Abstract
Objective
Non-right handedness (NRH) is reportedly more common in very preterm (VPT; <32 weeks’ gestation) children compared with term-born peers, but it is unclear whether neonatal brain injury or altered brain morphology and microstructure underpins NRH in this population. Given that NRH has been inconsistently reported to be associated with cognitive and motor difficulties, this study aimed to examine associations between handedness and neurodevelopmental outcomes in VPT 7 year-olds. Further, the relationship between neonatal brain injury and integrity of motor tracts (corpus callosum and corticospinal tract) with handedness at age 7 years in VPT children was explored.
Method
One hundred and seventy-five VPT and 69 term-born children completed neuropsychological and motor assessments and a measure of handedness at 7 years’ corrected age. At term-equivalent age, brain injury on MRI was assessed and diffusion tensor measures were obtained for the corpus callosum and posterior limb of the internal capsule.
Results
There was little evidence of stronger NRH in the VPT group compared with term controls (regression coefficient [b] −1.95, 95% confidence interval [CI] −5.67 to 1.77). Poorer academic and working memory outcomes were associated with stronger NRH in the VPT group. While there was little evidence that neonatal unilateral brain injury was associated with stronger NRH, increased area and fractional anisotropy of the corpus callosum splenium were predictive of stronger NRH in the VPT group.
Conclusions
VPT birth may alter the relationship between handedness and academic outcomes, and neonatal corpus callosum integrity predicts hand preference in VPT children at school age.
Keywords: Functional laterality, Premature birth, Cognition, Child development, Diffusion Tensor Imaging, Magnetic Resonance Imaging
Introduction
A higher prevalence of mixed and left (non-right) handedness has been reported in preterm (<37 weeks’ gestation) and low birthweight (<2500 g) children compared with term born peers (Marlow, Roberts, & Cooke, 1989; Powls, Botting, Cooke, & Marlow, 1996). Findings from a recent meta-analysis exploring handedness in preterm children found that, based on 18 case-control studies with a total of 1947 cases and 8170 controls, the rate of non-right handedness (NRH) in the preterm population was approximately 22%, compared with 12% in term children (Domellof, Johansson, & Ronnqvist, 2011).
NRH has been associated with atypical development and poorer outcomes in preterm and low birthweight populations (Domellof et al., 2011), however past research has been limited and conflicting. Some studies have reported NRH to be associated with lower IQ, language delays, poorer motor coordination and more difficult temperament (O'Callaghan, Burn, Mohay, Rogers, & et al., 1993; Ross, Lipper, & Auld, 1987), with stronger right-handed preference related to better cognitive development (Marlow, Hennessy, Bracewell, & Wolke, 2007). Other studies, however, have reported similar cognitive, academic, and ball and balance skill performances between non-right and right-handed very low and extremely low birthweight children (Powls et al., 1996; Saigal, Rosenbaum, Szatmari, & Hoult, 1992), with little evidence that hand preference is related to the presence or severity of cerebral palsy or neurological classification (Ross et al., 1987). These inconsistent findings may reflect differences in measures of handedness and age of participants (4-6 years olds versus 8-12 year olds), with studies involving older children finding little association. Hand preference may be more influenced by social, cultural or educational effects in older compared with younger children (Medland, Perelle, De Monte, & Ehrman, 2004). Furthermore, previous studies report on cohorts born several decades ago and may not be representative of more contemporary cohorts of VPT children in which survival is greater for those born at the limits of viability. This pattern of atypical development for NRH is not characteristically seen in the general population (Somers, Shields, Boks, Kahn, & Sommer, 2015). However, the literature remains mixed even within the general population, with some studies reporting lower levels of achievement and cognitive performance for left-handed individuals (Johnston, Nicholls, Shah, & Shields, 2009; Nicholls, Chapman, Loetscher, & Grimshaw, 2010; Resch et al., 1997).
Various theories have been proposed to explain the increased NRH prevalence and potential associated cognitive and motor deficits observed in preterm individuals, including genetic or hereditary factors. While the contribution of genetics and familial handedness to hand preference has mostly been explored in the general population (Annett, 1985; McManus, 1991; Medland et al., 2009), these factors may influence the process of lateralization in VPT populations alike. One study maintained a higher incidence of NRH in preterm compared with term-born children even after accounting for familial handedness (Ross et al., 1987). However, further research is required to investigate the extent of the contribution of heritability to NRH in VPT children.
Currently, the prominent theory for increased NRH in preterm individuals is that NRH may reflect a disturbance of cerebral lateralization and underlying neurological abnormality (Bakan, Dibb, & Reed, 1973; Coren & Porac, 1980; Satz, Orsini, Saslow, & Henry, 1985). However, previous findings have been inconsistent, with some studies illustrating an association between neurological abnormalities (abnormal neurological examination or cerebral ultrasound) and hand preference (Allin et al., 2006; Saigal et al., 1992), and others failing to detect evidence of such a relationship (Marlow et al., 1989; Powls et al., 1996).
It may be that the hemisphere involved in focal neurological damage is important to consider for NRH in preterm children, with one small study finding a trend for greater left-sided intraventricular hemorrhage in non-right handed preterm children based on cranial ultrasound (Powls et al., 1996). Neonatal magnetic resonance imaging (MRI) is a sensitive diagnostic tool for detecting white matter punctate lesions, cerebellar injury or subcortical insults in preterm children (Inder, Anderson, Spencer, Wells, & Volpe, 2003; Hiroyuki Kidokoro et al., 2014), however the relationship between these pathologies and handedness have not yet been explored. In older children, MRI studies (Caldu et al., 2006; Kesler et al., 2006; Lancefield et al., 2006; Nosarti et al., 2004), have found no association of handedness with fronto-occipital asymmetry (Lancefield et al., 2006), temporal gyral width (Kesler et al., 2006) or corpus callosum size (Nosarti et al., 2004) in VPT cohorts.
Given the possible association between neurological abnormality and NRH, utilization of diffusion weighted imaging of implicated motor tracts, such as the corpus callosum (CC) and corticospinal tract (CST), may help clarify the relationship between handedness and cerebral morphology. Diffusion weighted imaging, and more specifically diffusion tensor imaging, enables examination of the microstructural properties of cerebral white matter tracts, providing several measures indicative of tract integrity, maturity and organization. Specifically, fractional anisotropy (FA) is a measure of directional water diffusion and higher FA is indicative of greater white matter organization. The apparent diffusion coefficient, or mean diffusivity (MD), is an overall measure of diffusion, with axial diffusivity (AD) being a measure of diffusion parallel to white matter tracts, and radial diffusivity (RD) being an estimate of diffusion perpendicular to white matter tracts. Lower diffusivity is associated with greater maturation of white matter tracts and diffusivity is known to decrease with age (Huppi et al., 2001).
The corpus callosum (CC), a vital white matter structure that is regarded as a marker of functional laterality, has been associated with NRH in the normal population (Witelson, 1985). Some studies have shown that non-right handed individuals have a larger CC than their right handed counterparts (Tuncer, Hatipoglu, & Ozates, 2005; Witelson, 1985, 1989); however, this has not been consistently reported (Welcome et al., 2009; Westerhausen et al., 2004). Furthermore, left-handedness has been reported to be associated with higher FA and lower MD in the CC (Westerhausen et al., 2004; Westerhausen et al., 2003). The posterior limb of the internal capsule (PLIC) is another region thought to be associated with handedness (Westerhausen, Huster, Kreuder, Wittling, & Schweiger, 2007). Corticospinal tract (CST) fibres that arise from the primary motor and premotor areas and descend through the PLIC are known to be functionally involved in fine and gross movements (e.g., finger, hand and arm). While much is known about handedness-related asymmetries in the cortical motor areas, such as the premotor and sensorimotor regions (Herve, Crivello, Perchey, Mazoyer, & Tzourio-Mazoyer, 2006; Westerhausen et al., 2007), findings regarding handedness-related asymmetries of CST connections, such as the PLIC, are controversial, requiring further investigation (Seizeur et al., 2014; Westerhausen et al., 2007).
Preterm birth is thought to alter the microstructure and morphology of the CC and PLIC. Research suggests that CC size, shape, FA and diffusivity are reduced or altered in preterm populations compared with controls, particularly in the posterior subregions (Andrews et al., 2010; Caldu et al., 2006; Constable et al., 2008; Lawrence et al., 2010; Nosarti et al., 2004; Skiöld et al., 2010; Thompson et al., 2011). Similar findings have been reported within the CST, with VPT infants with moderate to severe brain abnormalities (intraventricular hemorrhage and ventriculomegaly on MRI) exhibiting impaired CST microstructural development as they reach term-equivalent age compared with VPT infants without abnormalities on MRI (Adams et al., 2010). However, studies examining the association between CC and PLIC morphology and microstructure and NRH in VPT children are scarce, although one study reported no relationship between handedness and CC size (Nosarti et al., 2004). We are not aware of any studies that have investigated the relationship between PLIC microstructural organization and NRH in VPT children.
The current study aimed to: (1) examine the association between handedness and neurodevelopmental outcomes at age 7 years and whether this relationship varies between VPT and term-born children; (2) explore the relationship between the hemisphere of neonatal brain injury and NRH in VPT children, and (3) evaluate the associations between neonatal CC and PLIC microstructural organization with NRH in VPT children.
It was hypothesized that stronger NRH would be associated with poorer neurodevelopmental outcomes. It was also expected that VPT children with left-sided neonatal cerebral injury would more likely exhibit stronger NRH at 7 years of age than VPT children with right-sided neonatal cerebral injury. Lastly, it was hypothesized that greater CC area, higher fractional anisotropy and lower diffusivity, particularly in the posterior CC subregions (i.e. isthmus and splenium), would be associated with stronger NRH in VPT children. Stronger rightward PLIC asymmetry (e.g. higher fractional anisotropy, lower diffusivity) was also expected to be associated with stronger NRH in VPT children.
Material and methods
Participants
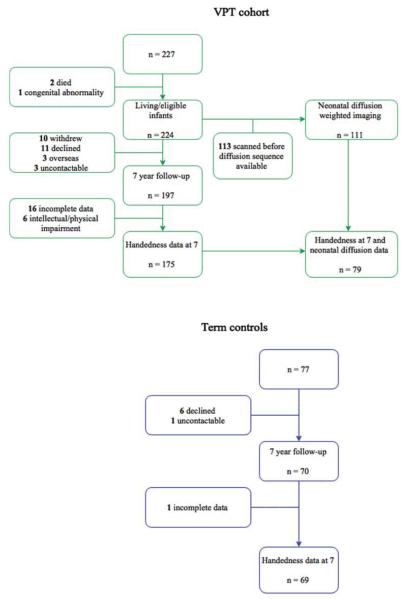
Participants were part of the Victorian Infant Brain Studies (VIBeS) cohort, a prospective, longitudinal study examining the development of children born VPT (<30 weeks’ gestational age (GA) and/or very low birthweight (<1250 g). Recruitment occurred between July 2001 and December 2003 from the Royal Women’s Hospital in Melbourne, Victoria, Australia. Two hundred and twenty seven eligible VPT infants without genetic or congenital abnormalities known to affect development were recruited; however two infants subsequently died, and 1 was later excluded due to a late diagnosis of congenital infection known to affect developmental outcome, leaving 224 VPT infants. A term control group (37 to 42 weeks’ GA and/or birthweight ≥2500g) consisting of 77 infants were recruited either at birth from the Royal Women’s Hospital or the community (n = 46), or at 2 years of age from Maternal and Child Health Centers within metropolitan Melbourne (n=31). Neonatal brain MRI was performed on all VPT and term infants who were recruited during the neonatal period. Within the VPT sample, 111 infants had diffusion weighted imaging. This smaller sample was due to the late inclusion of diffusion sequences in the neonatal scanning protocol. Those with diffusion data were similar to those without in their neonatal and demographic characteristics. Of these, 79 VPT infants had useable diffusion data and handedness data at age 7 years (Figure 1). Follow-up assessments were conducted at 2, 5 and 7 years of age corrected for prematurity for all children (Reidy et al., 2013; Roberts, Lim, Doyle, & Anderson, 2011; Thompson et al., 2008; Treyvaud et al., 2013). At 7 years of age, 197 VPT children (88% of survivors) and 70 term children (91%) were seen for follow-up. Handedness data were available for 175 VPT and 69 term children at age 7 years (Figure 1). Reasons for missing handedness information in both groups were due to incomplete handedness inventories (n = 17) or intellectual and/or physical disability that made inventory administration unreliable (n = 6) (Figure 1). The original and follow-up studies were approved by the Human Research Ethics Committee of the Royal Women’s Hospital and the Royal Children’s Hospital in Melbourne, Australia. Written consent as obtained from families prior to data collection.
Figure 1.
Flowchart of participants in the current study.
n.b. Data collection at the 2 and 5 year follow-up not included.
Procedure
During the perinatal period, a neonatal assessment and a term-equivalent MRI scan were performed. The 7-year follow-up included a neuropsychological assessment, and parent-report questionnaires relating to the child’s behavior and daily functioning.
Measures
Perinatal measures
Perinatal data were obtained from chart review and information pertaining to gestational age (GA), sex, birth weight, multiple birth, length of hospital stay, bronchopulmonary dysplasia, cystic periventricular leukomalacia, sepsis, necrotizing enterocolitis, patent ductus arteriosus and grade 3 or 4 intraventricular haemorrhage was recorded. Bronchopulmonary dysplasia was defined as the requirement for oxygen at or beyond 36 weeks’ GA. Necrotizing enterocolitis and sepsis comprised proven episodes, and intraventricular haemorrhage was recorded as the highest grade from serial ultrasound scans throughout the neonatal intensive care course (Shah et al., 2008).
MRI
At term-equivalent age, infants were scanned in a 1.5 General Electric MRI scanner (Signa LX Echospeed System; General Electric, Fairfield, Connecticut) at the Royal Children’s Hospital, without sedation. Infants underwent T1 (0.8 − 1.6 mm coronal slices; flip angle 45°; repetition time 35 ms; echo time 9 ms; field of view 21 × 15 cm2; matrix 256 × 192), T2 (1.7 - 3 mm coronal slices; repetition time 4000 ms; echo time 60 / 160 ms; field of view 22 × 16 cm2; matrix 256 × 192, interpolated 512 × 512) and line scan diffusion weighted imaging (4 - 6 mm axial slices; 2 images at b = 5 s/mm2; 6 non-collinear gradient directions at b = 700 s/mm2).
Hemispheric injury assessment
Presence of neonatal brain injury on MRI was assessed and reviewed by a neonatal neurologist using a previously described scoring system for all VPT infants (H. Kidokoro, Neil, & Inder, 2013). To determine the hemisphere of injury, a single blinded rater (J.C.) reviewed reported cases of cerebral white matter and cerebellar unilateral injury based on the MRI scoring assessment (specifically the presence of focal unilateral cystic lesions and/or focal punctuate signal abnormality). Focal unilateral injury in either the left or right hemispheres was classified as left or right hemispheric injury accordingly.
CC segmentation and diffusion analysis
For those VPT infants with diffusion weighted imaging, corpora callosa were manually delineated on the mid-sagittal slice of the anterior commissure-posterior commissure (AC-PC) aligned T1-weighted image using 3D slicer software (www.slicer.org). CC tracing was performed by a trained operator who was blinded to all perinatal data and neurodevelopmental results. The method used to determine the cross-sectional area of the CC has been previously described (Thompson et al., 2011; Thompson et al., 2012). Diffusion measures within the CC were obtained by overlaying the CC mask onto the diffusion map that had been co-registered with the AC-PC aligned T1 image. MD, FA, AD and RD were quantified within the whole CC as well as within the 6 CC subregions (genu, rostral body, anterior mid-body, posterior mid-body, isthmus and splenium) (Thompson et al., 2011).
PLIC diffusion analysis
MD, FA, AD and RD were calculated within manually selected regions of interest on an axial slice of the brain taken at the level through the basal ganglia and PLIC. Bilateral regions of interest included the middle third of the PLIC, as previously described (Cheong et al., 2009). PLIC asymmetry was examined by calculating an asymmetry quotient, , for the four diffusion measures (MD, FA, AD and RD). A negative asymmetry quotient indicates leftward asymmetry, while a positive asymmetry quotient indicates rightward asymmetry.
Neurodevelopmental measures
The neuropsychological assessment conducted at 7 years’ corrected age included measures of intellect, academic achievement, working memory, language, and gross and fine motor skills. All assessment results are reported using standardized scores based on the child’s corrected age to avoid bias in cognitive test scores (Wilson-Ching, Pascoe, Doyle, & Anderson, 2014). All assessments were performed by trained pediatric assessors, psychologists or pediatricians.
Handedness was assessed through a 12-item, self-report hand preference inventory (Briggs & Nebes, 1975) based on Annett’s (1967) questionnaire. Scores on this measure range from −24 to +24, with higher scores representing more right-handedness. An overall handedness score of 8 or less indicated NRH (scores of −9 to 8 are considered mixed-handed; scores of −24 to −9 are considered left handed), whereas a score of 9 and above signified right-handedness. Familial left-handedness was assessed via questionnaire where the primary caregiver was asked to report if either parent was left-handed (Briggs & Nebes, 1975). General intellectual functioning was assessed using the Full Scale IQ (FSIQ) score of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). Language ability was measured using the Core Language score from the Clinical Evaluation of Language Fundamentals – Fourth edition Australian (Semel, Wiig, & Secord, 2006). Reading, spelling and mathematical computational skills were measured through the Wide Range Achievement Test – Fourth edition (Wilkinson & Robertson, 2006). Verbal working memory was assessed using the Backward Digit Recall subtest from the Working Memory Test Battery for Children (Pickering & Gathercole, 2001). Each of these tests has a normative mean of 100 and standard deviation (SD) of 15. Motor skills were assessed using the Movement Assessment Battery for Children – version 2 (Henderson, Sugden, & Barnett, 2007), where standard scores for manual dexterity (fine motor), aiming and catching, and balance (gross motor), were combined to form a total movement score (global motor) (normative mean = 10, SD = 3). Parents rated their child’s behavior using the Strengths and Difficulties questionnaire – Australian English version (Goodman, 1997). The total difficulty score was used as an estimate of behavioral problems in daily life, with a higher score reflecting greater behavioral difficulty (range: 0-40).
Social Risk
Social risk at age 7 years was determined based on a composite measure assessing family structure, education of the primary caregiver, occupation and employment status of the primary income earner, language spoken at home, and maternal age when the child was born (Roberts et al., 2008), where higher scores indicated greater social risk.
Data Analysis
Data were analyzed using Stata 13.0 (StataCorp, 2013). To address our first aim, the main and interaction effects of handedness score and birth group on neurodevelopmental outcomes at 7 years were examined using linear regression. Analyses were adjusted for corrected age at assessment, social risk, and parental left-handedness. Wilcoxon-Mann-Whitney tests were used to compare the distribution of handedness between VPT children with left- versus right-hemisphere injuries in the cerebral white matter and cerebellum (Aim 2). Lastly, linear regression models were used to examine the association between CC area and diffusion measures and PLIC diffusion asymmetry and handedness at 7 years (Aim 3). Analyses were adjusted for age at scan and parental left-handedness. Analyses involving CC area were also adjusted for intracranial volume. Regression models were fitted using Generalised Estimating Equations with an exchangeable correlations structure and are reported with robust standard errors to allow for correlations between twins/triplets in the study (Carlin, Gurrin, Sterne, Morley, & Dwyer, 2005). When regression models could not be fitted using Generalized Estimating Equations, regression models were reported using robust (sandwich) estimates of standard errors to allow for correlations between twins/triplets. Due to the issue of multiple comparisons, results were interpreted based on the profile and magnitude of differences and associations, rather than simply relying on p-values.
Results
Sample characteristics (Table 1)
Table 1.
Perinatal and demographic characteristics of study participants.
| VPT n* = 175 |
Term Control n* = 69 |
||
|---|---|---|---|
| Perinatal | GA (weeks), M (SD) | 27.5 (1.9) | 39.1 (1.3) |
| Birth Weight (g), M (SD) | 969 (222) | 3313 (505) | |
| Singleton, n (%) | 100 (57.1) | 65 (94.2) | |
| Male, n (%) | 93 (53.1) | 34 (49.3) | |
| Bronchopulmonary dysplasia, n (%) | 58 (33.1) | n/a | |
| Cystic periventricular leukomalacia, n (%) | 7 (4.0) | n/a | |
| Intraventricular hemorrhage grade 3/4, n (%) | 5 (2.9) | n/a | |
| Proven sepsis, n (%) | 77 (44.0) | 1 (2.4) | |
| Necrotising enterocolitis (proven), n (%) | 9 (5.1) | n/a | |
| Patent ductus arteriosus, n (%) | 86 (49.1) | n/a | |
| Length of stay (days), M (SD) | 87 (32.7) | 5.2 (3.7) (n = 43) |
|
| 7 years | Corrected age at assessment (years), M (SD) | 7.5 (0.2) | 7.6 (0.3) |
| Social risk score, median (interquartile range) | 2 (1-3) (n = 168) |
1 (0-2) (n = 68) |
|
| Cerebral palsy, n (%) | 5 (2.9) | n/a | |
| Handedness M (SD) | 10.7 (13.5) | 13.8 (10.9) | |
| Range | − 23 to 24 | − 24 to 24 | |
| NRH, n (%) | 54 (30.9) | 15 (21.7) | |
| Left handed, n (%) | 23 (13.1) | 4 (5.8) | |
| Mixed handed, n (%) | 31 (17.7) | 11 (15.9) | |
| Right handed, n (%) | 121 (69.1) | 54 (78.3) | |
| Parental left handedness, n (%) | 36 (27.9) (n = 129) |
16 (29.6) (n = 54) |
For some variables, the sample size is less than the total sample due to missing data; actual sample reported in table NRH, non-right handedness; GA, gestational age; M, mean; SD, standard deviation
Regarding perinatal and demographic characteristics, participants with handedness data at age 7 years were representative of the original VPT and term-born cohorts, except that rates of cerebral palsy at 2 years of age were lower in VPT participants compared with VPT non-participants (4% vs. 24%). Of VPT children with handedness data, children who did not have neonatal diffusion data had lower birth weights (mean difference −84, 95% CI −149 to −18, p = 0.01) and poorer language performance at 7 years (mean difference −7.0, 95% CI −12.1 to −1.9, p = 0.007) compared with VPT children with diffusion imaging. As expected, the VPT group differed from controls on perinatal medical variables and the proportion of singletons. VPT children were also more socially disadvantaged. Rates of parental left-handedness were similar between VPT and controls. There was a trend for stronger NRH in the VPT group compared with controls (regression coefficient (b) −2.97, 95% CI −6.25 to 0.31, p = 0.08). Following adjustment for parental left-handedness this association disappeared (b −1.95, 95% CI −5.67 to 1.77, p = 0.30).
NRH and neurodevelopmental outcomes at 7 years of age (Table 2)
Table 2.
Association between handedness and neurodevelopmental outcomes by birth group at 7 years of age
| VPT (n = 175) | Term Control (n = 69) | ||||
|---|---|---|---|---|---|
| Outcome measure | b (95% CI)^ | p ^ | b (95% CI)^ | p ^ | p ^ * |
|
|
|
|
|||
| Full scale IQ | 0.08 (−0.05, 0.23) | 0.22 | 0.09 (−0.21, 0.38) | 0.55 | 0.10 |
| Reading | 0.26 (0.05, 0.47) | 0.02 | −0.17 (−0.51, 0.18) | 0.34 | 0.04 |
| Spelling | 0.26 (0.08, 0.44) | 0.005 | −0.15 (−0.48, 0.19) | 0.38 | 0.04 |
| Mathematics | 0.22 (0.02, 0.42) | 0.03 | 0.05 (−0.24, 0.33) | 0.74 | 0.34 |
| Language | 0.19 (−0.01, 0.39) | 0.06 | −0.01 (−0.27, 0.25) | 0.95 | 0.24 |
| Global motor ability | 0.01 (−0.05, 0.02) | 0.49 | 0.05 (−0.02, 0.13) | 0.17 | 0.13 |
| Fine motor skills | 0.01 (−0.02, 0.04) | 0.53 | 0.01 (−0.06, 0.08) | 0.71 | 0.94 |
| Gross motor skills | 0.01 (−0.06, 0.03) | 0.51 | 0.04 (−0.07, 0.16) | 0.46 | 0.36 |
| Working Memory | 0.27 (0.06, 0.49) | 0.01 | 0.06 (−0.38, 0.52) | 0.78 | 0.40 |
| Behavior difficulty | 0.01 (−0.08, 0.05) | 0.69 | 0.08 (−0.04, 0.19) | 0.20 | 0.19 |
Adjusted for social risk at age 7 years, corrected age at assessment and parental left-handedness
b = regression coefficient; a positive value indicates that a lower score is associated with stronger NRH. If the regression is statistically significant, the 95% CI will not contain 0.
p value for the interaction between birth group and handedness
As reported previously, VPT birth compared with birth at term was associated with poorer performance across all neurodevelopmental outcomes, even after adjusting for social risk and age at assessment (p <0.05 for all outcomes; data not presented) (Omizzolo et al., 2014; Reidy et al., 2013). For reading and spelling, there was evidence of interactions between handedness and birth group, with stronger NRH associated with poorer performance in the VPT group, but slightly better performance in the term controls. Poorer mathematics and working memory performances were associated with stronger NRH in the VPT group. There was a trend for poorer language outcomes and stronger NRH in the VPT group. There was little evidence of interactions between handedness and birth group in other neurodevelopmental outcomes.
Hemisphere of neonatal brain injury and NRH at 7 years of age in children born VPT (Table 3)
Table 3.
Frequency of neonatal MRI unilateral cerebral white matter and cerebellar injury based on NRH dichotomy in VPT children
| Region | Non-right n = 54 |
Right n= 121 |
||
|---|---|---|---|---|
| Cerebral white matter | Cystic lesions | Right sided, n (%) | 0 (0) | 2 (2)a |
| Left sided, n (%) | 1 (2) | 0 (0) | ||
| Signal abnormality | Right sided, n (%) | 0 (0) | 2 (2)a | |
| Left sided, n (%) | 2 (4) | 0 (0) | ||
| Cerebellar hemorrhage | Right sided, n (%) | 2 (4) | 0 (0) | |
| Left sided, n (%) | 0 (0) | 5 (4) |
1 right-handed child was found to have both right-sided cystic lesions and signal abnormality
Only a small number of children had severe unilateral brain injury on the neonatal MRI, and as such analyses were exploratory (see Table 3). Wilcoxon-Mann-Whitney tests found that right handed and non-right handed groups did not differ based on the hemisphere of unilateral cerebral white matter injury in VPT children (p > .05). However, there was a trend for a handedness effect for cerebellar hemorrhage (z = −1.94, p= 0.05), with a higher handedness rank (greater right-handedness) for left-sided cerebellar hemorrhage.
Neonatal CC area and diffusion and NRH at 7 years of age in children born VPT (Table 4)
Table 4.
Neonatal area and diffusion measures within the CC and its subregions in VPT infants and the association with handedness at 7 years of age
| b (95% CI) | p | ||
|---|---|---|---|
| Area (mm2) # | |||
| Whole CC | −0.24 (−0.60, 0.12) | 0.20 | |
| Genu | −0.35 (−1.41, 0.71) | 0.52 | |
| Rostral body | 0.20 (−2.01, 2.42) | 0.86 | |
| Anterior midbody | −0.83 (−2.44, 0.79) | 0.32 | |
| Posterior midbody | −0.62 (−2.63, 1.39) | 0.55 | |
| Isthmus | 0.07 (−2.72, 2.86) | 0.96 | |
| Splenium | −1.04 (−1.89, −0.19) | 0.02 | |
| Diffusion ^ | |||
| Whole CC | FA | −0.65 (−1.27, −0.04) | 0.04 |
| MD (×10−3 mm2/s) | 0.33 (−0.001, 0.66) | 0.05 | |
| AD (×10−3 mm2/s) | 0.08 (−0.17, 0.33) | 0.52 | |
| RD (×10−3 mm2/s) | 0.35 (0.08, 0.62) | 0.01 | |
| Genu | FA | −0.51 (−0.10, −0.03) | 0.04 |
| MD (×10−3 mm2/s) | 0.03 (−0.38, 0.45) | 0.88 | |
| AD (×10−3 mm2/s) | −0.11 (−0.30, 0.08) | 0.25 | |
| RD (×10−3 mm2/s) | 0.18 (−0.14, 0.51) | 0.27 | |
| Rostral body | FA | −0.02 (−0.45, 0.41) | 0.92 |
| MD (×10−3 mm2/s) | 0.09 (−0.08, 0.26) | 0.29 | |
| AD (×10−3 mm2/s) | 0.08 (−0.05, 0.21) | 0.24 | |
| RD (×10−3 mm2/s) | 0.08 (−0.09, 0.25) | 0.36 | |
| Anterior midbody | FA | 0.32 (−0.26, 0.91) | 0.28 |
| MD (×10−3 mm2/s) | 0.16 (−0.15, 0.46) | 0.31 | |
| AD (×10−3 mm2/s) | 0.13 (−0.09, 0.35) | 0.26 | |
| RD (×10−3 mm2/s) | 0.13 (−0.18, 0.43) | 0.42 | |
| Posterior midbody | FA | 0.31 (−0.21, 0.84) | 0.24 |
| MD (×10−3 mm2/s) | 0.10 (−0.18, 0.39) | 0.47 | |
| AD (×10−3 mm2/s) | 0.10 (−0.05, 0.25) | 0.19 | |
| RD (×10−3 mm2/s) | 0.05 (−0.29, 0.39) | 0.79 | |
| Isthmus | FA | −0.25 (−0.89, 0.40) | 0.45 |
| MD (×10−3 mm2/s) | 0.11 (−0.21, 0.43) | 0.50 | |
| AD (×10−3 mm2/s) | 0.04 (−0.13, 0.20) | 0.66 | |
| RD (×10−3 mm2/s) | 0.12 (−0.21, 0.45) | 0.48 | |
| Splenium | FA | −0.44 (−0.82, −0.07) | 0.02 |
| MD (×10−3 mm2/s) | 0.25 (0.06, 0.44) | 0.01 | |
| AD (×10−3 mm2/s) | 0.20 (−0.008, 0.40) | 0.06 | |
| RD (×10−3 mm2/s) | 0.19 (0.05, 0.33) | 0.01 |
Adjusted for age at scan, intracranial volume and parental left handedness
Adjusted for age at scan and parental left handedness
FA, fractional anisotropy; MD, mean diffusivity; AD, axial diffusivity; RD, radial diffusivity.
b = regression coefficient; a negative value indicates that higher score is associated with stronger NRH.
Increased FA and decreased RD in the whole CC, as well as increased FA within the genu, were associated with stronger NRH. Within the splenium, increased area, increased FA and decreased MD and RD were associated with stronger NRH.
Neonatal PLIC diffusion asymmetry and NRH at 7 years of age in children born VPT
There was a trend for greater rightward FA asymmetry in the PLIC being associated with greater NRH in VPT children (b −0.37, 95% CI −0.80, 0.06, p = 0.09). There was little evidence for associations between MD, AD and RD asymmetry quotients and NRH in VPT children (p > .05; data not presented).
Discussion
The current study aimed to explore neurodevelopmental outcomes and neural mechanisms associated with handedness in VPT children. While we found weak evidence that VPT birth was associated with stronger NRH, this was in line with findings from a meta-analysis where 17 studies also reported weak evidence of more NRH in preterm children compared with term-born children (Domellof et al., 2011). Detectable differences in NRH between VPT and term children may be related to the age at which handedness was assessed, as well as birth weight and gestational age characteristics of preterm participants. Absolute (right versus left) hand preference may emerge with age, leading to greater representation of left-handers in older compared with younger cohorts, and stronger NRH may be expected with decreasing gestational age and/or birthweight (Domellof et al., 2011). Interestingly, larger-scale population studies have reported no evidence for a link between birth stress (e.g. preterm birth and low birth weight factors) and hand preference (Johnston et al., 2009; Nicholls, Johnston, & Shields, 2012; Van der Elst et al., 2011).
NRH and neurodevelopmental outcomes at 7 years of age
NRH was associated with poorer neurodevelopmental outcomes in VPT children at age 7 years but not for term born controls. These findings are similar to Marlow and colleagues (2007) who noted an association between hand preference and cognitive development at age 6 years in children born <25 weeks’ gestation. Considering that findings regarding the association between NRH and neuropsychological outcomes have largely been inconsistent in preterm children, and several studies were unable to show links between NRH and cognitive function (Luciana, Lindeke, Georgieff, Mills, & Nelson, 1999; Powls et al., 1996; Saigal et al., 1992), our findings are important in providing further evidence that NRH is associated with poorer neuropsychological outcome in this population. The relationship between NRH and neuropsychological outcomes in VPT children in the current study was most pertinent for academic and working memory outcomes. In contrast to previous studies, we did not find motor abilities, in particular fine motor skills, were associated with handedness. It may be that the tasks of fine motor dexterity utilized were not sensitive to hand skill differences compared with other motor tasks (e.g. cutting, hammering) that could not be considered in the current study. Previous research has also suggested that the performance of the non-dominant hand should also be considered: Ross and colleagues (1992) found that non-right handed VPT children were less skilled at using their non-dominant hand compared with their right-handed peers. Regarding term-born children, our results are in contrast to a meta-analysis of typically developing children (Somers et al., 2015), which showed a small advantage in verbal and spatial ability for right-handed children. It is likely that our study lacked sufficient power or the appropriate sample composition to detect a handedness effect on cognitive outcomes for our comparison group.
Potential neural mechanisms of NRH in VPT children
Potential underlying neural mechanisms for NRH in VPT children were examined in the current study. Handedness was not related to the hemisphere of unilateral cerebral white matter injury, however there was a trend for stronger right-handedness in VPT children with left-sided cerebellar hemorrhage. While our results do not strongly support a pathological model of NRH, only a small number of VPT children had unilateral cerebral (n=6) and cerebellar (n=7) injuries, so the study was likely underpowered to detect relationships between lateralized brain injury and NRH. To our knowledge this is the first study to specifically investigate lateralized cerebral white matter and cerebellar brain injury (detected via MRI) in relation to handedness in VPT children specifically. While further investigation using advanced imaging techniques and larger samples are needed, our study highlights the possibility that stronger NRH in VPT children might be associated with early brain injury.
The rate of severe brain abnormalities detected on cranial ultrasound are decreasing in contemporary preterm and low birth weight cohorts (Wilson-Costello et al., 2007). In our study, 3-4% of VPT children had severe brain abnormalities (grade 3 or 4 intraventricular hemorrhage or cystic periventricular leukomalacia), compared to a rate of 6-7% in VPT infants born in the late 1990s (Heuchan, Evans, Henderson Smart, & Simpson, 2002). However, despite this decline in severe brain pathology, the rate of later neurodevelopmental difficulties continues to be high. This may suggest that more diffuse brain injury or subtle changes in brain development, such as alterations in neonatal brain morphology or microstructure, could explain some of the poorer functional outcomes that emerge in this population. Considering this, we investigated whether the expression of NRH in VPT children was a consequence of a disruption to normal brain asymmetry development, by examining the microstructural integrity of major white matter tracts implicated in motor development, such as the CC and PLIC. In contrast to previous research (Caldu et al., 2006; Nosarti et al., 2004), we found CC morphology and microstructure to be associated with NRH in our VPT cohort. Specifically, we found that larger neonatal splenium area was associated with stronger NRH in VPT children. We further noted that increased FA in the whole CC, specifically in the genu and splenium, and decreased MD and RD in the splenium, were related to stronger NRH, which is similar to findings by Westerhausen and colleagues (2004). Our findings support early research by Witelson (1989) that highlighted that non-right handed individuals have larger CC than their right-handed counterparts. It has been demonstrated that VPT birth interferes with the maturation and myelination processes of the CC, particularly for posterior subregions (Caldu et al., 2006; Constable et al., 2008; Lawrence et al., 2010; Nosarti et al., 2004; Skiöld et al., 2010; Thompson et al., 2011). During the late prenatal and early postnatal period, there is a naturally occurring process of pruning of callosal axons. This process is thought to result in pronounced functional lateralization and reduce the likelihood of left-handedness (Westerhausen et al., 2004). VPT birth may disrupt this process, leading to a greater number, or density, of callosal axons being present during the neonatal period, increasing the prevalence of NRH in the VPT population. Therefore, our findings suggest that the primary effect of preterm birth on CC microstructure and morphology subsequently influences the development and expression of hand preference at age 7.
The current study is the first to explicitly examine neonatal PLIC microstructure in relation to handedness in VPT children. While there was a trend for NRH in VPT children to be associated with stronger rightward PLIC FA asymmetry, our overall PLIC diffusion results provided little evidence to support an association between PLIC microstructure and handedness in this population. Our findings are in line with Westerhausen and colleagues (2007), who also reported little association between CST asymmetries and handedness. While others have noted an association between handedness and CST morphology, these studies either included small samples (Seizeur et al., 2014), or were based on other MRI techniques (i.e. volume based on apparent grey matter density) (Herve et al., 2009). Findings from our study therefore suggest that neonatal CST asymmetry characteristics are not early determinants of hand preference in VPT children. Handedness may instead reflect asymmetries of cortical motor areas rather than asymmetries of motor tracts or connections, such as the CST (Herve et al., 2006).
While the current study did not have the power to categorize and examine weak (i.e. mixed handedness) versus strong handedness laterality (i.e. left and right handedness), some research has suggested the “strength” may be more important than the “direction” of handedness (Welcome et al., 2009). It has been suggested that mixed handed individuals exhibit greater inter-hemispheric connectivity and subsequently have larger CC compared with left- or right-handed individuals (Welcome et al., 2009; Witelson & Goldsmith, 1991). Behavioral and cognitive discrepancies have been noted between mixed handed individuals and consistent-handed individuals (Welcome et al., 2009). It has also been suggested that mixed handedness may be associated with poorer behavior and intellectual ability in children, compared with left-handedness (Domellof et al., 2011; Rodriguez et al., 2010; Rodriguez & Waldenström, 2008). Considering this, it is imperative that future studies examine and highlight the influence of atypical handedness on outcomes in VPT children.
The current study has some limitations. The study used a questionnaire measure of hand preference and did not include measures of lateral performance when evaluating handedness. It is important to distinguish hand preference from hand performance as they have been acknowledged to be different and separate outcomes (Porac & Coren, 1981; Scharoun & Bryden, 2014). Further, a relatively small sample size due to loss to follow-up and available MRI data may have resulted in a lack of power to identify differences between lateralized unilateral brain injury and hand preference, and associations between PLIC microstructure and NRH.
Findings from the current study have contributed to our understanding of handedness in VPT children and its relationship with neurodevelopmental outcomes. This study was unique in its specific examination of potential neural mechanisms underpinning the development of NRH in VPT children using advanced imaging techniques and a standardized measure of hand preference. Considering findings from the current study, it is important to examine handedness over time via longitudinal studies, in order to determine whether expressions and correlates of NRH in VPT children persist or change dynamically with neurodevelopment. The data reported here suggest that handedness may be an important marker for some cognitive and academic difficulties in VPT children, and indicate that white matter connections may be differentially involved in hand preference. These findings have implications for VPT children particularly in the educational setting, where built-in support structures may be beneficial to assist non-right handed children strengthen and develop particular cognitive or developmental skills.
Acknowledgements
We acknowledge the input of the VIBeS research team, the Melbourne Children’s MRI Center, and all the families who participated in this study. This study was funded by Australia’s National Health & Medical Research Council [Project Grants (237117 & 491209), Early Career Award (1012236 to D.T.), research fellowship support (1081288 to P.A.; 1053787 to J.C.), Centre for Research Excellence in Newborn Medicine (1060733)]; and National Institutes of Health (HD058056). We also acknowledge the support provided by the Murdoch Childrens Research Institute, the Royal Children’s Hospital Foundation, and the Victorian Government's Operational Infrastructure Support Program.
Footnotes
This manuscript has never been published elsewhere and there are no conflicts of interest.
References
- Adams E, Chau V, Poskitt KJ, Grunau RE, Synnes A, Miller SP. Tractography-based quantitation of corticospinal tract development in premature newborns. J Pediatr. 2010;156(6):882–888. 888.e881. doi: 10.1016/j.jpeds.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allin M, Rooney M, Griffiths T, Cuddy M, Wyatt J, Rifkin L, Murray R. Neurological abnormalities in young adults born preterm. Journal of Neurology, Neurosurgery & Psychiatry. 2006;77(4):495–499. doi: 10.1136/jnnp.2005.075465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews JS, Ben-Shachar M, Yeatman JD, Flom LL, Luna B, Feldman HM. Reading performance correlates with white-matter properties in preterm and term children. Dev Med Child Neurol. 2010;52(6):e94–100. doi: 10.1111/j.1469-8749.2009.03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annett M. The binomial distribution of right, mixed and left handedness. Quarterly Journal of Experimental Psychology. 1967;19(4):327–333. doi: 10.1080/14640746708400109. [DOI] [PubMed] [Google Scholar]
- Annett M. Left, right, hand and brain: The right shift theory. Lawrence Erlbaum; London: 1985. [Google Scholar]
- Bakan P, Dibb G, Reed P. Handedness and birth stress. Neuropsychologia. 1973;11(3):363–366. doi: 10.1016/0028-3932(73)90050-x. [DOI] [PubMed] [Google Scholar]
- Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex. 1975;11(3):230–238. doi: 10.1016/s0010-9452(75)80005-0. [DOI] [PubMed] [Google Scholar]
- Caldu X, Narberhaus A, Junque C, Gimenez M, Vendrell P, Bargallo N, Botet F. Corpus callosum size and neuropsychologic impairment in adolescents who were born preterm. J Child Neurol. 2006;21(5):406–410. doi: 10.1177/08830738060210050801. [DOI] [PubMed] [Google Scholar]
- Carlin JB, Gurrin LC, Sterne JA, Morley R, Dwyer T. Regression models for twin studies: a critical review. International Journal of Epidemiology. 2005;34(5):1089–1099. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- Cheong JL, Thompson DK, Wang HX, Hunt RW, Anderson PJ, Inder TE, Doyle LW. Abnormal white matter signal on MR imaging is related to abnormal tissue microstructure. AJNR Am J Neuroradiol. 2009;30(3):623–628. doi: 10.3174/ajnr.A1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable RT, Ment LR, Vohr BR, Kesler SR, Fulbright RK, Lacadie C, Reiss AR. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121(2):306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- Coren S, Porac C. Birth factors and laterality: effects of birth order, parental age, and birth stress on four indices of lateral preference. Behav Genet. 1980;10(2):123–138. doi: 10.1007/BF01066263. [DOI] [PubMed] [Google Scholar]
- Domellof E, Johansson AM, Ronnqvist L. Handedness in preterm born children: a systematic review and a meta-analysis. Neuropsychologia. 2011;49(9):2299–2310. doi: 10.1016/j.neuropsychologia.2011.04.033. [DOI] [PubMed] [Google Scholar]
- Goodman R. The Strengths and Difficulties Questionnaire: a research note. Journal of child psychology and psychiatry. 1997;38(5):581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Henderson SE, Sugden DA, Barnett AL. Movement assessment battery for children-2: Movement ABC-2: Examiner's manual. Pearson; 2007. [Google Scholar]
- Herve PY, Crivello F, Perchey G, Mazoyer B, Tzourio-Mazoyer N. Handedness and cerebral anatomical asymmetries in young adult males. Neuroimage. 2006;29(4):1066–1079. doi: 10.1016/j.neuroimage.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Herve PY, Leonard G, Perron M, Pike B, Pitiot A, Richer L, Paus T. Handedness, motor skills and maturation of the corticospinal tract in the adolescent brain. Hum Brain Mapp. 2009;30(10):3151–3162. doi: 10.1002/hbm.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuchan AM, Evans N, Henderson Smart DJ, Simpson JM. Perinatal risk factors for major intraventricular haemorrhage in the Australian and New Zealand Neonatal Network, 1995–97. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2002;86(2):F86–F90. doi: 10.1136/fn.86.2.F86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppi PS, Murphy B, Maier SE, Zientara GP, Inder TE, Barnes PD, Volpe JJ. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107(3):455–460. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- Inder TE, Anderson NJ, Spencer C, Wells S, Volpe JJ. White Matter Injury in the Premature Infant: A Comparison between Serial Cranial Sonographic and MR Findings at Term. American Journal of Neuroradiology. 2003;24(5):805–809. [PMC free article] [PubMed] [Google Scholar]
- Johnston DW, Nicholls ME, Shah M, Shields MA. Nature’s Experiment? Handedness and Early Childhood Development. Demography. 2009;46(2):281–301. doi: 10.1353/dem.0.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Vohr B, Schneider KC, Katz KH, Makuch RW, Reiss AL, Ment LR. Increased temporal lobe gyrification in preterm children. Neuropsychologia. 2006;44(3):445–453. doi: 10.1016/j.neuropsychologia.2005.05.015. doi: http://dx.doi.org/10.1016/j.neuropsychologia.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Kidokoro H, Anderson PJ, Doyle LW, Woodward LJ, Neil JJ, Inder TE. Brain Injury and Altered Brain Growth in Preterm Infants: Predictors and Prognosis. Pediatrics. 2014;134(2):e444–e453. doi: 10.1542/peds.2013-2336. [DOI] [PubMed] [Google Scholar]
- Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol. 2013;34(11):2208–2214. doi: 10.3174/ajnr.A3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancefield K, Nosarti C, Rifkin L, Allin M, Sham P, Murray R. Cerebral asymmetry in 14 year olds born very preterm. Brain Res. 2006;1093(1):33–40. doi: 10.1016/j.brainres.2006.03.097. [DOI] [PubMed] [Google Scholar]
- Lawrence EJ, Allen GM, Walshe M, Allin M, Murray R, Rifkin L, Nosarti C. The corpus callosum and empathy in adults with a history of preterm birth. J Int Neuropsychol Soc. 2010;16(4):716–720. doi: 10.1017/s1355617710000500. [DOI] [PubMed] [Google Scholar]
- Luciana M, Lindeke L, Georgieff M, Mills M, Nelson CA. Neurobehavioral evidence for working-memory deficits in school-aged children with histories of prematurity. Dev Med Child Neurol. 1999;41(8):521–533. doi: 10.1017/s0012162299001140. [DOI] [PubMed] [Google Scholar]
- Marlow N, Hennessy EM, Bracewell MA, Wolke D. Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics. 2007;120(4):793–804. doi: 10.1542/peds.2007-0440. [DOI] [PubMed] [Google Scholar]
- Marlow N, Roberts BL, Cooke RW. Laterality and prematurity. Archives of Disease in Childhood. 1989;64(12):1713–1716. doi: 10.1136/adc.64.12.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus IC. The inheritance of left-handedness. Ciba Found Symp. 1991;162:251–267. doi: 10.1002/9780470514160.ch15. discussion 267-281. [DOI] [PubMed] [Google Scholar]
- Medland SE, Duffy DL, Wright MJ, Geffen GM, Hay DA, Levy F, Boomsma DI. Genetic influences on handedness: Data from 25,732 Australian and Dutch twin families. Neuropsychologia. 2009;47(2):330–337. doi: 10.1016/j.neuropsychologia.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medland SE, Perelle I, De Monte V, Ehrman L. Effects of culture, sex, and age on the distribution of handedness: an evaluation of the sensitivity of three measures of handedness. Laterality. 2004;9(3):287–297. doi: 10.1080/13576500342000040. [DOI] [PubMed] [Google Scholar]
- Nicholls ME, Chapman HL, Loetscher T, Grimshaw GM. The relationship between hand preference, hand performance, and general cognitive ability. J Int Neuropsychol Soc. 2010;16(4):585–592. doi: 10.1017/s1355617710000184. [DOI] [PubMed] [Google Scholar]
- Nicholls ME, Johnston DW, Shields MA. Adverse birth factors predict cognitive ability, but not hand preference. Neuropsychology. 2012;26(5):578–587. doi: 10.1037/a0029151. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Rushe TM, Woodruff PW, Stewart AL, Rifkin L, Murray RM. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain. 2004;127(Pt 9):2080–2089. doi: 10.1093/brain/awh230. [DOI] [PubMed] [Google Scholar]
- O'Callaghan MJ, Burn YR, Mohay HA, Rogers Y, et al. Handedness in extremely low birth weight infants: Aetiology and relationship to intellectual abilities, motor performance and behaviour at four and six years. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior. 1993;29(4):629–637. doi: 10.1016/s0010-9452(13)80286-9. [DOI] [PubMed] [Google Scholar]
- Omizzolo C, Scratch SE, Stargatt R, Kidokoro H, Thompson DK, Lee KJ, Anderson PJ. Neonatal brain abnormalities and memory and learning outcomes at 7 years in children born very preterm. Memory. 2014;22(6):605–615. doi: 10.1080/09658211.2013.809765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering S, Gathercole SE. Working memory test battery for children (WMTB-C) Psychological Corporation; 2001. [Google Scholar]
- Porac C, Coren S. Lateral Preferences and Human Behavior. Springer; New York: 1981. [Google Scholar]
- Powls A, Botting N, Cooke RWI, Marlow N. Handedness in very low birthweight (VLBW) children at 12 years of age: relation to perinatal and outcome variables. Developmental Medicine & Child Neurology. 1996;38(7):594–602. doi: 10.1111/j.1469-8749.1996.tb12124.x. [DOI] [PubMed] [Google Scholar]
- Reidy N, Morgan A, Thompson DK, Inder TE, Doyle LW, Anderson PJ. Impaired language abilities and white matter abnormalities in children born very preterm and/or very low birth weight. J Pediatr. 2013;162(4):719–724. doi: 10.1016/j.jpeds.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch F, Haffner J, Parzer P, Pfueller U, Strehlow U, Zerahn-Hartung C. Testing the hypothesis of the relationships between laterality and ability according to Annett's right-shift theory: findings in an epidemiological sample of young adults. Br J Psychol. 1997;88(Pt 4):621–635. doi: 10.1111/j.2044-8295.1997.tb02661.x. [DOI] [PubMed] [Google Scholar]
- Roberts G, Howard K, Spittle AJ, Brown NC, Anderson PJ, Doyle LW. Rates of early intervention services in very preterm children with developmental disabilities at age 2 years. J Paediatr Child Health. 2008;44(5):276–280. doi: 10.1111/j.1440-1754.2007.01251.x. [DOI] [PubMed] [Google Scholar]
- Roberts G, Lim J, Doyle LW, Anderson PJ. High rates of school readiness difficulties at 5 years of age in very preterm infants compared with term controls. J Dev Behav Pediatr. 2011;32(2):117–124. doi: 10.1097/DBP.0b013e318206d5c9. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Kaakinen M, Moilanen I, Taanila A, McGough JJ, Loo S, Järvelin M-R. Mixed-handedness is linked to mental health problems in children and adolescents. Pediatrics. 2010;125(2):e340–e348. doi: 10.1542/peds.2009-1165. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Waldenström U. Fetal origins of child non right handedness and mental health. Journal of child psychology and psychiatry. 2008;49(9):967–976. doi: 10.1111/j.1469-7610.2008.01923.x. [DOI] [PubMed] [Google Scholar]
- Ross G, Lipper E, Auld PAM. Hand preference, prematurity and developmental outcome at school age. Neuropsychologia. 1992;30(5):483–494. doi: 10.1016/0028-3932(92)90095-4. [DOI] [PubMed] [Google Scholar]
- Ross G, Lipper EG, Auld PA. Hand preference of four-year-old children: its relationship to premature birth and neurodevelopmental outcome. Dev Med Child Neurol. 1987;29(5):615–622. doi: 10.1111/j.1469-8749.1987.tb08503.x. [DOI] [PubMed] [Google Scholar]
- Saigal S, Rosenbaum P, Szatmari P, Hoult L. Non-right handedness among ELBW and term children at eight years in relation to cognitive function and school performance. Developmental Medicine & Child Neurology. 1992;34(5):425–433. doi: 10.1111/j.1469-8749.1992.tb11455.x. [DOI] [PubMed] [Google Scholar]
- Satz P, Orsini DL, Saslow E, Henry R. The pathological left-handedness syndrome. Brain and Cognition. 1985;4(1):27–46. doi: 10.1016/0278-2626(85)90052-1. doi: http://dx.doi.org/10.1016/0278-2626(85)90052-1. [DOI] [PubMed] [Google Scholar]
- Scharoun SM, Bryden PJ. Hand preference, performance abilities, and hand selection in children. Frontiers in Psychology. 2014;5:82. doi: 10.3389/fpsyg.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seizeur R, Magro E, Prima S, Wiest-Daessle N, Maumet C, Morandi X. Corticospinal tract asymmetry and handedness in right- and left-handers by diffusion tensor tractography. Surg Radiol Anat. 2014;36(2):111–124. doi: 10.1007/s00276-013-1156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E, Wiig E, Secord W. Clinical Evaluation of Language Fundamentals - Fourth Edition, Australian Standardised Edition. Harcourt Assessment; Marrackville, Australia: 2006. [Google Scholar]
- Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, Inder TE. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153(2):170–175. 175.e171. doi: 10.1016/j.jpeds.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Skiöld B, Horsch S, Hallberg B, Engström M, Nagy Z, Mosskin M, Ådén U. White matter changes in extremely preterm infants, a population-based diffusion tensor imaging study. Acta Pædiatrica. 2010;99(6):842–849. doi: 10.1111/j.1651-2227.2009.01634.x. [DOI] [PubMed] [Google Scholar]
- Somers M, Shields LS, Boks MP, Kahn RS, Sommer IE. Cognitive benefits of right-handedness: a meta-analysis. Neurosci Biobehav Rev. 2015;51:48–63. doi: 10.1016/j.neubiorev.2015.01.003. [DOI] [PubMed] [Google Scholar]
- StataCorp . Stata Statistical Software: Release 13. StataCorp LP; College Station, TX: 2013. [Google Scholar]
- Thompson DK, Inder TE, Faggian N, Johnston L, Warfield SK, Anderson PJ, Egan GF. Characterization of the corpus callosum in very preterm and full-term infants utilizing MRI. Neuroimage. 2011;55(2):479–490. doi: 10.1016/j.neuroimage.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Inder TE, Faggian N, Warfield SK, Anderson PJ, Doyle LW, Egan GF. Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. Neuroimage. 2012;59(4):3571–3581. doi: 10.1016/j.neuroimage.2011.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Wood SJ, Doyle LW, Warfield SK, Lodygensky GA, Anderson PJ, Inder TE. Neonate hippocampal volumes: prematurity, perinatal predictors, and 2-year outcome. Ann Neurol. 2008;63(5):642–651. doi: 10.1002/ana.21367. [DOI] [PubMed] [Google Scholar]
- Treyvaud K, Ure A, Doyle LW, Lee KJ, Rogers CE, Kidokoro H, Anderson PJ. Psychiatric outcomes at age seven for very preterm children: rates and predictors. J Child Psychol Psychiatry. 2013;54(7):772–779. doi: 10.1111/jcpp.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncer MC, Hatipoglu ES, Ozates M. Sexual dimorphism and handedness in the human corpus callosum based on magnetic resonance imaging. Surg Radiol Anat. 2005;27(3):254–259. doi: 10.1007/s00276-004-0308-1. [DOI] [PubMed] [Google Scholar]
- Van der Elst W, Hurks PP, Wassenberg R, Meijs CJ, Van Boxtel MP, Jolles J. On the association between lateral preferences and pregnancy/birth stress events in a nonclinical sample of school-aged children. J Clin Exp Neuropsychol. 2011;33(1):1–8. doi: 10.1080/13803391003757825. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. Psychological Corporation; New York: 1999. [Google Scholar]
- Welcome SE, Chiarello C, Towler S, Halderman LK, Otto R, Leonard CM. Behavioral correlates of corpus callosum size: Anatomical/behavioral relationships vary across sex/handedness groups. Neuropsychologia. 2009;47(12):2427–2435. doi: 10.1016/j.neuropsychologia.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhausen R, Huster RJ, Kreuder F, Wittling W, Schweiger E. Corticospinal tract asymmetries at the level of the internal capsule: is there an association with handedness? Neuroimage. 2007;37(2):379–386. doi: 10.1016/j.neuroimage.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Kreuder F, Dos Santos Sequeira S, Walter C, Woerner W, Wittling RA, Wittling W. Effects of handedness and gender on macro- and microstructure of the corpus callosum and its subregions: a combined high-resolution and diffusion-tensor MRI study. Brain Res Cogn Brain Res. 2004;21(3):418–426. doi: 10.1016/j.cogbrainres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Walter C, Kreuder F, Wittling RA, Schweiger E, Wittling W. The influence of handedness and gender on the microstructure of the human corpus callosum: a diffusion-tensor magnetic resonance imaging study. Neuroscience Letters. 2003;351(2):99–102. doi: 10.1016/j.neulet.2003.07.011. doi: http://dx.doi.org/10.1016/j.neulet.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. WRAT 4: Wide Range Achievement Test - professional manual. Psychological Assessment Resources, Incorporated; Lutz, Florida: 2006. [Google Scholar]
- Wilson-Ching M, Pascoe L, Doyle LW, Anderson PJ. Effects of correcting for prematurity on cognitive test scores in childhood. J Paediatr Child Health. 2014;50(3):182–188. doi: 10.1111/jpc.12475. [DOI] [PubMed] [Google Scholar]
- Wilson-Costello D, Friedman H, Minich N, Siner B, Taylor G, Schluchter M, Hack M. Improved Neurodevelopmental Outcomes for Extremely Low Birth Weight Infants in 2000–2002. Pediatrics. 2007;119(1):37–45. doi: 10.1542/peds.2006-1416. [DOI] [PubMed] [Google Scholar]
- Witelson SF. The brain connection: the corpus callosum is larger in left-handers. Science. 1985;229(4714):665–668. doi: 10.1126/science.4023705. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. 1989;112 doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Goldsmith CH. The relationship of hand preference to anatomy of the corpus callosum in men. Brain Research. 1991;545(1-2):175–182. doi: 10.1016/0006-8993(91)91284-8. [DOI] [PubMed] [Google Scholar]