Abstract
Objective
To describe antenatal findings and evaluate prenatal risk parameters for adverse outcome or need for intervention in fetuses with congenital pulmonary airway malformation (CPAM).
Methods
In our retrospective study all fetuses with a prenatal diagnosis of CPAM detected in our tertiary referral center between 2002 and 2013 were analyzed. Sonographic findings were noted and measurements of mass-to-thorax-ratio (MTR), congenital pulmonary airway malformation volume-ratio (CVR) and observed to expected lung-to head-ratio (o/e LHR) were conducted and correlated to fetal or neonatal morbidity and mortality and/or need for prenatal intervention.
Results
67 fetuses with CPAM were included in the study. Hydropic fetuses were observed in 16.4% (11/67) of cases, prenatal intervention was undertaken in 9 cases; 7 pregnancies were terminated. The survival rate of non-hydropic fetuses with conservatively managed CPAM was 98.0% (50/51), the survival rate for hydropic fetuses with intention to treat was 42.9% (3/7). 10 (18.2%) children needed respiratory assistance. Fetuses with a CVR of <0.91 were significantly less likely to experience adverse outcome or need for prenatal intervention with sensitivity, specificity and positive/negative predictive value of 0.89, 0.71, 0.62 and 0.93, respectively. A MTR (mass-to-thorax-ratio) of < 0.51 had a positive predictive value of 0.54 and a negative predictive value of 0.96 of adverse events with a sensitivity of 0.95 and a specificity of 0.63. The negative predictive value for o/e LHR of 45% was 0.84 with sensitivity, specificity and positive predictive value of 0.73, 0.68 and 0.52, respectively.
Conclusions
The majority of cases with CPAM have a favorable outcome. MTR and CVR are able to identify fetuses at risk, the o/e LHR is less sensitive.
Introduction
Prenatal diagnosis of echogenic lung lesions can be achieved using high resolution ultrasound during the second trimester. The most common causative lesions are congenital pulmonary airway malformation (CPAM) and pulmonary sequestration (BPS). Few studies have described prognosis, prenatal and postnatal management of these lesions [1,2,3,4,5,6,7,8,9,10]. Though the majority of fetuses with CPAM usually have a favorable outcome, the prognosis of rare cases with large lesions complicated by hydrops and need for prenatal intervention is remarkably worse. In order to provide more information on the prognosis of these fetuses, we conducted a retrospective single-center study of 67 consecutive cases of CPAM over the last 12 years, focusing on intrauterine prognostic markers such as MTR, CVR and o/e LHR predicting need for prenatal intervention, respiratory assistance and fetal mortality.
Methods
This is a retrospective study of data routinely achieved and anonymously analyzed. All participants (mothers of the fetuses) signed in a form, that the data may be used anonymously for research use. Our local ethics committee (Ethik Kommission der Medizinischen Fakultät der Universität Bonn) does not generate a protocol in this form of study but exempted us from approval in a formal waver. The mothers were contacted at the routinely performed sonography, not especially for the study. Sonographic findings were extracted from the medical records and stored in an anonymized data base by the authors, as well as information to postnatal therapy and outcome. After data extraction no identifying information was stored by the researchers. Our hospital gave permission to the publication of patient`s medical images.
The study was carried out between 2002 and 2013. In this period 59,082 cases were sent to our tertiary referral center for a detailed second trimester scan. The antenatal findings of 67 fetuses with prenatal diagnosis of CPAM stored in our computerized database were analyzed. Location and size of the lesion, presence of mediastinal shifting and associated malformations were noted. Follow up scans with regard to regression or progression of the lesion were included. All patients received a complete anatomic scan and Doppler sonography using ATL HDI 5000 and IU22, Philips (Hamburg, Germany) or Voluson E8, GE Healthcare (Solingen, Germany) devices. In cases of fetal intervention the different methods (single or repeated thoracocentesis, shunting) were noted as well as success of the intervention in the course of pregnancy. Shunting was performed with a double pigtail catheter (Harrison fetal bladder Stent Set, Cook Medical, Bloomington, IN, USA). The type of lesion was recorded using the classification described by Stocker [11]. To calculate the CVR (CPAM volume ratio), a volumetric index of mass size that allows for comparison of fetuses at different gestational ages [1], the length, width and depth of the mass were multiplied by a 0.52 correction factor and divided by the head circumference. Moreover the transverse diameter of the mass and the transverse diameter of the thorax were taken and the mass-to-thorax-ratio (MTR) was calculated [12]. Finally the observed to expected lung area to head circumference ratio, showing the LHR in relation to the fetal head circumference independant of the gestational age and usually performed as a prognostic marker in cases with congenital diaphragmatic hernia, was measured [13]. Furthermore adverse events during pregnancy, mode of delivery, postnatal respiratory adaptation of the newborns and long term outcome were analyzed based on obstetrical and pediatric charts. Postnatal confirmation of the diagnosis was obtained by chest x-ray, contrast computerized tomography or magnetic resonance imaging. In cases of postnatal surgery histopathological findings were analyzed.
Statistical analyses were performed with the Fisher exact test with the SPSS software package (SPSS Inc, Chicago IL). Receiver operating characteristic curves were calculated based on the CVR, MTR or o/e LHR in relationship to outcome and/or need for intervention. Adverse outcome/need for intervention was defined as perinatal death, termination of pregnancy, hydrops, need for prenatal intervention such as pleural drainage and/or thoracocentesis or need for postnatal respiratory assistance. Significance was defined as a probability value of < .05.
Results
In the study period 67 fetuses (38 male/ 29 female fetuses) of CPAM were diagnosed, including three cases with hybrid lesions. Median gestational age at diagnosis was at 22 weeks (range 17–29). The microcystic, macrocystic and mixed type of lesion was diagnosed in 34 (50.7%), 14 (20.9%) and 19 (28.4%) cases, respectively. There was no predominance of left sided or right sided lesions (47.8% vs. 52.2%). Mediastinal shifting occured in 56 (83.6%) fetuses; the shift was discrete, moderate and severe in 21 (31.3%), 23 (34.3%) and 12 (17.9%) cases, respectively. Cases with macrocystic lesions showed predominately moderate (28.6%) and severe (57.1%) shifting.
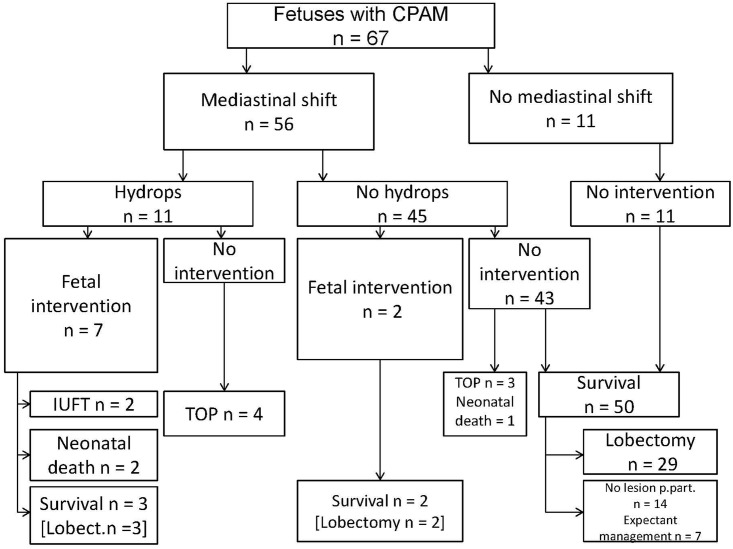
Hydropic fetuses with CPAM were observed in 16.4% (11/67 cases). Of these, 81.8% (9/11) were macrocystic and two microcystic. Five further fetuses had unilateral pleural effusions without hydrops (Fig 1).
Fig 1. Flow chart showing management and pregnancy outcome of 67 fetuses with prenatally diagnosed CPAM.
Karyotyping was performed in 19 (28.4%) cases and revealed trisomy 21 in one case with an additional perimembranous outlet ventricular septal defect. Other additional malformations were noted in 5 (7.5%) cases (two cases with muscular ventricular septal defect, one case with unilateral hydronephrosis, one case with bilateral hydronephrosis and one case with hydrocephalus internus). In 7 cases (10.4%) the parents opted for termination of pregnancy, including the fetus with trisomy 21 and 4 cases with fetal hydrops.
Of the remaining 60 cases, 33 (55%) showed regression in the course of pregnancy (22; 36,6% partial, 11; 18.3% complete). 25 (41.7%) lesions remained unchanged, and two showed progression. Spontaneous regression in cases of uncomplicated CPAM without need for intervention was observed in more than half of continued pregnancies (33/60, 55%), predominantly in microcystic and mixed lesions. In 11 macrocystic lesions none of the cases regressed and progression was noted in two. In contrast, 73.5% (25/34) of microcystic lesions showed partial (41.2%, 14/34) or complete (32.3%, 11/34) regression.
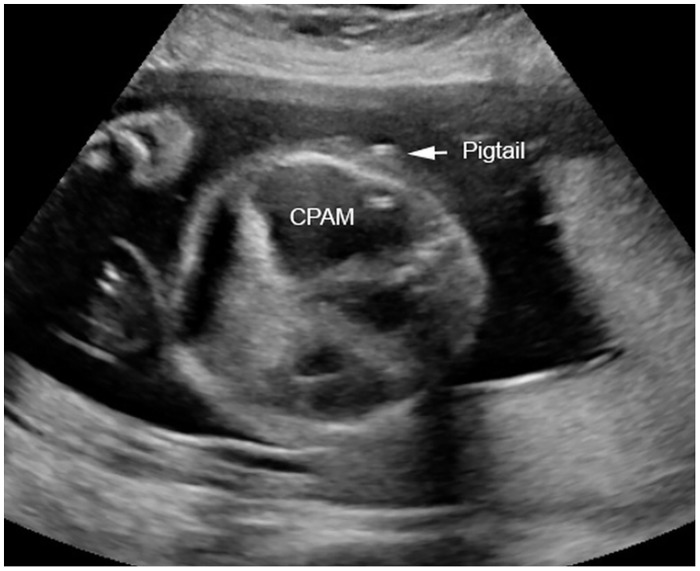
9 fetuses (7 of those with hydrops) received an intrauterine intervention: 6 shunt insertions (Fig 2), two instillations of OK-432 and one transplacental treatment with dexamethasone. Besides hydrops inclusion criteria for intervention for the fetuses without hydrops (n = 2) were in one case a large single cyst with moderate mediastinal shift and ascites and in the second case administration of steroids to the mother in presence of severe mediastinal shift due to a large mixed lesion (MTR 0.76, CVR 2.30 and o/e LHR 34.2%), ascites and no possibility of shunting in absence of a large single cyst. Details are given in Table 1.
Fig 2. Transverse section of the fetal thorax at 25 weeks of gestation demonstrating a macrocystic congenital pulmonary airway malformation (CPAM) drained by an inserted double pigtail catheter (Harrison Fetal Bladder Stent Set).
Table 1. Outcome of fetuses with intrauterine treatment of congenital pulmonary airway malformation.
| Case | Hydrops | Type of the lesion* | GA (week) at intervention | Type of Intervention | resolution | complication | GA (week) delivery | outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | no | I | 24 | 1 cyst puncture | yes | - | 39 | alive |
| instillation of OK-432 | ||||||||
| 2 | yes | I | 21,22,23, | 5 cyst punctures | yes | dislocation of the shunt in 29 weeks | 38 | alive |
| 24,27 | 1 thoraco-amniotic shunt | no reintervention neccessary | ||||||
| 3 | yes | I | 22,23,24 | 3 cyst punctures | no | IUFT | 32 | IUFT |
| 3 instillation of OK-432 | ||||||||
| 2 thoraco-amniotic shunts | ||||||||
| 4 | yes | I | 25,28,29,30, | 2 cyst punctures | yes | dislocation of 4 shunts | 36 | alive |
| 32,33,36 | 7 thoraco-amniotic shunts | |||||||
| 5 | yes | I | 25,28 | 2 thoraco-amniotic shunts | yes | dislocation of shunt 1 | 28 | neonatal death |
| fetal distress after 2nd. intervention, emergency cesarean section | ||||||||
| 6 | yes | I | 22 | 1 thoraco-amniotic shunt | yes | Spontaneous abortion | 22 | IUFT |
| 7 | yes | I | 28 | 4 cyst punctures | yes | triplet pregnancy, cesarean section at 31 weeks | 31 | neonatal death |
| 1 thoraco-amniotic shunt | ||||||||
| 8 | yes | I | 26 | 1 thoraco-amniotic shunt | yes | - | 39 | alive |
| 9 | no | II | 22 | maternal steroids | yes | - | 38 | alive |
* Classification of Stocker [11],
GA = gestational age, IUFT = intrauterine death
Pregnancy outcome
The survival rate of fetuses with CPAM was 91.7% (55/60) of those with intention to treat and 98.0% (50/51) for fetuses without hydrops managed conservatively (three cases of TOP excluded). The survival rate for hydropic fetuses with intention to treat was 3/7 (42.9%).
The mean age of delivery for fetuses with CPAM was 37 weeks of gestation (range: 22–40 weeks of gestation). All surviving neonates were born by planned delivery in a tertial center and in presence of neonatologists. 81.8% (45/55) of the neonates showed unremarkable postnatal respiratory function, 10 (18.2%) children needed respiratory assistance. Continuous positive airway pressure was necessary in 5 cases, in 5 cases mechanical ventilation was performed, in no case extracorporal membrane oxygenation-therapy was necessary.
The diagnosis was postnatally confirmed in 64.3% of cases (36/56) by chest X-ray and additional contrast computerized tomography or MRT including one case with early postnatal death. In 2 cases histopathological examination revealed sequestration, in 4 cases congenital lobal emphysema was detected, whereas in all other cases of postnatal surgery (28/34) the diagnosis of CPAM was histologically confirmed. No case of teratoma was detected. In 14 neonates no lesion could be demonstrated in the postpartal period. Postnatal surgery was performed in 61.8% of surviving fetuses (34/55), 7 parents opted for conservative management.
Sonographic parameters for risk assessment
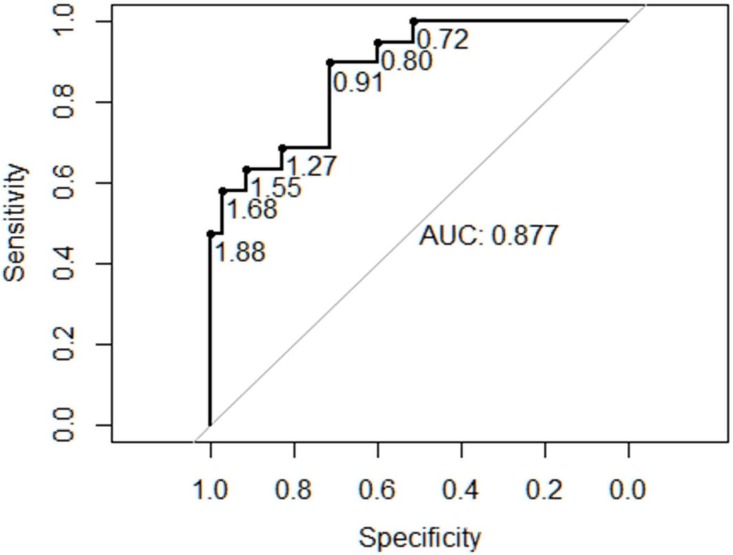
The CVR measurement (Fig 3), available in 62/67 fetuses, correlated strongly with perinatal death, termination of pregnancy, hydrops, need for prenatal intervention such as pleural drainage and/or thoracocentesis or need for postnatal respiratory assistance (p<0.001). The area under the curve of receiver operator characteristic curves was 0.877 (Fig 4). Fetuses with a CVR of <0.91 were significantly less likely to experience some kind of adverse outcome or need for prenatal intervention with a sensitivity of 0.89 and a specificity of 0.71, a positive predictive value of 0.62 and a negative predictive value of 0.93. A CVR ratio of 1.68 had a sensitivity, specificity, positive predictive value and negative predictive value of 0.58, 0.97, 0.91 and 0.81, respectively.
Fig 3. Transverse and longitudinal section of the fetal thorax demonstrating a large microcystic congenital pulmonary airway malformation (CPAM) with mediastinal shift at 19 weeks of gestation.
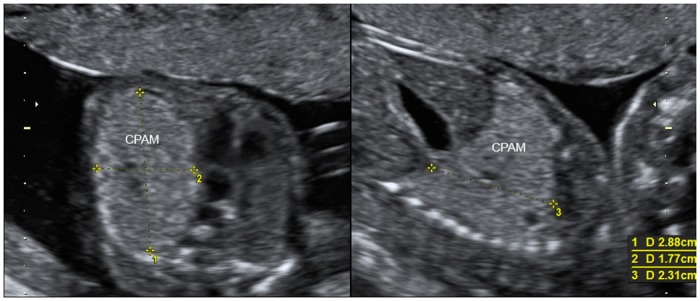
CVR (CPAM volume ratio): 2.88 x 1.77 x 2.31 x 0.52/16.1 (head circumference) = 0.38.
Fig 4. Receiver operator characteristics curve for the congenital pulmonary airway malformation-volume ratio (CVR) and composite adverse outcome or need for intervention.
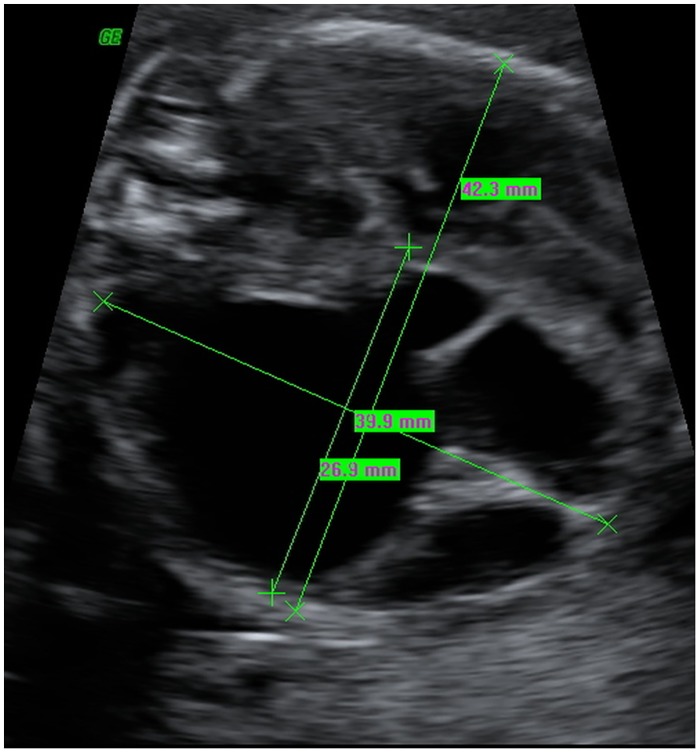
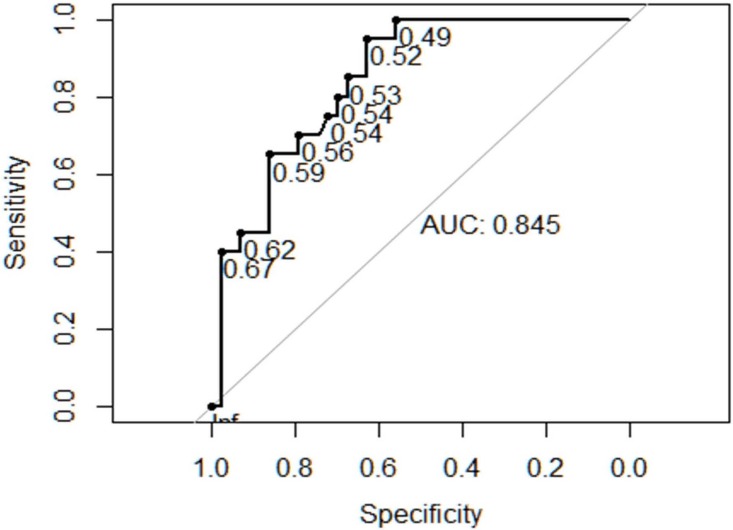
As well MTR (mass-to-thorax-ratio) (Fig 5) and adverse outcome were strongly associated (p<0.001). The measurement of the MTR was available in all cases. The area under the curve of receiver operator characteristics curves was 0.845 (Fig 6). A MTR of < 0.51 had a positive predictive value of 0.54 and a negative predictive value of 0.96 of adverse events with a sensitivity of 0.95 and a specificity of 0.63.
Fig 5. Transverse section of the fetal thorax at 19 weeks of gestation demonstrating a large macrozystic congenital pulmonary airway malformation (CPAM) with severe mediastinal shift.
MTR (Mass-to-thorax ratio): 26.9 / 42.3 = 0.63.
Fig 6. Receiver operator characteristics curve for Mass-to-thorax ratio (MTR) and composite adverse outcome or need for intervention.
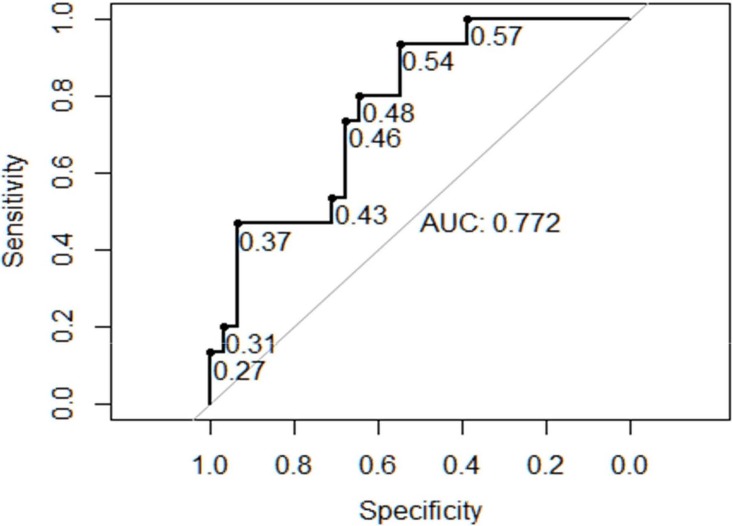
The measurement of the observed to expected lung area to head circumference (o/e LHR) was less informative than the other two already established prognostic markers, although significant (p = 0.0012). Due to strict criteria of measurement (three images at the exact plane of the four chamber view of the heart) not all of the retrospectively analyzed images could be included (50/67), in one case with hydrocephalus internus and elevated head circumference the ratios could not be calculated. The area under the curve was 0.877 and 0.845 for CVR and MTR in contrast to 0.772 for o/e LHR (Fig 7). An o/e LHR of 45% had a sensitivity of 0.73, a specificity of 0.68 and a positive predictive value of 0.52, a negative predictive value of 0.84 of prediction of adverse outcome. The cutoff value of 25%, which defines fetuses with poor outcome in cases of congenital diaphragmatic hernia, had a positive predictive value of 1.00 with a specificity of 1.00 in the cases with CPAM, but a very low sensitivity of 0.06.
Fig 7. Receiver operator characteristics curve for observed to expected lung-to-head ratio (o/e LHR) and composite adverse outcome or need for intervention.
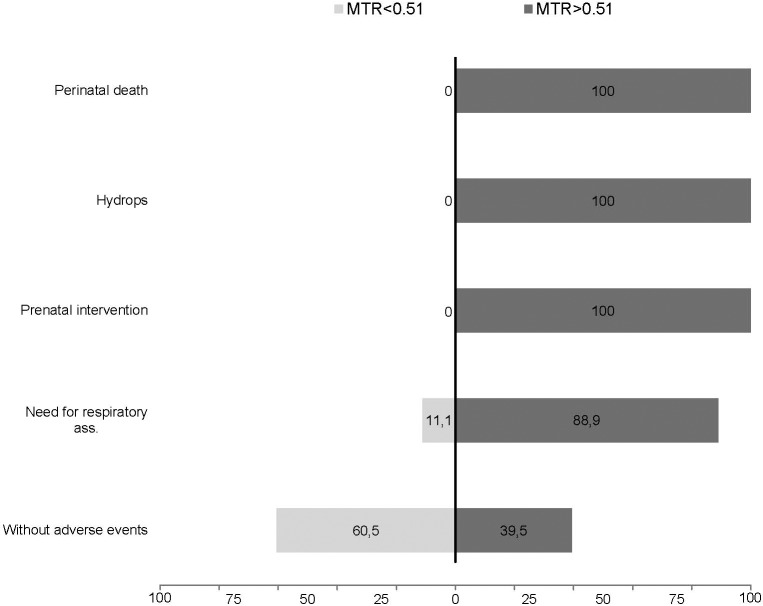
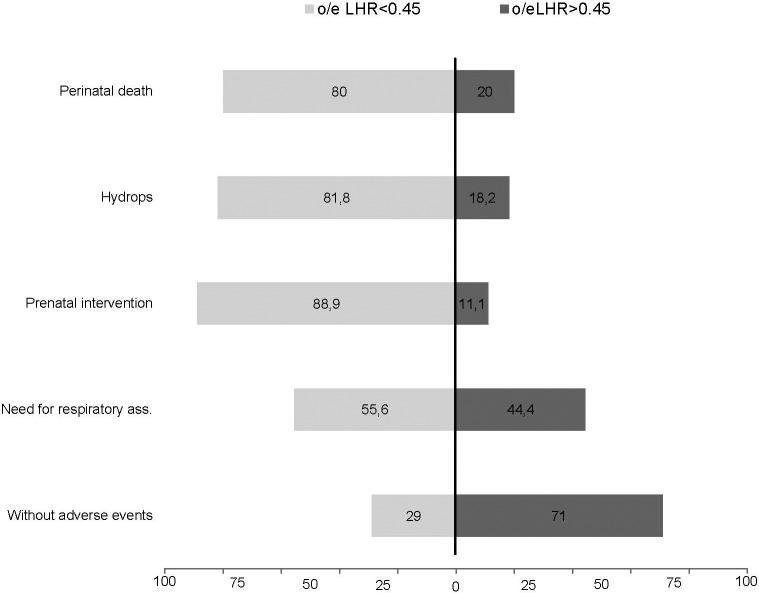
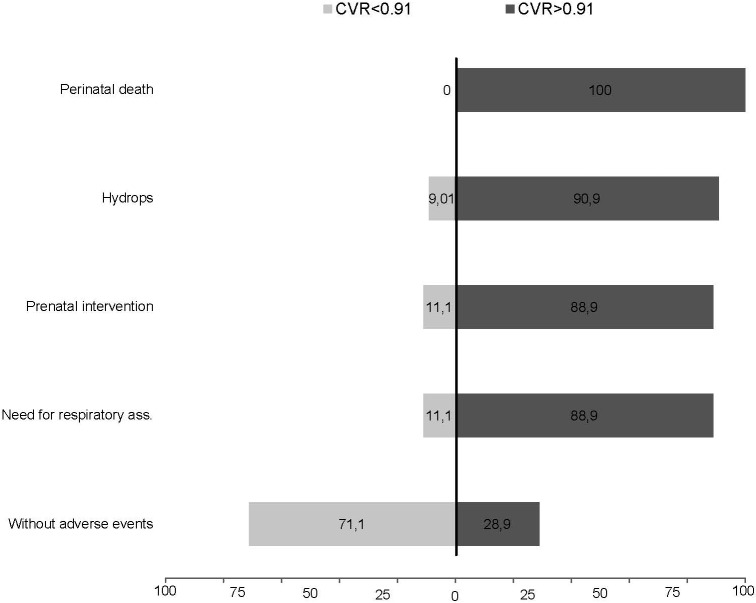
The proportion (%) of cases with each adverse event (perinatal death, hydrops, prenatal intervention, need for respiratory assistance) below and above the selected cutoff (MTR 0.51, CVR 0.91 and o/e LHR 45%) compared to the group of fetuses without adverse events is shown in Figs 8–10.
Fig 8. Detectionrate in % of adverse events (perinatal death, hydrops, prenatal intervention or need for respiratory assistance) at a cutoff of 0.51 mass-to-thorax rate (MTR) in comparison to fetuses without adverse events.
Fig 10. Detectionrate in % of adverse events (perinatal death, hydrops, prenatal intervention or need for respiratory assistance) at a cutoff of 45% observed to expected Lung to head ratio (LHR) in comparison to fetuses without adverse events.
Fig 9. Detectionrate in % of adverse events (perinatal death, hydrops, prenatal intervention or need for respiratory assistance) at a cutoff of 0.91 CPAM volume ratio (CVR) in comparison to fetuses without adverse events.
Logistic regression of a combination of CVR and MTR did not reveal additional information, and a model of the combined score CVR-MTR plus o/e LHR showed no advantage as well.
Discussion
Congenital pulmonary airway malformation and bronchopulmonary sequestration are the most common lung lesions identified by fetal ultrasound. As ultrasound resolution improves even smaller CPAMs and BPS are now being diagnosed with increasing frequency; the current incident is approximately 1 in 12.000–15.000 live births [14,15]. Moreover Doppler studies have contributed to the distinction between CPAM and BPS by identification of a feeding vessel [16].
CPAM is characterized by abnormal airway patterning during lung branching morphogenesis and is formed by abnormal branching of the immature bronchioles. Growth of the CPAM is unpredictable between 18–26 weeks of gestation. The fastest growth occurs between 20–25 weeks of gestation, a plateau is usually reached at 25 weeks and regression is observed after 29 weeks of gestation [1,3,6,17]. Whereas in the majority of cases the lesion either regresses throughout the pregnancy or remains unchanged and leads to favorable outcome, fetuses with hydrops and severe mediastinal shifting are at risk of intrauterine demise or neonatal death [2,3,12,18,19,20,21,22]. In order to predict fetuses at risk for adverse outcome several sonographic obtained measurements have been established [15,23,24].
Cromblehome et al. developed the CPAM volume to head circumference ratio (CVR) to create a gestational age corrected volume ratio for prognostic predication [1,25]. Fetuses with a CVR >1.6 were considered to be at increased risk for developing hydrops. Recently a study of 64 cases of lung lesions found the ratio of CVR >1.6 had a sensitivity of 75% and a positive predictive value of 60%, a negative predictive value of 98% for the detection of hydrops. The authors state that although an initial CVR >1.6 placed a fetus at an increased risk for poorer outcome in general, the cutoff of 1.6 was not sensitive enough to determine fetuses at risk. Therefore they propose a CRV cutoff value of <1.0, predicting a probability of near 100% for being asymptomatic at birth. Yong et al found that even a CVR <0.56 was predictive of good prognosis after birth (negative predictive value 100%) [23]. Our data support these findings suggesting slightly different cutoffs for negative prediction. As reported in the literature a CVR of >1.68 was associated with adverse outcome in our study, but already a CVR of < 0.91 seems to be effective to predict a good outcome with a sensitivity of 89% and a negative predictive value of 93%. In our cohort fetuses with a CVR of <0.56 had a negative predictive value of 1.00 with a sensitivity of 1.00 and a specificity of 0.40 for experiencing some kind of adverse outcome or need for intervention. Summarizing these findings a CVR less than 0.91 calculated at first presentation of the patient predicts a favorable outcome, and sonographic reevaluation can be carried out in larger intervals than in cases with more elevated CVR.
Other prognostic markers for survival of fetuses with CPAM are described by Vu et al. who report the outcome of 36 fetuses with large CPAM, including 27 hydropic fetuses [12]. The presence of hydrops was the most important prognostic indicator for poor outcome in fetuses with CPAMs. Additionally Vu et al. calculated the mass-to-thorax-ratio (MTR), measured on an axial image of the fetal chest, on which the four-chamber view of the heart was displayed and found that it was highly correlated with the risk of developing hydrops. An MTR cutoff value of 0.56 had an odds ratio of 13.0 for hydrops. Our data support the effectiveness of this marker for prediction of adverse outcome or need for intervention. A MTR of < 0.51 had a positive predictive value of 0.54 and a negative predictive value of 0.96 for adverse events with a sensitivity of 0.95 and a specificity of 0.63. As an easily obtained measurement during a sonographic scan it could be a reassuring tool for predicting outcome and counseling of the parents.
The observed to expected lung area to head ratio (o/e LHR) was first established in the prediction of survival in fetuses with isolated diaphragmatic hernia [13]. The authors found that survival was very poor for fetuses with isolated left sided hernia when the o/e LHR was ≤ 25%. Due to considerable overlap of individual fetuses regarding the value of o/e LHR in affected and normal fetuses, the sensitivity of o/e LHR in the prediction of survival was only 46% with a false positive rate of 10%. Since then the reliability and reproducibility of the o/e LHR was discussed controversial [26,27,28] and compared to fetal MR lung volumetry, which seems to be of slightly better predictive value [29,30]. The o/e LHR has not been used to predict survival in fetuses with intrathoracal lesions as CPAM before, and prognosis or tendency for regression are quite different in CPAM versus congenital diaphragmatic hernia. Whereas the extent of a herniation of intaabdominal organs into the thorax does not regress in the course of pregnancy, CPAM, especially microcystic lesions, have a considerable amount of regression after 29 weeks of gestation. Moreover the survival of fetuses with CDH is remarkably worse than those with CPAM. This might be the reason, that our results regarding LHR are less sensitive as CVR or MTR. Additionally the measurement of o/e LHR was conducted retrospectively in our cohort, which might be less exact than a prospective evaluation.
Regarding other sonographic findings such as the severity of mediastinal shift or presence of polyhydramnios no correlation with the risk of hydrops were found in most studies [12,21,31], whereas macrocystic lesions are known to be associated with hydrops and poor prognosis [1,4,12] probably due to faster expansion of large cysts. Regression of the lesion is reported in 20%-76% in previous series [7,31,32,33], mostly in the microcystic type[2,34]. These findings are congruent to our own observations. The majority of fetuses with CPAM and hydrops had macrocystic lesions with no tendency of regression, whereas regression occurred in 55% in our cohort, predominantly in microcystic lesions.
In cases of non-hydropic fetuses with CPAM the prognosis is excellent with high survival rates and low postnatal morbidity, whereas fetuses with hydrops managed expectantly usually die either before or after delivery [2,3,35]. Therefore numerous attempts of fetal therapy have been made such as thoraco-amniotic shunting or thoracocentesis [5,22,36], prenatal open surgery with hysterotomy and lobectomy [3,6,37] and ultrasound-guided laser ablation [22,38,39], recently described by fetal bronchoscopy[40], or percutaneous radiofrequency [2]. Finally the treatment of fetuses with maternal steriod application is described in small retrospective trials and seems to be effective in microcystic lesions in presence of hydrops, where survival rates with expectant management are described as very low [41,42,43,44]. However, the spontaneous regression rate of microcystic lesions is high and prospective randomized trials are lacking.
Cumulating the recent studies of Cavoretto et al. and Lima et al. with our own findings results in an excellent survival rate for uncomplicated, conservatively managed CPAM (97.3%, 694/713) [2,45] and also for non-hydropic fetuses treated with thoraco-amniotic shunting (22/25, 88%), whereas hydropic fetuses with thoraco-amniotic shunting and intention to treat survive in about 58.7% (54/92) of cases.
Shunt-related complications such as shunt dislocation or shunt occlusion are described in most series. A recent overview on 286 shunt insertions for treatment of fetal pleural effusion revealed 53 shunt-related complications, of which 18 migrations of the shunt into the thorax or the amniotic cavity were noted[22]. However, in our series shunt dislocation occurred much more frequently (6 /15 procedures), one case presented with four consecutive shunt dislocations. In all cases we used the double pigtail Harrison fetal bladder stent (Cook Medical, Bloomington, IN, USA), which is similar to the Rocket KCH ™ Fetal bladder drainage catheter used by other series [46] and provides no anchor system. Recently different types of fetal bladder stents including an anchor system were developed, such as the double basket device [47] or the Somatex intrauterine shunt (Somatex Medical Technologies, Berlin,Germany), which is fixed in the fetal thoracic wall with self-developing parasols. In recent cases of necessity for fetal drainage we use the Somatex sten in order to reduce shunt complications.
Surgical resection is the standard of postnatal care for symptomatic lung lesions; however, the management of asymptomatic CPAM remains controversial [48,49,50,51]. The majority of authors recommend surgical resection to avoid the possible development of complications such as recurrent infection, pneumothorax or rarely development of malignancy [1,4,37,52,53,54,55,56,57,58,59].
In summary the prognosis of antenatally diagnosed CPAM is excellent in the absence of complications such as hydrops fetalis. Fetuses at risk for intrauterine or postnatal death, need for fetal intervention or postnatal respiratory assistance can be detected by CVR, MTR and less sensitive by o/e LHR. Short-term follow up scans, if necessary intrauterine shunting and planned delivery in a tertiary care center are mandatory in these cases.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1.Crombleholme TM, Coleman B, Hedrick H, Liechty K, Howell L, Flake AW, et al. (2002) Cystic adenomatoid malformation volume ratio predicts outcome in prenatally diagnosed cystic adenomatoid malformation of the lung. J Pediatr Surg 37: 331–338. [DOI] [PubMed] [Google Scholar]
- 2.Cavoretto P, Molina F, Poggi S, Davenport M, Nicolaides KH (2008) Prenatal diagnosis and outcome of echogenic fetal lung lesions. Ultrasound Obstet Gynecol 32: 769–783. 10.1002/uog.6218 [DOI] [PubMed] [Google Scholar]
- 3.Adzick NS, Harrison MR, Crombleholme TM, Flake AW, Howell LJ (1998) Fetal lung lesions: management and outcome. Am J Obstet Gynecol 179: 884–889. [DOI] [PubMed] [Google Scholar]
- 4.Davenport M, Warne SA, Cacciaguerra S, Patel S, Greenough A, Nicolaides K (2004) Current outcome of antenally diagnosed cystic lung disease. J Pediatr Surg 39: 549–556. [DOI] [PubMed] [Google Scholar]
- 5.Witlox RS, Lopriore E, Oepkes D (2011) Prenatal interventions for fetal lung lesions. Prenat Diagn 31: 628–636. 10.1002/pd.2778 [DOI] [PubMed] [Google Scholar]
- 6.Wilson RD, Hedrick HL, Liechty KW, Flake AW, Johnson MP, Bebbington M, et al. (2006) Cystic adenomatoid malformation of the lung: review of genetics, prenatal diagnosis, and in utero treatment. Am J Med Genet A 140: 151–155. [DOI] [PubMed] [Google Scholar]
- 7.Laberge JM, Flageole H, Pugash D, Khalife S, Blair G, Filiatrault D, et al. (2001) Outcome of the prenatally diagnosed congenital cystic adenomatoid lung malformation: a Canadian experience. Fetal Diagn Ther 16: 178–186. [DOI] [PubMed] [Google Scholar]
- 8.Wall J, Coates A (2014) Prenatal imaging and postnatal presentation, diagnosis and management of congenital lung malformations. Curr Opin Pediatr 26: 315–319. 10.1097/MOP.0000000000000091 [DOI] [PubMed] [Google Scholar]
- 9.Stoiber B, Moehrlen U, Kurmanavicius J, Meuli M, Haslinger C, Zimmermann R, et al. (2015) Congenital Lung Lesion: Prenatal Course, Therapy and Predictors of Perinatal Outcome. Ultraschall Med. [DOI] [PubMed] [Google Scholar]
- 10.Durell J, Lakhoo K (2014) Congenital cystic lesions of the lung. Early Hum Dev 90: 935–939. 10.1016/j.earlhumdev.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 11.Stocker JT, Madewell JE, Drake RM (1977) Congenital cystic adenomatoid malformation of the lung. Classification and morphologic spectrum. Hum Pathol 8: 155–171. [DOI] [PubMed] [Google Scholar]
- 12.Vu L, Tsao K, Lee H, Nobuhara K, Farmer D, Harrison M, et al. (2007) Characteristics of congenital cystic adenomatoid malformations associated with nonimmune hydrops and outcome. J Pediatr Surg 42: 1351–1356. [DOI] [PubMed] [Google Scholar]
- 13.Jani J, Nicolaides KH, Keller RL, Benachi A, Peralta CF, Favre R, et al. (2007) Observed to expected lung area to head circumference ratio in the prediction of survival in fetuses with isolated diaphragmatic hernia. Ultrasound Obstet Gynecol 30: 67–71. [DOI] [PubMed] [Google Scholar]
- 14.Adzick NS, Harrison MR (1993) Management of the fetus with a cystic adenomatoid malformation. World J Surg 17: 342–349. [DOI] [PubMed] [Google Scholar]
- 15.Ehrenberg-Buchner S, Stapf AM, Berman DR, Drongowski RA, Mychaliska GB, Treadwell MC, et al. (2013) Fetal lung lesions: can we start to breathe easier? Am J Obstet Gynecol 208: 151 e151–157. [DOI] [PubMed] [Google Scholar]
- 16.Achiron R, Hegesh J, Yagel S (2004) Fetal lung lesions: a spectrum of disease. New classification based on pathogenesis, two-dimensional and color Doppler ultrasound. Ultrasound Obstet Gynecol 24: 107–114. [DOI] [PubMed] [Google Scholar]
- 17.Kunisaki SM, Barnewolt CE, Estroff JA, Ward VL, Nemes LP, Fauza DO, et al. (2007) Large fetal congenital cystic adenomatoid malformations: growth trends and patient survival. J Pediatr Surg 42: 404–410. [DOI] [PubMed] [Google Scholar]
- 18.Baird R, Puligandla PS, Laberge JM (2014) Congenital lung malformations: informing best practice. Semin Pediatr Surg 23: 270–277. 10.1053/j.sempedsurg.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 19.Thorpe-Beeston JG, Nicolaides KH (1994) Cystic adenomatoid malformation of the lung: prenatal diagnosis and outcome. Prenat Diagn 14: 677–688. [DOI] [PubMed] [Google Scholar]
- 20.Rice HE, Estes JM, Hedrick MH, Bealer JF, Harrison MR, Adzick NS (1994) Congenital cystic adenomatoid malformation: a sheep model of fetal hydrops. J Pediatr Surg 29: 692–696. [DOI] [PubMed] [Google Scholar]
- 21.Bunduki V, Ruano R, da Silva MM, Miguelez J, Miyadahira S, Maksoud JG, et al. (2000) Prognostic factors associated with congenital cystic adenomatoid malformation of the lung. Prenat Diagn 20: 459–464. [DOI] [PubMed] [Google Scholar]
- 22.Grabowska K, Wilson RD. Fetal lung growth, development, and lung fluid. Clinical management of pleural effusion and pulmonary pathology In: Kilby M, Johnson A, Oepkes D (2013) Fetal therapy: scientific basis and critical appraisal of clinical benefits. Cambridge: New York: Cambridge University Press; xv, 453 p. p. [Google Scholar]
- 23.Yong PJ, Von Dadelszen P, Carpara D, Lim K, Kent N, Tessier F, et al. (2012) Prediction of pediatric outcome after prenatal diagnosis and expectant antenatal management of congenital cystic adenomatoid malformation. Fetal Diagn Ther 31: 94–102. 10.1159/000331936 [DOI] [PubMed] [Google Scholar]
- 24.Hadchouel A, Benachi A, Revillon Y, Rousseau V, Martinovic J, Verkarre V, et al. (2011) Factors associated with partial and complete regression of fetal lung lesions. Ultrasound Obstet Gynecol 38: 88–93. 10.1002/uog.8909 [DOI] [PubMed] [Google Scholar]
- 25.Adzick NS, Flake AW, Crombleholme TM (2003) Management of congenital lung lesions. Semin Pediatr Surg 12: 10–16. [DOI] [PubMed] [Google Scholar]
- 26.Heling KS, Wauer RR, Hammer H, Bollmann R, Chaoui R (2005) Reliability of the lung-to-head ratio in predicting outcome and neonatal ventilation parameters in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 25: 112–118. [DOI] [PubMed] [Google Scholar]
- 27.Odibo AO, Najaf T, Vachharajani A, Warner B, Mathur A, Warner BW (2010) Predictors of the need for extracorporeal membrane oxygenation and survival in congenital diaphragmatic hernia: a center's 10-year experience. Prenat Diagn 30: 518–521. 10.1002/pd.2508 [DOI] [PubMed] [Google Scholar]
- 28.Cruz-Martinez R, Figueras F, Moreno-Alvarez O, Martinez JM, Gomez O, Hernandez-Andrade E, et al. (2010) Learning curve for lung area to head circumference ratio measurement in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 36: 32–36. 10.1002/uog.7577 [DOI] [PubMed] [Google Scholar]
- 29.Kilian AK, Busing KA, Schuetz EM, Schaible T, Neff KW (2009) Fetal MR lung volumetry in congenital diaphragmatic hernia (CDH): prediction of clinical outcome and the need for extracorporeal membrane oxygenation (ECMO). Klin Padiatr 221: 295–301. 10.1055/s-0029-1192022 [DOI] [PubMed] [Google Scholar]
- 30.Bebbington M, Victoria T, Danzer E, Moldenhauer J, Khalek N, Johnson M, et al. (2014) Comparison of ultrasound and magnetic resonance imaging parameters in predicting survival in isolated left-sided congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 43: 670–674. 10.1002/uog.13271 [DOI] [PubMed] [Google Scholar]
- 31.Ierullo AM, Ganapathy R, Crowley S, Craxford L, Bhide A, Thilaganathan B (2005) Neonatal outcome of antenatally diagnosed congenital cystic adenomatoid malformations. Ultrasound Obstet Gynecol 26: 150–153. [DOI] [PubMed] [Google Scholar]
- 32.Dommergues M, Louis-Sylvestre C, Mandelbrot L, Aubry MC, Revillon Y, Jarreau PH, et al. (1997) Congenital adenomatoid malformation of the lung: when is active fetal therapy indicated? Am J Obstet Gynecol 177: 953–958. [DOI] [PubMed] [Google Scholar]
- 33.Adzick NS (2009) Management of fetal lung lesions. Clin Perinatol 36: 363–376, x 10.1016/j.clp.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 34.Kunisaki SM, Ehrenberg-Buchner S, Dillman JR, Smith EA, Mychaliska GB, Treadwell MC (2015) Vanishing fetal lung malformations: Prenatal sonographic characteristics and postnatal outcomes. J Pediatr Surg 50: 978–982. 10.1016/j.jpedsurg.2015.03.025 [DOI] [PubMed] [Google Scholar]
- 35.Grethel EJ, Wagner AJ, Clifton MS, Cortes RA, Farmer DL, Harrison MR, et al. (2007) Fetal intervention for mass lesions and hydrops improves outcome: a 15-year experience. J Pediatr Surg 42: 117–123. [DOI] [PubMed] [Google Scholar]
- 36.Schrey S, Kelly EN, Langer JC, Davies GA, Windrim R, Seaward PG, et al. (2012) Fetal thoracoamniotic shunting for large macrocystic congenital cystic adenomatoid malformations of the lung. Ultrasound Obstet Gynecol 39: 515–520. 10.1002/uog.11084 [DOI] [PubMed] [Google Scholar]
- 37.Adzick NS (2010) Open fetal surgery for life-threatening fetal anomalies. Semin Fetal Neonatal Med 15: 1–8. 10.1016/j.siny.2009.05.003 [DOI] [PubMed] [Google Scholar]
- 38.Ruano R, da Silva MM, Salustiano EM, Kilby MD, Tannuri U, Zugaib M (2012) Percutaneous laser ablation under ultrasound guidance for fetal hyperechogenic microcystic lung lesions with hydrops: a single center cohort and a literature review. Prenat Diagn 32: 1127–1132. 10.1002/pd.3969 [DOI] [PubMed] [Google Scholar]
- 39.Cruz-Martinez R, Mendez A, Duenas-Riano J, Ordorica-Flores R, Nieto-Zermeno J, Malagon-Salazar P, et al. (2015) Fetal laser surgery prevents fetal death and avoids the need for neonatal sequestrectomy in cases with bronchopulmonary sequestration. Ultrasound Obstet Gynecol 46: 627–628. 10.1002/uog.14921 [DOI] [PubMed] [Google Scholar]
- 40.Cruz-Martinez R, Mendez A, Perez-Garcilita O, Monroy A, Aguilar-Vidales K, Cruz-Martinez MA, et al. (2015) Fetal bronchoscopy as a useful procedure in a case with prenatal diagnosis of congenital microcystic adenomatoid malformation. Fetal Diagn Ther 37: 75–80. 10.1159/000361015 [DOI] [PubMed] [Google Scholar]
- 41.Curran PF, Jelin EB, Rand L, Hirose S, Feldstein VA, Goldstein RB, et al. (2010) Prenatal steroids for microcystic congenital cystic adenomatoid malformations. J Pediatr Surg 45: 145–150. 10.1016/j.jpedsurg.2009.10.025 [DOI] [PubMed] [Google Scholar]
- 42.Morris LM, Lim FY, Livingston JC, Polzin WJ, Crombleholme TM (2009) High-risk fetal congenital pulmonary airway malformations have a variable response to steroids. J Pediatr Surg 44: 60–65. 10.1016/j.jpedsurg.2008.10.012 [DOI] [PubMed] [Google Scholar]
- 43.Loh KC, Jelin E, Hirose S, Feldstein V, Goldstein R, Lee H (2012) Microcystic congenital pulmonary airway malformation with hydrops fetalis: steroids vs open fetal resection. J Pediatr Surg 47: 36–39. 10.1016/j.jpedsurg.2011.10.015 [DOI] [PubMed] [Google Scholar]
- 44.Yamashita A, Hidaka N, Yamamoto R, Nakayama S, Sasahara J, Ishii K, et al. (2015) In utero resolution of microcystic congenital cystic adenomatoid malformation after prenatal betamethasone therapy: A report of three cases and a literature review. J Clin Ultrasound 43: 451–457. 10.1002/jcu.22214 [DOI] [PubMed] [Google Scholar]
- 45.Lima JS, Camargos PA, Aguiar RA, Campos AS, Aguiar MJ (2014) Pre and perinatal aspects of congenital cystic adenomatoid malformation of the lung. J Matern Fetal Neonatal Med 27: 228–232. 10.3109/14767058.2013.807236 [DOI] [PubMed] [Google Scholar]
- 46.Yinon Y, Grisaru-Granovsky S, Chaddha V, Windrim R, Seaward PG, Kelly EN, et al. (2010) Perinatal outcome following fetal chest shunt insertion for pleural effusion. Ultrasound Obstet Gynecol 36: 58–64. 10.1002/uog.7507 [DOI] [PubMed] [Google Scholar]
- 47.Miyoshi T, Katsuragi S, Ikeda T, Horiuchi C, Kawasaki K, Kamiya CA, et al. (2013) Retrospective review of thoracoamniotic shunting using a double-basket catheter for fetal chylothorax. Fetal Diagn Ther 34: 19–25. 10.1159/000348776 [DOI] [PubMed] [Google Scholar]
- 48.Ng C, Stanwell J, Burge DM, Stanton MP (2014) Conservative management of antenatally diagnosed cystic lung malformations. Arch Dis Child 99: 432–437. 10.1136/archdischild-2013-304048 [DOI] [PubMed] [Google Scholar]
- 49.Stanton M, Njere I, Ade-Ajayi N, Patel S, Davenport M (2009) Systematic review and meta-analysis of the postnatal management of congenital cystic lung lesions. J Pediatr Surg 44: 1027–1033. 10.1016/j.jpedsurg.2008.10.118 [DOI] [PubMed] [Google Scholar]
- 50.Sauvat F, Michel JL, Benachi A, Emond S, Revillon Y (2003) Management of asymptomatic neonatal cystic adenomatoid malformations. J Pediatr Surg 38: 548–552. [DOI] [PubMed] [Google Scholar]
- 51.Aziz D, Langer JC, Tuuha SE, Ryan G, Ein SH, Kim PC (2004) Perinatally diagnosed asymptomatic congenital cystic adenomatoid malformation: to resect or not? J Pediatr Surg 39: 329–334; discussion 329–334. [DOI] [PubMed] [Google Scholar]
- 52.Laberge JM, Puligandla P, Flageole H (2005) Asymptomatic congenital lung malformations. Semin Pediatr Surg 14: 16–33. [DOI] [PubMed] [Google Scholar]
- 53.Granata C, Gambini C, Balducci T, Toma P, Michelazzi A, Conte M, et al. (1998) Bronchioloalveolar carcinoma arising in congenital cystic adenomatoid malformation in a child: a case report and review on malignancies originating in congenital cystic adenomatoid malformation. Pediatr Pulmonol 25: 62–66. [DOI] [PubMed] [Google Scholar]
- 54.Papagiannopoulos K, Hughes S, Nicholson AG, Goldstraw P (2002) Cystic lung lesions in the pediatric and adult population: surgical experience at the Brompton Hospital. Ann Thorac Surg 73: 1594–1598. [DOI] [PubMed] [Google Scholar]
- 55.Nasr A, Himidan S, Pastor AC, Taylor G, Kim PC (2010) Is congenital cystic adenomatoid malformation a premalignant lesion for pleuropulmonary blastoma? J Pediatr Surg 45: 1086–1089. 10.1016/j.jpedsurg.2010.02.067 [DOI] [PubMed] [Google Scholar]
- 56.Li J, Chen GS, Zhang X, Moore L, Cheng H (2014) Congenital cystic adenomatoid malformation with associated mucinous bronchioloalveolar carcinoma in a neonate. Fetal Pediatr Pathol 33: 29–34. 10.3109/15513815.2013.842272 [DOI] [PubMed] [Google Scholar]
- 57.Ozcan C, Celik A, Ural Z, Veral A, Kandiloglu G, Balik E (2001) Primary pulmonary rhabdomyosarcoma arising within cystic adenomatoid malformation: a case report and review of the literature. J Pediatr Surg 36: 1062–1065. [DOI] [PubMed] [Google Scholar]
- 58.Priest JR, Williams GM, Hill DA, Dehner LP, Jaffe A (2009) Pulmonary cysts in early childhood and the risk of malignancy. Pediatr Pulmonol 44: 14–30. 10.1002/ppul.20917 [DOI] [PubMed] [Google Scholar]
- 59.Miniati DN, Chintagumpala M, Langston C, Dishop MK, Olutoye OO, Nuchtern JG, et al. (2006) Prenatal presentation and outcome of children with pleuropulmonary blastoma. J Pediatr Surg 41: 66–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.