Abstract
Over the years, aldosterone has been a favorite topic of renal physiologists given its role in the maintenance of the body fluids. Investigators are only recently coming to appreciate a second pro-inflammatory and pro-fibrotic role for this hormone. Mineralocorticoids, like aldosterone, trigger a pro-fibrotic process, which in many respects mimics the early phase of wound healing. Depending on the type of cell involved, aldosterone may activate the pro-fibrotic process through classical mineralocorticoid receptors (MR), non-classical membrane associated MR, and/or glucocorticoid receptors. In the kidney, the actions of aldosterone can be attenuated by 11-dehydro metabolites of endogenous glucocorticoids generated by isoforms of the enzyme 11β-hydroxysteroid dehydrogenase (11β-HSD-1 and 11β-HSD-2). Thus the renal 11β-HSD isoforms may have two functions; to prevent endogenous glucocorticoids from inappropriately binding to and activating MR and to synthesize agents that limit the actions of aldosterone. While sodium in the diet has been implicated in aggravating aldosterone induced renal fibrotic processes, preliminary findings are consistent with the view that aldosterone alone can initiate the matrix production in renal tissue even in the absence of active sodium transport. Thus, there is a growing body of laboratory and clinical evidence supporting the use of inhibitors of aldosterone action in patients with both glomerular and tubular diseases.
BACKGROUND
Since its isolation and characterization in the early 1950’s, aldosterone has captivated renal physiologists. From their early efforts, we now appreciate how picomolar concentrations of aldosterone produced in the adrenal cortex glomerulosa cells regulate our electrolyte balance, our extracellular fluid volume, and our very survival. Hostetter and his colleagues were among the first to suggest that this life-preserving hormone might also have a darker side by demonstrating that aldosterone can contribute to renal injury1. The idea that aldosterone has an additional function as a pro-inflammatory and/or pro-fibrotic agent was actually proposed years earlier by Hans Selye shortly after aldosterone itself was discovered2. His hypothesis and observations were largely set aside until relatively recently. The fundamental question is why would a hormone so essential for our survival be also linked to inflammation and scarring? The question is obviously rhetorical; one can only surmise that during our earliest times, the two overwhelming and immediate external threats to existence were loss of circulating volume and an insufficient response to a wound injury. Could one hormone address both threats?
The idea that aldosterone is pro-inflammatory and pro-fibrotic even raises the possibility of the mineralocorticoid playing a role in wound healing. Recently, investigators have suggested that aldosterone is actively involved in the inflammatory and fibrotic responses associated with normal surgical wound healing3, 4. Aldosterone has been shown to activate a key kinase pathway called mTOR (mammalian Target of Rapamycin), which promotes cell proliferation5 and this same pathway is turned on during the early phases of wound healing6 and of renal repair after injury7. Excessive or prolonged activation of mTOR appears to promote interstitial inflammation and fibrosis7. It turns out that mTOR also plays a role in activating the sodium channel in renal collecting duct cells by phosphorylating SGK1 (serum and glucocorticoid induced kinase 1)8; SGK1 is a key enzyme triggered by aldosterone during sodium transport9. As a corollary, inhibitors of the mTOR pathway like rapamycin are known to significantly delay or blunt wound healing6.
CASE VIGNETTE
A 5 year old African American girl was noted to have a blood pressure of 140/90 during a routine yearly visit to her pediatrician. She had been receiving no medications but she had been eating a moderately high salt diet. Her only complaint was that she had occasional headaches and felt tired at times. There was no edema or acute weight change noted. The immediate family history was negative for renal disease and hypertension. Her height and weight were in the 60th and 50th percentile respectively and the blood pressure of 140/90 sitting at rest was confirmed. Her initial laboratory evaluation revealed a serum potassium of 2.7 mEq/L and a serum bicarbonate of 33 mmol/L. Her plasma renin on a moderately high salt diet was 0.1 ng/ml/hour (0.028 ng/L/sec) (normal 1.1 ± 0.8 ng/ml/hour or 0.31 ± 0.22 ng/L/sec) and serum aldosterone was 49 ng/dl (1359 pmol/L) (normal 2 to 9 ng/dl or 55.5 to 249 pmol/L). Her renal function was normal with a serum creatinine of 0.5 mg/dl (44.5 µmol/L) (Schwartz pediatric formula10 e-GFR 130 ml/min/1.73m2)and a random urine analysis demonstrated a specific gravity of 1.013 with a pH of 7, trace protein and no cells or casts. Other studies included a normal 24 hour urinary cortisol and an elevated 18 hydroxycorticosterone. A CT scan initially and again at six months showed no discrete changes in either adrenal gland.
The patient was treated with spironolactone resulting in a modest reduction in blood pressure to 132/84. Approximately 6 months after her presentation, she underwent a scan using 131I labeled methylcholesterol (131I-NP-59) with dexamethasone and potassium iodide pretreatment. The scan localized the activity to the left adrenal gland and the patient electively then underwent surgical removal of that gland. The pathology revealed diffuse nodular changes consistent with adrenal hyperplasia. Although her blood pressure and serum potassium initially normalized after her surgery, her hypertension and electrolyte abnormalities re-occurred 2 years later. She was restarted on spironolactone and other antihypertensive agents but compliance with medications and dietary sodium restriction was limited. By early adolescence, her serum creatinine had risen to 1.0 mg/dl (89.0 µmol/L) (Schwartz pediatric formula10 e-GFR 92 ml/min/1.73m2) and she developed low grade but persistent proteinuria. (This case vignette was based on case previously reported by Brem et al11)
PATHOGENESIS
Over the last 10 or 15 years, aldosterone’s roles in inflammation and injury have been rigorously examined in two major disease model systems, cardiovascular and renal disease. Rocha12, Weber13, Young14, Brown15, Funder16 and numerous others have each elegantly expanded the current understanding of aldosterone’s role in the pathogenesis of cardiovascular disease. Recent reviews have specifically summarized the role aldosterone plays in renal injury yet many questions remain to be answered17–19. Rather than dwell on what has already been said, we plan to focus this review on questions largely unaddressed in previous publications: is aldosterone capable of actually promoting disease on its own or is it an adjunct player modifying pre-existing renal disease, which receptor pathways might be involved, and what mechanisms normally might keep the fibrotic pathways in check. A very brief general description of what is known about aldosterone and its role in the response to renal injury seems appropriate before we address these questions.
Renal Responses to Injury
Recent reviews20–23 have characterized the various steps in the renal responses to injury and we will only briefly present the relevant highlights from those reviews. Following renal injury, pro-inflammatory chemokines like MCP-1 (monocyte chemoattractant protein-1) and RANTES are released from damaged cells24. The increase in COX-2 expression, considered to be a pro-inflammatory marker, leads to the local generation of vaso-dilating prostaglandins and a net increase in blood flow to the injured area. These prostaglandins counter ischemia-inducing peptides like endothelin-I and angiotensin II, which are released from damaged vascular beds. Mononuclear cells are also recruited to the damaged area by various chemoattractant and leukocyte adhesion molecules. A series of chemical reactions lead to the local production of reactive oxygen species (ROS), which ultimately result in the disruption of foreign proteins5. ROS are bactericidal and are also part of the initial inflammatory process. As the development of initial injury matures, the process of fibrogenesis follows a similar pattern to normal wound healing. Activated fibroblasts (myofibroblasts) either migrate to the injured area or are formed in situ from residual renal tubular epithelial cells in a process known as epithelial to mesenchymal transition (EMT). Activated pro-fibrotic growth factors including insulin-like-growth factor-1, connective tissue growth factor, angiotensin II, and transforming growth factor-beta are elaborated from the damaged tissue to promote the production of more myofibroblasts and the net generation of extracellular matrix.
In the next phase, damaged tissues can either heal with a return to normal structure and function or be converted into non-functional fibrotic scar. The course of events largely is determined by the extent of the injury and by whether factors favor excessive matrix synthesis with excessive maladaptive EMT or factors limit matrix accumulation by promoting matrix degradation accompanied by the regeneration of normal cells. Fibrosis may then result from over amplification of the fibrogenic factors following a single severe injury or repeated bouts of injury occurring over time. Normally matrix provides the structural support for replacement cells and consists of proteins including collagens I and III so its generation is a necessary element of both healing and repair. However in damaged kidneys, the matrix also contains novel chains of collagen IV and fibronectin. This type of excess matrix forms cross linkages, which makes it difficult to break down and leads to scar rather than normal replacement tissue.
RECENT ADVANCES
The Role of Aldosterone in Renal Fibrosis
Human and whole animal studies have clearly implicated aldosterone as player in renal injury, inflammation, and ensuing fibrosis25, 26. However, most of these studies describe a beneficial effect of aldosterone antagonists in pre-existing forms of renal injury1, 27–29. What is the evidence that aldosterone itself can contribute to injury, inflammation, and the fibrotic response (see figure 1)? Aldosterone and deoxycorticosterone (DOCA), a cousin mineralocorticoid, can both activate circulating lymphocytes30 and locally increase superoxide production (ROS) through activation of NADPH oxidase in mesangial cells5, 31. Activation of T cells and superoxide production are part of the first phase of inflammation. In mesangial cells32 and in collecting duct epithelial cells33, aldosterone promotes the phosphorylation of serum glucocorticoid kinase-1 (SGK-1), which then leads to phosphorylaton of IΚB and its subsequent inactivation and activation of NF-ΚB. The NF-ΚB translocates to the nucleus and stimulates additional genes responsible for the inflammatory and fibrotic processes including CTGF and TGF-β. Mineralocorticoids also have been implicated in promoting EMT and apoptosis in proximal renal tubules34 as well as renal interstitial fibrosis35. Interestingly, many of the studies mentioned above used supra-physiological concentrations of mineralocorticoid coupled with induced injury to achieve these effects. Recently, our laboratory demonstrated that low concentrations of aldosterone (10 nM) can directly induce early fibrotic changes in cultured renal collecting duct cells (CCD) in as little as 48 hours36. This response in CCD cells, which contain MR and 11β-HSD-2 is consistent with activation of genomic processes. Moreover, the observed significant increase in extracellular matrix (collagen) occurred in the absence of inflammatory cells. In contrast, we did not observe any changes in cultured proximal tubule epithelial cells, which lack a mineralocorticoid receptor.
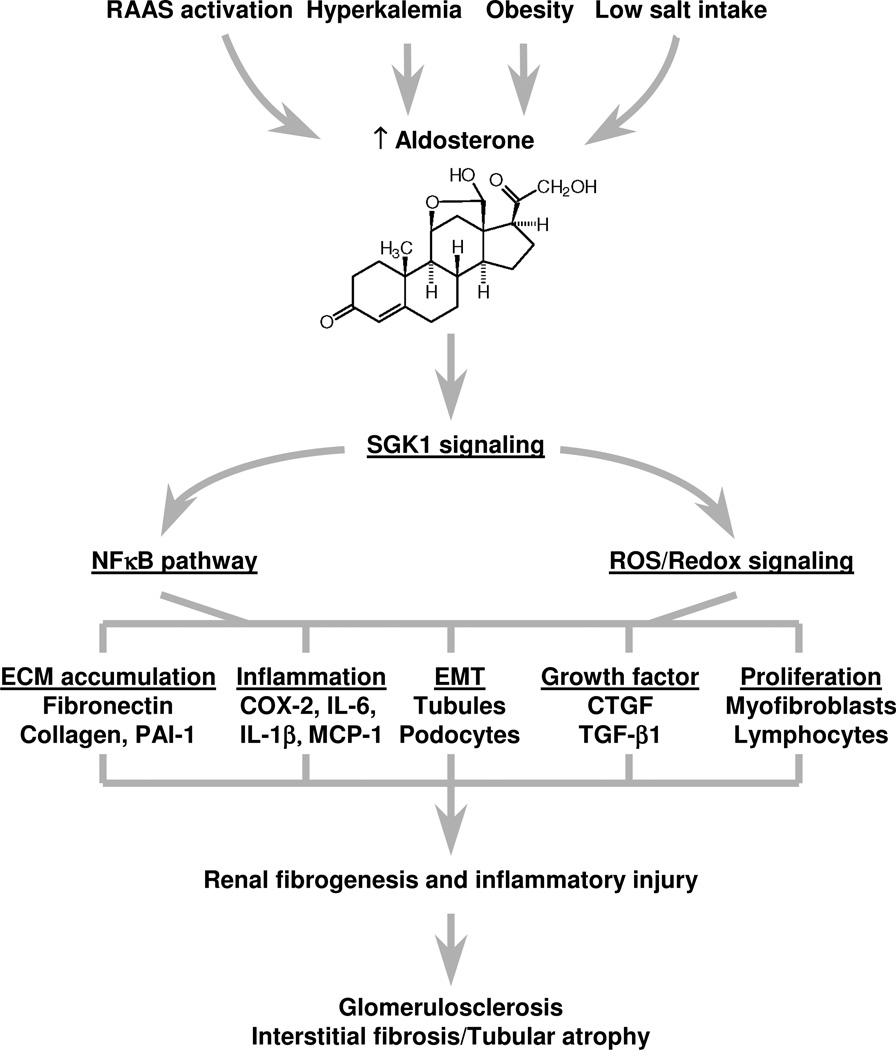
Figure 1.
Chronically stimulate Aldosterone secretion can lead to activation of pro-inflammatory and pro-fibrotic pathways in mineralocorticoid responsive cells. In the kidney, activation of these pathways is associated with both glomerular sclerosis and interstitial fibrosis with tubular atrophy.
From the discussion above, it is clear aldosterone can contribute to renal injury and inflammation but can the hormone itself actually induce fibrotic changes in the kidney in vivo? There are several lines of evidence that under certain conditions, aldosterone alone can. Reungui and co-workers37 demonstrated that over a 20 study week period, treatment with thiazide diuretics was associated with an increase in osteopontin expression and an increase in oxidative stress in rats. These changes did not correlate with hypokalemia but were related to hyperaldosteronism37. Leroy and her associates33 showed that in rats fed a very sodium restricted diet, the NF-κB pathway and PAI-1 mRNA were activated in renal cortical collecting ducts after only one week. The effects were produced by endogenous aldosterone. These effects could be blocked if the animals were treated with spironolactone. Our laboratory explored this question in a murine model36. Mice were either adrenalectomized or left adrenally intact and treated with aldosterone 8 µgrams/kg/day delivered via an Alzet pump for one week. This intervention gives levels of aldosterone that are high physiologic approximating what one might see with chronic administration of diuretics. Early fibrotic changes characterized by increased interstitial collagen accumulation and mesangial hypercellularity were observed in aldosterone treated adrenally intact mice but were much more noticeable in the aldosterone treated adrenalectomized mice (figure 2). These changes directly correlated with elevated expression of collagen, fibronectin, and connective tissue growth factor relative to controls. If the aldsterone treated mice were also given the aldosterone inhibitor RU-318 or the 11β-HSD end product 11-dehydrocorticosterone, the aldosterone induced fibrotic changes were attenuated. The mice in the study were not hypertensive and did not have pre-existing renal disease or injury. Thus it appears as though aldosterone alone can, under selected circumstances, induce fibrotic changes in the kidney in vivo.
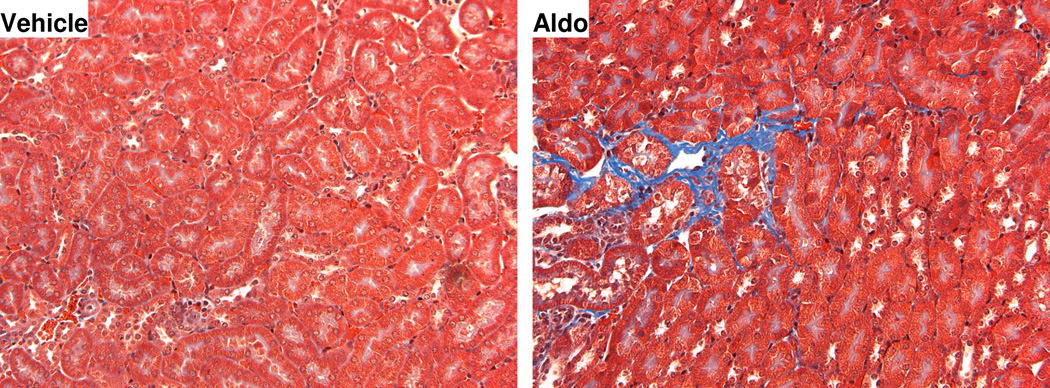
Figure 2.
Compared to controls (left panel), Aldosterone (8 micrograms/kg/day) (right panel) is capable of inducing early fibrotic interstitial changes in both cortex and medulla after only one week in an adrenalectomized mice without pre-existing hypertension or renal injury. The blue staining represents collagen matrix (magnification × 400). See reference 34 for details.
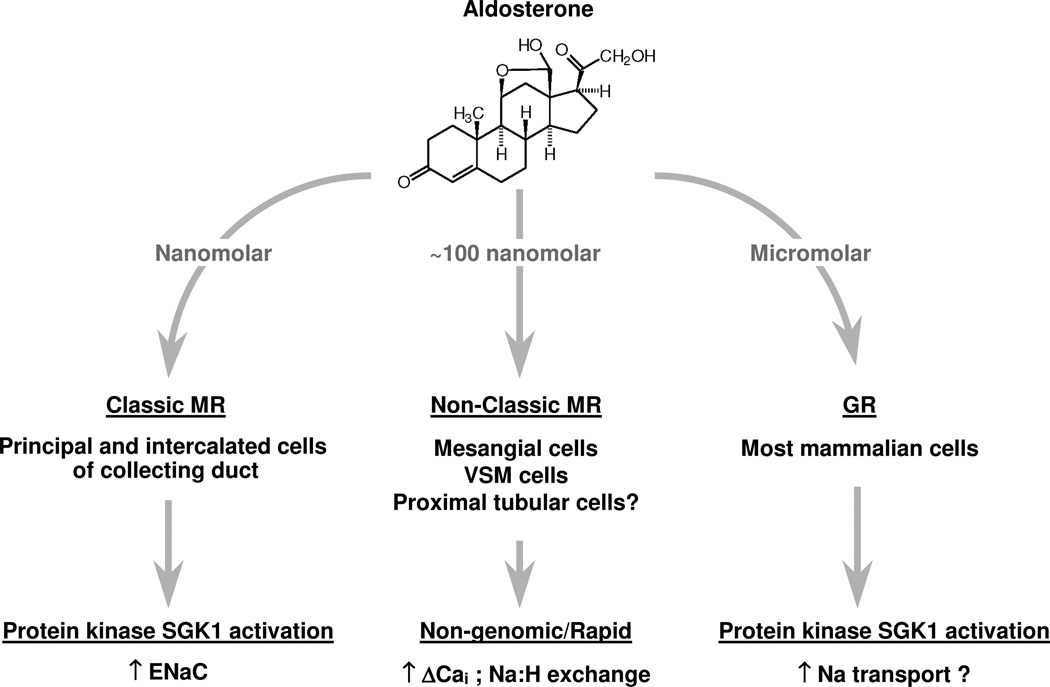
Despite the growing number of publications in this area, certain issues remain unresolved. Most notably, cells like lymphocytes30, vascular muscle cells30, and mesangial cells5 appear to express putative mineralocorticoid receptors (MRs) but those receptors do not appear to function as classical MRs (figure 3). Classical MRs, as exist in renal collecting duct epithelial cells, bind aldosterone in the cytosol, then the MR-hormone complex translocates to the nucleus, and induces genes that produce specific proteins; a process requiring at least 90 minutes or so before biologic events begin to occur38–41. Atypical or non-classical MRs, which appear to exist in white blood cells30, vascular tissue30, 42, mesangial cells5, and podocytes43, are located in the cell membrane and bind aldosterone with a binding affinity for aldosterone that appears to be an order of magnitude higher than cytosolic classical MRs30. Once bound to this atypical membrane-associated receptor, aldosterone almost immediately induces effects that are non genomic. These non-classical MRs are involved in cell signaling and when activated, generate a spike in cytosolic calcium occurring in seconds accompanied by a fall in intracellular pH followed by a rise in sodium-hydrogen ion exchange activity with an increase in intracellular sodium and pH30, 42. In mesangial cells, inhibition of protein synthesis does not appear to affect the changes induced by aldosterone, consistent with the idea that non-classical MR activation does not lead to gene induction and generation of new proteins5. The question then is how do aldosterone activated processes, presumably mediated through non-classical MR and with signals lasting seconds to minutes, lead to chronic irreversible fibrotic changes?
Figure 3.
Aldosterone can bind to several different receptors including classical MR, non-classical or atypical membrane associated MR, and GR depending on the cell type and function.
In mesangial32 and perhaps other renal cells like podocytes43, non-classical MR activation is associated with up-regulation of serum and glucocorticoid kinase 1 (SGK-1), an enzyme that aldosterone also stimulates when classical MRs are activated for sodium transport in renal collecting duct epithelial cells9, 44. Gene pathways, which are likely pro-fibrotic including upregulation of the NF-κB pathway and the expression of connective tissue growth factor, fibronectin, and collagen 1, are also activated in sodium transporting renal collecting duct epithelial cells after more prolonged aldosterone exposure33, 36, 45. In most of the above studies involving cells with non-classical MRs, the maximum biologic response required concentrations of aldosterone of 100 nM or more and commenced within an hour of exposure to the hormone. When activated, these non-classical MRs clearly are associated with fibrotic changes in the kidney and may contribute indirectly to genomic processes32, 46. However, the potential for fibrosis to occur probably depends on the continued presence of non-classical MRs in the cell membrane and exposure to high concentrations of aldosterone over time to maintain the signal. Most cells normally recycle membrane bound receptors and limit the exposure of those receptors in the cell membrane when the ligand levels are excessive. If non-classical MRs behave as one might expect, aldosterone induced pro-fibrotic signaling should normally be limited and may only occur under conditions where the hormone levels are excessively high.
Whether and/or how aldosterone may influence fibrotic changes in renal proximal tubules is a question that has a complicated answer. Aldosterone has been reported to induce apoptosis and possibly to promote epithelial to mesenchymal transition in proximal tubule epithelial cells34. However in our laboratory, high but physiologically attainable concentrations of aldosterone (10 nM) appear to have no effect on either cell transformation or extracellular matrix production after 48 hours36. It is generally well accepted that classical MRs do not exist in this segment and the existence of non-classical MRs is controversial. Since aldosterone can bind to glucocorticoid receptors (GRs) when concentrations exceed 100 nM47, some mineralocorticoid effects may be mediated through the hormone binding to glucocorticoid receptors present in cells46 (figure 3). Activation of GRs leads to up-regulation of connective tissue growth factor (CTGF), a known early pro-fibrotic protein48. CTGF and other pro-fibrotic pathways can be partially blocked by the GR antagonist RU-486 so it does appear that some of the aldosterone induced pro-fibrotic events could be mediated by GRs.
Renal collecting duct epithelial cells containing classical MRs normally respond to aldosterone by increasing sodium transport and potassium secretion, an effect that only lasts a period of hours after exposure to the hormone38. Pro-fibrotic changes recently described in cultured renal collecting duct cells require a more prolonged period of aldosterone exposure (48 hours) before they occur36 but these changes can be induced with concentrations of aldosterone that induce sodium transport and are in the high physiologic range. What then prevents aldosterone from inducing fibrosis all the time? As we also have reviewed elsewhere49, some years ago, various investigators observed a paradox involving aldosterone and its effects on electrolyte transport: the impact of aldosterone on sodium transport was evident in preparations of cell membrane from toad bladder50 and isolated perfused renal collecting ducts51. However, aldosterone-mediated renal sodium reabsorption was not reproducibly seen in animal with intact adrenal glands52, 53. If animals were adrenalectomized prior to treatment with physiological doses of aldosterone, the expected antinatriuretic effect was clearly present. These observations lead to the idea that aldosterone must be physiologically regulated by substances most likely generated by or derived from the adrenal gland.
Uete and Venning54, described candidate adrenal steroids, which were able to interfere with the renal antinatriuretic actions of mineralocorticoid. Administration of cortisol or its 11-dehydro (11-keto) metabolite, cortisone, attenuated the antinatriuretic response to both DOCA and aldosterone in the adenalectomized rat model. This blunting effect of cortisol and cortisone was dose dependent. Later, Alberti and Sharp55 suggested a mechanism by demonstrating that cortisone functioned as an aldosterone antagonist. Cortisone had no direct effect on sodium transport by itself but it was able to limit aldosterone nuclear binding even though cortisone does not bind well to either MRs or GRs.
Toad urinary bladders are a model of the mammalian collecting duct, which contain MRs and 11β-HSD-2 with no reductase activity. In our laboratory, toad bladders exposed to the 11-dehydro (11-keto) product of corticosterone, 11-dehydrocorticosterone, and then stimulated with aldosterone exhibited a markedly suppressed response in trans-epithelial sodium transport compared to controls stimulated with aldosterone alone56. A similar pattern was seen when bladders were exposed to corticosterone and then stimulated with aldosterone. Both corticosterone and 11-dehydrocorticosterone also suppressed aldosterone induced renal sodium retention in the adrenalectomized rat model57. In separate studies, we were able to show that 11-dehydrocorticosterone alone does not directly activate mineralocorticoid receptors but it is able to blunt aldosterone activation of these same receptors58. Interestingly, 11-dehydrocorticosterone has no effect on dexamethasone binding to glucocorticoid receptors. Odermatt and his colleagues extended these receptor binding studies in demonstrating that both 11-dehydrocorticosterone and cortisone in concentrations as low as 50 nM block aldosterone 10 nM induced MR activation in transfected HEK-293 cells59. Taken together then, ample evidence exists that the 11-dehydro metabolites of the endogenous glucocorticoids, cortisol and corcorticosterone, blunt aldosterone stimulated electrolyte transport not by directly binding to MR but by somehow preventing aldosterone from translocating to its nuclear binding site. Our laboratory now has extended this idea and we have demonstrated that 11-dehydrocorticosterone also blunts the pro-fibrotic actions of aldosterone in a dose dependent fashion36.
Are 11-dehydro metabolites of endogenous glucocorticoids locally generated by the kidney in concentrations that would be biologically significant? The endogenously produced glucocorticoids cortisol and corticosterone are metabolized in the kidney by isoforms of the enzyme 11β-Hydroxysteroid Dehydrogenase (11β-HSD). Mammalian renal proximal tubules contain 11β-HSD-1, which uses NADP as a cofactor and has a Km in the range of 1 µM for glucocorticoids60–63. While in most other sites in the body this isoform of the enzyme is bi-directional, in the kidney it appears to function only as a dehydrogenase generating 11-dehydro products60, 61. Renal collecting duct epithelial cells containing MRs also have 11β-HSD-2, an isoform that is dependent on NAD as a co-factor and has a Km in the 10 nM range64. Could these two enzymes locally produce enough 11-dehydro metabolite to adequately attenuate the effects of aldosterone? In an attempt to answer this question, a few calculations need to be combined with what is already known. The average plasma concentration of cortisol in the human is 10 µgram/dl (the actual range is 5 to 20 µgram/dl) and approximately 10% of that cortisol normally exists in a non-protein bound free state. Assume the average glomerular filtration rate for a human is 100 ml/minute (1 dl/min) then the kidney filters about 1,440 µgrams of free cortisol in a 24-hour period. We know that only about 50 µgrams of cortisol can be directly recovered in a 24 hour urine collection (about 3.5% of the filtered cortisol) and that the major metabolites are 11-dehydro glucocorticoids and their Ring A reduced derivatives65. Thus it is quite likely that adequate concentrations of cortisone and/or 11-dehydrocorticosterone are produced.
The 11-dehydro metabolites of cortisol and corticosterone are effective endogenously produced inhibitors of aldosterone action in tissues containing classical MRs. How they perform this task remains to be fully elucidated. Yet one still can ask the question; do they also work on atypical or non-classical membrane bound MRs? To date, the binding studies necessary to answer this question haven’t been done. In preliminary experiments conducted in the murine model, it does appear that 11-dehydrocorticosterone can limit aldosterone induced increase in mesangial cell number and accompanying matrix36. This is significant since is appears likely that mesangial cells contain non-classical MRs as previously discussed.
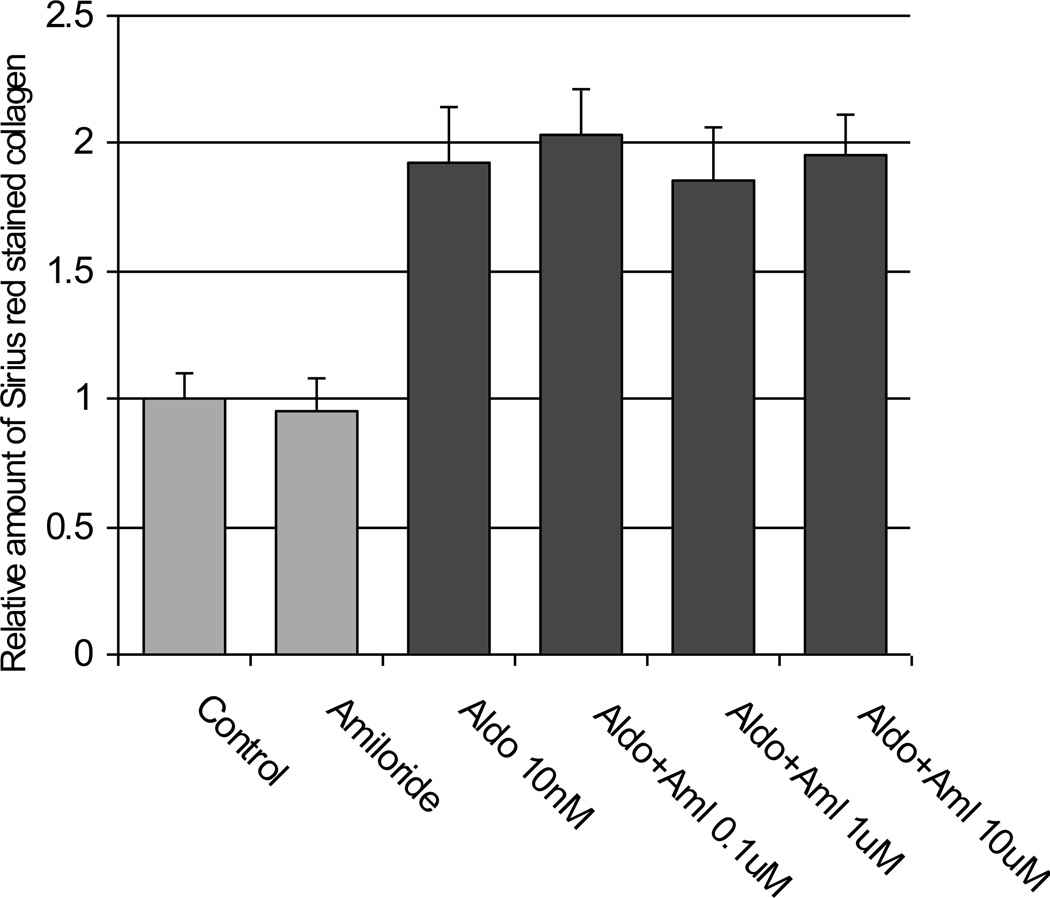
Lastly, is sodium somehow linked to aldosterone-induced fibrosis? The many papers have noted that previously injured renal tissue demonstrated an enhanced degree of fibrosis if the animals were maintained on a high salt diet and treated with mineralocorticoids66–69. Reduction in dietary sodium intake seemed to be protective especially when combined with aldosterone antagonists. In addition to stimulating sodium transport in renal collecting duct epithelial cells via classical MRs, aldosterone acutely increases sodium hydrogen ion antiporter activity in cells with non-classical MRs42, 70 so there is a link between aldsterone and sodium, either through transepithelial transport or cell signaling. To try and separate the effects on sodium from the effects on transport, we cultured renal collecting duct epithelial cells (IMCD) in plastic dishes. The cells grew with their basolateral surface adhering to the plastic dish so that no real transepithelial sodium transport could take place. Under these conditions, we observed that aldosterone was able to induce pro-fibrotic changes with increased extracellular matrix production. We also repeated this experiment but cultured the IMCD on semi-permeable supports so the cells could maintain polarity and transport sodium. The cells were stimulated with aldosterone 10 nM for 48 hours but this time amiloride was added to the medium bathing the apical surface to block the aldosterone sensitive sodium channel (ENaC). As before, extracellular matrix increased in the absence of active sodium transmembrane transport (figure 4). These preliminary findings lead us to the view that in IMCD, active aldosterone stimulated sodium transport is not needed in order to see the fibrotic changes. Whether sodium somehow enhances that fibrotic process though cell signaling can’t be answered from present data.
Figure 4.
IMCD generated extracellular matrix measured by Sirius Red staining when the cells were exposed to Aldosterone 10 nM for 48 hours. The cells were grown on semi-permeable supports and were polarized to allow for transmembrane sodium transport. When amiloride was added to the medium bathing the apical cell surface inhibiting the sodium channel (ENaC), the Aldosterone induced increase in matrix occurred even with sodium transport blocked.
SUMMARY
In re-examining our case vignette, it became clear that our patient’s hyperaldosteronism was going to be a life long condition. The natural history of her chronic disease could be seen as our patient reached adolescence with the subtle fall in GFR and development of low-grade proteinuria. Treatment options included the use of multiple anti-hypertensive agents and attempts to directly block the actions of aldosterone with spironolactone. As with any chronic condition, the ultimate success or failure of treatment depends on the effectiveness of drugs and the willingness and ability of the patient to comply with the therapy. In our case, compliance became a real compounding issue. Although the patient was never biopsied, it seems likely that fibrotic changes developed within both kidneys. As fibrosis replaced normal renal tissue, it also is likely that the endogenous renal protective effects of 11β-HSD metabolites against aldosterone action became less as well.
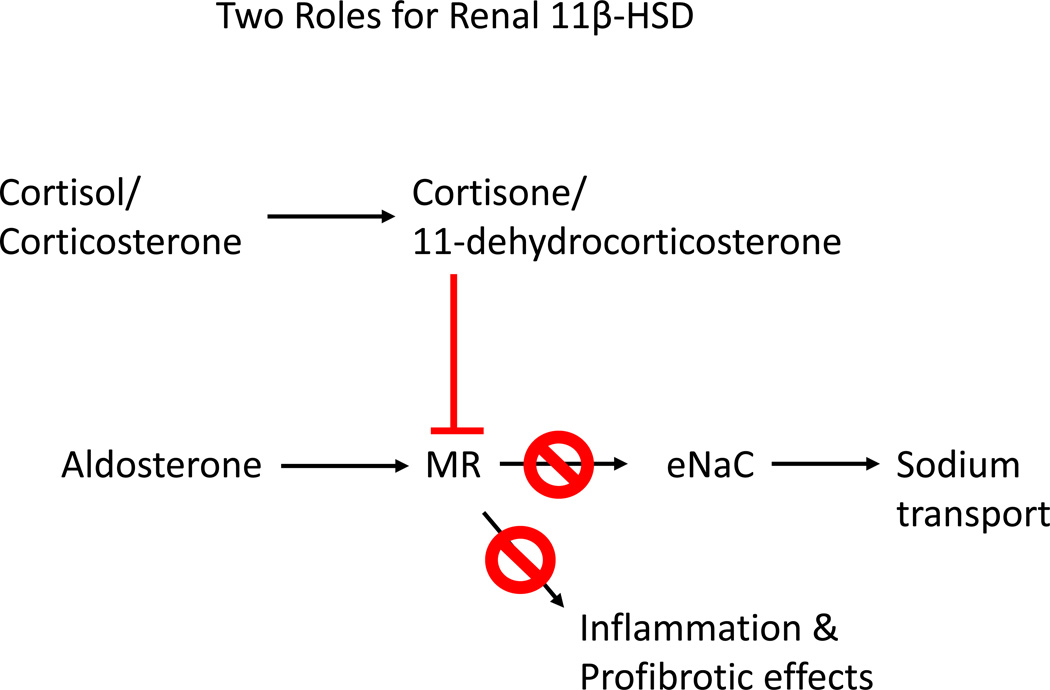
At the present moment, we know that aldosterone clearly can be a pro-fibrotic agent and can exert this effect in fairly low concentrations approaching the high physiological range. What prevents the fibrotic change from occurring is the fact that a prolonged and/or repetitive exposure seems to be necessary. Recognizing that aldosterone has a pro-fibrotic as well as a physiologic role regulating salt and water balance, an endogenous system is in place to counter excessive and unnecessary aldosterone activity, the 11-dehydro (11-keto) metabolites of cortisol and corticosterone. Renal 11β-HSD-2 present in collecting duct cells may then perform two functions; first to prevent inappropriate glucocorticoid activation of MRs and second, to locally generate 11-dehydro metabolites, which limit aldosterone activity (figure 5). These dual roles can be extended to 11β-HSD-1 contained in renal proximal tubules; this isoform provides a source of 11-dehydro-metabolites that are released in a paracrine fashion into the urine or blood and gain access to the collecting duct epithelial cells downstream. With these concepts in mind, it seems reasonable to consider the use of aldosterone inhibitors like spironolactone and agents, which might enhance renal 11β-HSD activity (if and when they become available) in patients either at risk of or with active chronic kidney disease.
Figure 5.
Renal 11β-Hydroxysteroid Dehydrogenase isoforms perform two functions; first to prevent endogenous glucocorticoids from inappropriately binding to MR and second to generate 11-dehydro (11-keto) glucocorticoid metabolites, which serve to attenuate the actions of aldosterone in the kidney.
References
- 1.Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest. 1996;98:1063–1068. doi: 10.1172/JCI118867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selye H. Anticortisol action of aldosterone. Science. 1955;121:368–369. doi: 10.1126/science.121.3141.368. [DOI] [PubMed] [Google Scholar]
- 3.Klinge U, Theuer S, Krott E, Fiebeler A. Absence of circulating aldosterone attenuates foreign body reaction around surgical sutures. Langenbecks Arch Surg. 2009;395:429–435. doi: 10.1007/s00423-009-0473-0. [DOI] [PubMed] [Google Scholar]
- 4.Slight SH, Chilakamarri VK, Nasr S, Dhalla AK, Ramires FJ, Sun Y, Ganjam VK, Weber KT. Inhibition of tissue repair by spironolactone: role of mineralocorticoids in fibrous tissue formation. Mol Cell Biochem. 1998;189:47–54. doi: 10.1023/a:1006844010371. [DOI] [PubMed] [Google Scholar]
- 5.Huang S, Zhang A, Ding G, Chen R. Aldosterone-induced mesangial cell proliferation is mediated by EGF receptor transactivation. Am J Physiol Renal Physiol. 2009;296:F1323–F1333. doi: 10.1152/ajprenal.90428.2008. Epub 2009 Apr 1321. [DOI] [PubMed] [Google Scholar]
- 6.Squarize CH, Castilho RM, Bugge TH, Gutkind JS. Accelerated wound healing by mTOR activation in genetically defined mouse models. PLoS. 5:e10643. doi: 10.1371/journal.pone.0010643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol. 2009;20:2493–2502. doi: 10.1681/ASN.2008111186. Epub 2009 Oct 2429. [DOI] [PubMed] [Google Scholar]
- 8.Lu M, Wang J, Jones KT, Ives HE, Feldman ME, Yao LJ, Shokat KM, Ashrafi K, Pearce D. mTOR complex-2 activates ENaC by phosphorylating SGK1. J Am Soc Nephrol. 2010;21:811–818. doi: 10.1681/ASN.2009111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naray-Fejes-Toth A, Helms MN, Stokes JB, Fejes-Toth G. Regulation of sodium transport in mammalian collecting duct cells by aldosterone-induced kinase, SGK1: structure/function studies. Mol Cell Endocrinol. 2004;217:197–202. doi: 10.1016/j.mce.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 11.Brem AS, Oyer CE, Noto RB. Progressive hypertension associated with hypokalemic alkalosis. J Pediatr. 1991;118:479–484. doi: 10.1016/s0022-3476(05)82172-9. [DOI] [PubMed] [Google Scholar]
- 12.Rocha R, Stier CT, Jr, Kifor I, Ochoa-Maya MR, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141:3871–3878. doi: 10.1210/endo.141.10.7711. [DOI] [PubMed] [Google Scholar]
- 13.Weber KT, Sun Y, Guarda E. Structural remodeling in hypertensive heart disease and the role of hormones. Hypertension. 1994;23:869–877. doi: 10.1161/01.hyp.23.6.869. [DOI] [PubMed] [Google Scholar]
- 14.Young MJ, Funder JW. Mineralocorticoids, salt, hypertension: effects on the heart. Steroids. 1996;61:233–235. doi: 10.1016/0039-128x(96)00020-7. [DOI] [PubMed] [Google Scholar]
- 15.Brown NJ. Aldosterone and end-organ damage. Curr Opin Nephrol Hypertens. 2005;14:235–241. doi: 10.1097/01.mnh.0000165889.60254.98. [DOI] [PubMed] [Google Scholar]
- 16.Funder J. Mineralocorticoids and cardiac fibrosis: the decade in review. Clin Exp Pharmacol Physiol. 2001;28:1002–1006. doi: 10.1046/j.1440-1681.2001.03586.x. [DOI] [PubMed] [Google Scholar]
- 17.Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney Int. 2004;66:1–9. doi: 10.1111/j.1523-1755.2004.00701.x. [DOI] [PubMed] [Google Scholar]
- 18.Remuzzi G, Cattaneo D, Perico N. The aggravating mechanisms of aldosterone on kidney fibrosis. J Am Soc Nephrol. 2008;19:1459–1462. doi: 10.1681/ASN.2007101079. Epub 2008 Jun 1411. [DOI] [PubMed] [Google Scholar]
- 19.Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat. 6:261–273. doi: 10.1038/nrneph.2010.30. Epub 2010 Mar 2016. [DOI] [PubMed] [Google Scholar]
- 20.Eddy AA. Progression in chronic kidney disease. Adv Chronic Kidney Dis. 2005;12:353–365. doi: 10.1053/j.ackd.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Iwano M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. Curr Opin Nephrol Hypertens. 2004;13:279–284. doi: 10.1097/00041552-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Eddy AA, Fogo AB. Plasminogen Activator Inhibitor-1 in Chronic Kidney Disease: Evidence and Mechanisms of Action. J Am Soc Nephrol. 2006;17:2999–3012. doi: 10.1681/ASN.2006050503. Epub 2006 Oct 2911. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 24.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63:1791–1800. doi: 10.1046/j.1523-1755.2003.00929.x. [DOI] [PubMed] [Google Scholar]
- 25.Sato A, Saruta T, Funder JW. Combination therapy with aldosterone blockade and renin-angiotensin inhibitors confers organ protection. Hypertens Res. 2006;29:211–216. doi: 10.1291/hypres.29.211. [DOI] [PubMed] [Google Scholar]
- 26.Hostetter TH, Rosenberg ME, Ibrahim HN, Juknevicius I. Aldosterone in progressive renal disease. Semin Nephrol. 2001;21:573–579. doi: 10.1053/snep.2001.26797. [DOI] [PubMed] [Google Scholar]
- 27.Han KH, Kang YS, Han SY, Jee YH, Lee MH, Han JY, Kim HK, Kim YS, Cha DR. Spironolactone ameliorates renal injury and connective tissue growth factor expression in type II diabetic rats. Kidney Int. 2006;70:111–120. doi: 10.1038/sj.ki.5000438. Epub 2006 May 2024. [DOI] [PubMed] [Google Scholar]
- 28.Aldigier JC, Kanjanbuch T, Ma LJ, Brown NJ, Fogo AB. Regression of existing glomerulosclerosis by inhibition of aldosterone. J Am Soc Nephrol. 2005;16:3306–3314. doi: 10.1681/ASN.2004090804. Epub 2005 Sep 3328. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda H, Tsuruya K, Toyonaga J, Masutani K, Hayashida H, Hirakata H, Iida M. Spironolactone suppresses inflammation and prevents L-NAME-induced renal injury in rats. Kidney Int. 2009;75:147–155. doi: 10.1038/ki.2008.507. Epub 2008 Oct 2015. [DOI] [PubMed] [Google Scholar]
- 30.Christ M, Wehling M. Rapid actions of aldosterone: lymphocytes, vascular smooth muscle and endothelial cells. Steroids. 1999;64:35–41. doi: 10.1016/s0039-128x(98)00103-2. [DOI] [PubMed] [Google Scholar]
- 31.Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, Kimura S, Yukimura T, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol. 2005;16:2906–2912. doi: 10.1681/ASN.2005040390. Epub 2005 Aug 2931. [DOI] [PubMed] [Google Scholar]
- 32.Terada Y, Kuwana H, Kobayashi T, Okado T, Suzuki N, Yoshimoto T, Hirata Y, Sasaki S. Aldosterone-stimulated SGK1 activity mediates profibrotic signaling in the mesangium. J Am Soc Nephrol. 2008;19:298–309. doi: 10.1681/ASN.2007050531. Epub 2008 Jan 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leroy V, De Seigneux S, Agassiz V, Hasler U, Rafestin-Oblin ME, Vinciguerra M, Martin PY, Feraille E. Aldosterone activates NF-kappaB in the collecting duct. J Am Soc Nephrol. 2009;20:131–144. doi: 10.1681/ASN.2008020232. Epub 2008 Nov 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang A, Jia Z, Guo X, Yang T. Aldosterone induces epithelial-mesenchymal transition via ROS of mitochondrial origin. Am J Physiol Renal Physiol. 2007;293:F723–F731. doi: 10.1152/ajprenal.00480.2006. Epub 2007 Jun 2027. [DOI] [PubMed] [Google Scholar]
- 35.Klanke B, Cordasic N, Hartner A, Schmieder RE, Veelken R, Hilgers KF. Blood pressure versus direct mineralocorticoid effects on kidney inflammation and fibrosis in DOCA-salt hypertension. Nephrol Dial Transplant. 2008;23:3456–3463. doi: 10.1093/ndt/gfn301. Epub 2008 May 3430. [DOI] [PubMed] [Google Scholar]
- 36.Brem AS, Morris DJ, Ge Y, Dworkin LD, Tolbert E, Gong R. Direct Fibrogenic Effects of Aldosterone on Normotensive Kidney: An Effect Modified by 11{beta}-HSD Activity. Am J Physiol Renal Physiol. 2010;298:F1178–F1187. doi: 10.1152/ajprenal.00532.2009. [DOI] [PubMed] [Google Scholar]
- 37.Reungjui S, Hu H, Mu W, Roncal CA, Croker BP, Patel JM, Nakagawa T, Srinivas T, Byer K, Simoni J, Wesson D, Sitprija V, Johnson RJ. Thiazide-induced subtle renal injury not observed in states of equivalent hypokalemia. Kidney Int. 2007;72:1483–1492. doi: 10.1038/sj.ki.5002564. Epub 2007 Oct 1410. [DOI] [PubMed] [Google Scholar]
- 38.Porter GA, Bogoroch R, Edelman IS. On the Mechanism of Action of Aldosterone On Sodium Transport: the Role of Rna Synthesis. Proc Natl Acad Sci U S A. 1964;52:1326–1333. doi: 10.1073/pnas.52.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marver D, Stewart J, Funder JW, Feldman D, Edelman IS. Renal aldosterone receptors: studies with (3H)aldosterone and the anti-mineralocorticoid (3H)spirolactone (SC-26304) Proc Natl Acad Sci U S A. 1974;71:1431–1435. doi: 10.1073/pnas.71.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossier BC, Wilce PA, Edelman IS. Spironolactone antagonism of aldosterone action on Na+ transport and RNA metabolism in toad bladder epithelium. J Membr Biol. 1977;32:177–194. doi: 10.1007/BF01905216. [DOI] [PubMed] [Google Scholar]
- 41.Farman N, Kusch M, Edelman IS. Aldosterone receptor occupancy and sodium transport in the urinary bladder of Bufo marinus. Am J Physiol. 1978;235:C90–C96. doi: 10.1152/ajpcell.1978.235.3.C90. [DOI] [PubMed] [Google Scholar]
- 42.Wehling M, Bauer MM, Ulsenheimer A, Schneider M, Neylon CB, Christ M. Nongenomic effects of aldosterone on intracellular pH in vascular smooth muscle cells. Biochem Biophys Res Commun. 1996;223:181–186. doi: 10.1006/bbrc.1996.0866. [DOI] [PubMed] [Google Scholar]
- 43.Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension. 2007;49:355–364. doi: 10.1161/01.HYP.0000255636.11931.a2. Epub 2007 Jan 2002. [DOI] [PubMed] [Google Scholar]
- 44.Naray-Fejes-Toth A, Fejes-Toth G. The sgk, an aldosterone-induced gene in mineralocorticoid target cells, regulates the epithelial sodium channel. Kidney Int. 2000;57:1290–1294. doi: 10.1046/j.1523-1755.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- 45.Husted RF, Sigmund RD, Stokes JB. Mechanisms of inactivation of the action of aldosterone on collecting duct by TGF-beta. Am J Physiol Renal Physiol. 2000;278:F425–F433. doi: 10.1152/ajprenal.2000.278.3.F425. [DOI] [PubMed] [Google Scholar]
- 46.Kellner M, Peiter A, Hafner M, Feuring M, Christ M, Wehling M, Falkenstein E, Losel R. Early aldosterone up-regulated genes: new pathways for renal disease? Kidney Int. 2003;64:1199–1207. doi: 10.1046/j.1523-1755.2003.00216.x. [DOI] [PubMed] [Google Scholar]
- 47.Mishina T, Scholer DW, Edelman IS. Glucocorticoid receptors in rat kidney cortical tubules enriched in proximal and distal segments. Am J Physiol. 1981;240:F38–F45. doi: 10.1152/ajprenal.1981.240.1.F38. [DOI] [PubMed] [Google Scholar]
- 48.Okada H, Kikuta T, Inoue T, Kanno Y, Ban S, Sugaya T, Takigawa M, Suzuki H. Dexamethasone induces connective tissue growth factor expression in renal tubular epithelial cells in a mouse strain-specific manner. Am J Pathol. 2006;168:737–747. doi: 10.2353/ajpath.2006.050656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brem AS. The Janus effect: two faces of aldosterone. Kidney Int. 2009;75:137–139. doi: 10.1038/ki.2008.567. [DOI] [PubMed] [Google Scholar]
- 50.Porter GA, Edelman IS. The Action of Aldosterone and Related Corticosteroids On Sodium Transport Across the Toad Bladder. J Clin Invest. 1964;43:611–620. doi: 10.1172/JCI104946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stanton BA. Role of adrenal hormones in regulating distal nephron structure and ion transport. Fed Proc. 1985;44:2717–2722. [PubMed] [Google Scholar]
- 52.Barger AC, Berlin RD, Tulenko JF. Infusion of aldosterone, 9-alpha-fluorohydrocortisone, and antidiuretic hormone into the renal artery of normal and adrenalectomized unanesthetized dogs: effect on electrolyte and water excretion. Endocrinology. 1958;62:804–815. doi: 10.1210/endo-62-6-804. [DOI] [PubMed] [Google Scholar]
- 53.Morris DJ, Berek JS, Davis RP. The physiological response to aldosterone in adrenalectomized and intact rats and its sex dependence. Endocrinology. 1973;92:989–993. doi: 10.1210/endo-92-4-989. [DOI] [PubMed] [Google Scholar]
- 54.Uete T, Venning EH. The effect of cortisone, hydrocortisone, and 9-alpha-fluoro-16-alpha-hydroxy-delta-hydrocortisone on the action of desoxycorticosterone and aldosterone with respect to electrolyte excretion. Endocrinology. 1960;67:62–69. doi: 10.1210/endo-67-1-62. [DOI] [PubMed] [Google Scholar]
- 55.Alberti KGMM, Sharp GWG. Identification of four types of steroid by their interaction with mineralocorticoid receptors in the toad bladder. J Endocrinology. 1970;48:563–574. doi: 10.1677/joe.0.0480563. [DOI] [PubMed] [Google Scholar]
- 56.Brem AS, Matheson KL, Barnes JL, Morris DJ. 11-Dehydrocorticosterone, a glucocorticoid metabolite, inhibits aldosterone action in toad bladder. American Journal of Physiology. 1991;261:F873–F879. doi: 10.1152/ajprenal.1991.261.5.F873. [DOI] [PubMed] [Google Scholar]
- 57.Souness GW, Myles K, Morris DJ. Other physiological considerations of protective mechanisms of mineralocorticoid action. Steroids. 1994;59:142–147. doi: 10.1016/0039-128x(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 58.Morris DJ, Souness GW, Brem AS, Oblin ME. Interactions of mineralocorticoids and glucocorticoids in epithelial target tissues. Kidney Int. 2000;57:1370–1373. doi: 10.1046/j.1523-1755.2000.00977.x. [DOI] [PubMed] [Google Scholar]
- 59.Odermatt A, Arnold P, Frey FJ. The intracellular localization of the mineralocorticoid receptor is regulated by 11beta-hydroxysteroid dehydrogenase type 2. J Biol Chem. 2001;276:28484–28492. doi: 10.1074/jbc.M100374200. Epub 22001 May 28411. [DOI] [PubMed] [Google Scholar]
- 60.Gong R, Morris DJ, Brem AS. Human renal 11beta-hydroxysteroid dehydrogenase 1 functions and co-localizes with COX-2. Life Sci. 2008;82:631–637. doi: 10.1016/j.lfs.2007.12.019. Epub 2008 Jan 2002. [DOI] [PubMed] [Google Scholar]
- 61.Brem AS, Bina RB, King T, Chobanian MC, Morris DJ. Influence of dietary sodium on the renal isoforms of 11 beta- hydroxysteroid dehydrogenase [In Process Citation] Proc Soc Exp Biol Med. 1997;214:340–345. doi: 10.3181/00379727-214-44101. [DOI] [PubMed] [Google Scholar]
- 62.Brem AS, Bina RB, Fitzpatrick C, King T, Tang SS, Ingelfinger JR. Glucocorticoid metabolism in proximal tubules modulates angiotensin II-induced electrolyte transport. Proc Soc Exp Biol Med. 1999;221:111–117. doi: 10.1046/j.1525-1373.1999.d01-63.x. [DOI] [PubMed] [Google Scholar]
- 63.Brem AS, Bina B, Matheson KL, Barnes JL, Morris DJ. Developmental changes in rat renal 11 beta-hydroxysteroid dehydrogenase. Kidney International. 1994;45:679–683. doi: 10.1038/ki.1994.91. [DOI] [PubMed] [Google Scholar]
- 64.Naray-Fejes-Toth A, Fejes-Toth G. 11 beta-Hydroxysteroid dehydrogenase in renal collecting duct cells. [Review] Steroids. 1994;59:105–110. doi: 10.1016/0039-128x(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 65.Best R, Walker BR. Additional value of measurement of urinary cortisone and unconjugated cortisol metabolites in assessing the activity of 11 beta-hydroxysteroid dehydrogenase in vivo. Clin Endocrinol (Oxf) 1997;47:231–236. doi: 10.1046/j.1365-2265.1997.2471061.x. [DOI] [PubMed] [Google Scholar]
- 66.Vallon V, Wyatt AW, Klingel K, Huang DY, Hussain A, Berchtold S, Friedrich B, Grahammer F, Belaiba RS, Gorlach A, Wulff P, Daut J, Dalton ND, Ross J, Jr, Flogel U, Schrader J, Osswald H, Kandolf R, Kuhl D, Lang F. SGK1-dependent cardiac CTGF formation and fibrosis following DOCA treatment. J Mol Med. 2006;84:396–404. doi: 10.1007/s00109-005-0027-z. Epub 2006 Apr 2008. [DOI] [PubMed] [Google Scholar]
- 67.Artunc F, Amann K, Nasir O, Friedrich B, Sandulache D, Jahovic N, Risler T, Vallon V, Wulff P, Kuhl D, Lang F. Blunted DOCA/high salt induced albuminuria and renal tubulointerstitial damage in gene-targeted mice lacking SGK1. J Mol Med. 2006;84:737–746. doi: 10.1007/s00109-006-0082-0. Epub 2006 Aug 2010. [DOI] [PubMed] [Google Scholar]
- 68.Lam EY, Funder JW, Nikolic-Paterson DJ, Fuller PJ, Young MJ. Mineralocorticoid receptor blockade but not steroid withdrawal reverses renal fibrosis in deoxycorticosterone/salt rats. Endocrinology. 2006;147:3623–3629. doi: 10.1210/en.2005-1527. Epub 2006 Apr 3620. [DOI] [PubMed] [Google Scholar]
- 69.Lea WB, Kwak ES, Luther JM, Fowler SM, Wang Z, Ma J, Fogo AB, Brown NJ. Aldosterone antagonism or synthase inhibition reduces end-organ damage induced by treatment with angiotensin and high salt. Kidney Int. 2009;75:936–944. doi: 10.1038/ki.2009.9. Epub 2009 Feb 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Young M, Funder J. Mineralocorticoid action and sodium-hydrogen exchange: studies in experimental cardiac fibrosis. Endocrinology. 2003;144:3848–3851. doi: 10.1210/en.2003-0039. [DOI] [PubMed] [Google Scholar]