investigations with a 10-ms time resolution adapted to measurements of neuronal receptor function in single cells. Biochemistry. 1992 Jun 23;31(24):5488–5499. doi: 10.1021/bi00139a010. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H., Ellis-Davies G. C. Photolabile chelators for the rapid photorelease of divalent cations. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. H., Forbush B., 3rd, Hoffman J. F. Rapid photolytic release of adenosine 5'-triphosphate from a protected analogue: utilization by the Na:K pump of human red blood cell ghosts. Biochemistry. 1978 May 16;17(10):1929–1935. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H. Photochemical manipulation of divalent cation levels. Annu Rev Physiol. 1990;52:897–914. doi: 10.1146/annurev.ph.52.030190.004225. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Matsubara N., Billington A. P., Hess G. P. How fast does an acetylcholine receptor channel open? Laser-pulse photolysis of an inactive precursor of carbamoylcholine in the microsecond time region with BC3H1 cells. Biochemistry. 1992 Jun 23;31(24):5507–5514. doi: 10.1021/bi00139a012. [DOI] [PubMed] [Google Scholar]
- McCray J. A., Herbette L., Kihara T., Trentham D. R. A new approach to time-resolved studies of ATP-requiring biological systems; laser flash photolysis of caged ATP. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7237–7241. doi: 10.1073/pnas.77.12.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn T., Matsubara N., Billington A. P., Udgaonkar J. B., Walker J. W., Carpenter B. K., Webb W. W., Marque J., Denk W., McCray J. A. Synthesis, photochemistry, and biological activity of a caged photolabile acetylcholine receptor ligand. Biochemistry. 1989 Jan 10;28(1):49–55. doi: 10.1021/bi00427a008. [DOI] [PubMed] [Google Scholar]
- Niu L., Hess G. P. An acetylcholine receptor regulatory site in BC3H1 cells: characterized by laser-pulse photolysis in the microsecond-to-millisecond time region. Biochemistry. 1993 Apr 20;32(15):3831–3835. doi: 10.1021/bi00066a001. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Neale E., Henkart M., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J Neurophysiol. 1977 Sep;40(5):1132–1150. doi: 10.1152/jn.1977.40.5.1132. [DOI] [PubMed] [Google Scholar]
- Udgaonkar J. B., Hess G. P. Chemical kinetic measurements of a mammalian acetylcholine receptor by a fast-reaction technique. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8758–8762. doi: 10.1073/pnas.84.24.8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. W., Somlyo A. V., Goldman Y. E., Somlyo A. P., Trentham D. R. Kinetics of smooth and skeletal muscle activation by laser pulse photolysis of caged inositol 1,4,5-trisphosphate. Nature. 1987 May 21;327(6119):249–252. doi: 10.1038/327249a0. [DOI] [PubMed] [Google Scholar]



